Abstract
Invasive fusariosis is a highly aggressive fungal infection associated with high mortality in heavily immunocompromised patients. Although posaconazole is efficacious as salvage therapy against infections caused by Fusarium species, concerns remain regarding this agent in the setting of reduced potency. To evaluate the efficacy of posaconazole as treatment or prophylaxis against invasive fusariosis caused by Fusarium solani, we utilized a neutropenic murine model of disseminated disease. ICR mice were administered escalating doses of posaconazole (6.25, 12.5, 25, or 50 mg/kg of body weight twice daily [BID]) by oral gavage beginning 2 days prior to inoculation in the prophylaxis studies or beginning 12 h after inoculation as treatment. Therapy was continued until day 9 postinoculation, and animals were monitored off therapy until day 15 for survival. Fungal burden was assessed as CFU in the kidneys. A clear dose-response relationship was observed, as the highest dose of posaconazole (50 mg/kg) was the most effective in prolonging survival and reducing tissue fungal burden both as prophylaxis and as treatment. This dose response was associated with high posaconazole serum concentrations as measured by bioassay. However, the extent of efficacy was also dependent on the infecting inoculum, as greater increases in survival and reductions in fungal burden were observed with the lower inocula tested. In this model high dosages of posaconazole were effective as treatment and prophylaxis against disseminated fusariosis caused by F. solani.
Fusarium species are among the leading causes of invasive mold infections among patients with hematologic malignancies and those undergoing hematopoietic stem cell transplantation (HSCT), with mortality exceeding 70% (1, 2, 15). Risk factors for disseminated infection include neutropenia, lymphopenia, graft-versus-host disease, corticosteroids, and immunosuppressive therapy (1, 2). In vitro studies have reported moderate to negligible activity of available antifungals against Fusarium, with amphotericin B and itraconazole having minimal activity (3, 8, 16). Reduced in vitro potency has also been reported for voriconazole and posaconazole when measured by standard microdilution methodology (3, 8, 16). However, both voriconazole and posaconazole have been shown to be effective in animal models of invasive fusariosis, including infections caused by isolates with reduced in vitro susceptibility (4, 10, 20).
Modifications to testing parameters used in determining the in vitro activity (e.g., inoculum size and incubation period) may greatly affect the MIC against Fusarium isolates (8, 16). This suggests that the in vitro activity as measured by standard microdilution methodology may not be predictive of in vivo efficacy. Our objective was to assess the in vivo efficacy of posaconazole against disseminated fusariosis caused by Fusarium solani, both as treatment and as prophylaxis, and to compare this activity to the in vitro potency measured at 24 and 48 h against different starting inocula. In addition, posaconazole concentrations were measured in animals that were administered the same dosages used for treatment and prophylaxis.
MATERIALS AND METHODS
Isolate.
F. solani species complex clinical isolate 95-2478 was obtained from the University of Texas Health Science Center at San Antonio. Conidia were inoculated onto potato dextrose agar and allowed to incubate at 37°C for 10 days. A mixture of micro- and macroconidia were harvested by flooding the plates with sterile physiologic saline and gentle scraping.
Antifungals.
For in vitro testing, posaconazole (Schering-Plough, Kenilworth, PA) was dissolved in dimethyl sulfoxide (DMSO), and further dilutions were made in RPMI medium supplemented with 2% glucose and buffered with 0.165 M 4-morpholinepropanesulfone acid (pH 7.0). For in vivo studies, the commercially available posaconazole oral suspension (Noxafil; Schering-Plough) was used.
In vitro susceptibility and pharmacodynamics.
The in vitro potency and pharmacodynamics of posaconazole were measured using the CLSI M38-A broth microdilution and the tetrazolium salt 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT) colorimetric assays, respectively (8, 11, 13). Briefly, conidia were added to microtiter trays containing posaconazole (final concentration range, 0 to 8 μg/ml) in RPMI with 2% glucose to achieve a starting inoculum of either 1 × 104 or 1 × 105 conidia/ml. After 24 and 48 h of incubation, the MIC was read as the lowest concentration of posaconazole that resulted in complete visual inhibition of growth compared to the growth control. The XTT assay was performed in triplicate as previously reported (12). Absorbance was read at 492 nm using a microplate spectrophotometer, and readings were converted to percent absorbance (growth control wells set as 100%, medium control wells set as 0%).
Bioassay.
Serum concentrations of posaconazole were measured in uninfected and nonimmunosuppressed mice following 5 doses of posaconazole at 6.25, 12.5, 25, or 50 mg/kg of body weight administered twice daily (BID) 4 h following administration of the last dose. Posaconazole concentrations were measured by bioassay using previously described methods (9, 19). Briefly, 100 μl of serum was instilled in duplicate into wells cored into potato dextrose agar that had been inoculated with 1 × 104 cells/ml of Candida kefyr ATCC 66028. After 48 h of incubation, zones of inhibition were measured, and posaconazole concentrations were interpolated from a standard curve.
Infection model.
An established murine model of invasive fusariosis was used for all experiments (4). Outbred ICR mice were rendered temporarily neutropenic by a single dose of 5-fluorouracil (150 mg/kg intravenously [i.v.]) 1 day prior to inoculation. Mice were infected intravenously using an 0.2-ml volume of inoculum. Different starting inocula were tested and ranged from 7.5 × 104 to 2.9 × 106 conidia/mouse. This protocol was approved by the Institutional Animal Care and Use Committee at the University of Texas Health Science Center at San Antonio, and animals were maintained in accordance with the standards of the American Association for Accreditation of Laboratory Animal Care (14).
Prophylaxis and treatment.
For treatment and prophylaxis studies four posaconazole regimens (6.25, 12.5, 25, and 50 mg/kg administered by oral gavage BID) were compared to untreated controls. In prophylaxis studies (n ≥ 10 animals per regimen within each regimen), posaconazole was begun 2 days prior to inoculation, while in treatment studies (n ≥ 20 animals per regimen) therapy began 12 h after inoculation. Animals that appeared moribund prior to the end of the study were euthanized, the kidneys were harvested, and death was recorded as occurring the next day. Kidneys were homogenized in sterile saline by using a tissue homogenizer, and serial dilutions were prepared in sterile saline and plated onto potato dextrose agar. Following 48 h of incubation, the number of CFU per gram of kidney tissue for each animal was calculated. Treatment studies were repeated on separate occasions to ensure reproducibility.
Data analysis.
The XTT reduction assay data were fitted to a four-parameter inhibitory sigmoid model (modified Hill equation) using computer curve-fitting software (Prism 5; GraphPad Software, Inc.) to derive concentrations resulting in 50% and 90% inhibition (IC50 and IC90, respectively). Goodness of fit was assessed by R2 and standard error of the IC50 value. Survival was plotted by Kaplan-Meier analysis, and differences in median survival time and percent survival were analyzed by the log rank test and Fisher's exact test, respectively. Differences in fungal burden endpoints between control and each antifungal regimen were assessed for significance by the Mann-Whitney test. A P value of ≤0.05 was considered statistically significant.
RESULTS
In vitro susceptibility and pharmacodynamics.
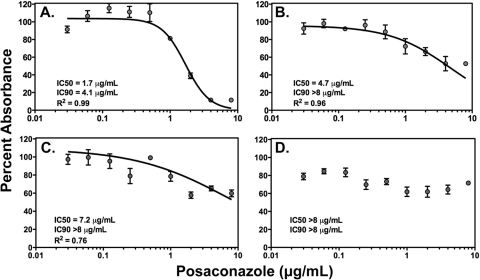
Similarly to previous studies we found discordant in vitro susceptibility and pharmacodynamic results for posaconazole between the two incubation periods and starting inocula (16). At the lower inoculum, a 4-fold increase in the MIC from 2 to 8 μg/ml was observed between the 24- and 48-h time points. Similarly, when the concentration-response curve was obtained using the XTT colorimetric assay, a 2.75-fold increase in the posaconazole IC50 value was also measured between the two incubation periods, and the IC90 value was also increased (4.1 μg/ml versus >8 μg/ml, respectively) (Fig. 1A and B). When the starting inoculum was increased to 1 × 105 conidia/ml, the potency of posaconazole against this isolate was markedly reduced at both 24 and 48 h as measured by elevated MICs (>8 μg/ml at both time points) and further increases in the calculated IC50 and IC90 values (Fig. 1C and D).
FIG. 1.
In vitro dose-response curves for posaconazole against Fusarium solani clinical isolate 95-2478 at 1 × 104 conidia/ml (A and B) and 1 × 105 conidia/ml (C and D) after 24 h (A and C) and 48 h (B and D) of incubation. Curves were generated by fitting XTT absorbance data to a four-parameter inhibitory sigmoid model. The concentrations resulting in 50% and 90% inhibition of growth (IC50 and IC90) and coefficients of determination (R2) are reported.
Serum concentrations.
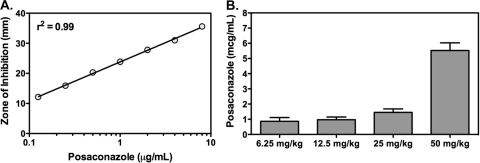
Posaconazole serum concentrations were measured by bioassay at 4 h following the fifth dose in uninfected and nonimmunosuppressed mice. This time point was chosen as it has been reported that a posaconazole concentration of ≥0.7 μg/ml drawn 3 to 5 h postdose is associated with improved prophylactic efficacy (7). In addition, average serum concentrations of >1.2 μg/ml were associated with improved efficacy when posaconazole was used as salvage therapy for invasive aspergillosis (22). As shown in Fig. 2, the highest posaconazole dosage group (50 mg/kg BID) achieved a median posaconazole serum concentration of 5.3 μg/ml (range, 4.9 to 6.2 μg/ml), and decreasing concentrations were observed as the dosages were lowered.
FIG. 2.
Posaconazole bioassay standard curve (A) and serum concentrations (B) measured in uninfected and nonimmunosuppressed mice. Blood was collected 4 h after the fifth dose in each regimen (posaconazole at 6.25, 12.5, 25, and 50 mg/kg by oral gavage BID) used in the treatment and prophylactic studies. Candida kefyr ATCC 66028 served as the indicator organism for the bioassay, and zones of inhibition were measured after 48 h of incubation.
Treatment.
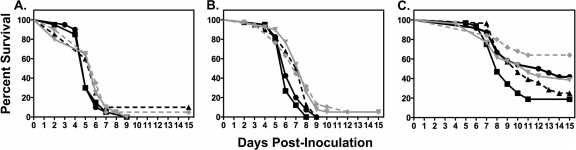
In treatment studies an inoculum effect and a small dose-response relationship were observed for posaconazole. In the initial study, no improvement in survival (Fig. 3) or reduction in tissue fungal burden within the kidneys (Table 1) was observed following inoculation with 2.9 × 106 conidia/animal. Based on these results this inoculum was used only once (n = 10 animals per group) and lower infecting inocula were evaluated for the remainder of the study. In the mid-inoculum studies (6.5 × 105 to 6.8 × 105 conidia/animal), median survival increased in animals that received BID posaconazole doses at 12.5 mg/kg (7.5 days), 25 mg/kg (8 days), and 50 mg/kg (7 days) compared to untreated controls (6 days; P < 0.05), although the percentage of animals in these groups surviving to the end of the study period was not improved (range, 0 to 5%). These results were consistent with the modest reductions in kidney tissue fungal burden within the 25- and 50-mg/kg dosage groups compared to untreated controls (Table 1). No differences in tissue fungal burden were observed in untreated controls that succumbed early to infection (≤5 days postinoculation) compared to those that survived longer (≥7 days).
FIG. 3.
Kaplan-Meier survival curves for the different posaconazole treatment dosage groups at the high inoculum (2.9 × 106 conidia/animal) (A), mid-inoculum range (6.5 × 105 to 6.8 × 105 conidia/animal) (B), and low inoculum range (7.7 × 104 to 7.8 × 104 conidia/animal) (C). Twice-daily dosing with oral posaconazole (black circles, 6.25 mg/kg; black triangles, 12.5 mg/kg; gray inverted triangles, 25 mg/kg; gray diamonds, 50 mg/kg) was initiated 12 h following intravenous inoculation in neutropenic mice, and values were compared to those for untreated controls (black squares).
TABLE 1.
Fusarium solani kidney tissue fungal burden in mice that received posaconazole as treatment beginning 12 h after inoculationa
| Inoculum (no. of conidia/animal; no. of animals/group) | Median log10 CFU/g in kidneys (range) for group: |
||||
|---|---|---|---|---|---|
| Control | PSC at mg/kg: |
||||
| 6.25 | 12.5 | 25 | 50 | ||
| High (2.9 × 106; 10) | 5.8 (4.9-6.1) | 5.6 (5.1-6.2) | 5.3 (4.7-5.5) | 5.3 (4.8-5.9) | 5.2 (4.4-5.9) |
| Middle (6.5 × 105-6.8 × 105; >20) | 6.1 (3.4-6.4) | 6.0 (4.4-6.5) | 5.8 (4.8-6.5) | 5.6 (2.4-6.4)* | 5.4 (3.7-6.4)* |
| Low (7.7 × 104-7.8 × 104; >20) | 5.3 (3.1-6.3) | 5.0 (2.7-5.9) | 5.1 (1.2-6.2) | 5.0 (1.5-5.9)* | 4.6 (1.2-6.0)* |
Median log10 CFU/g values and ranges are reported for each dosage group. P values are reported for posaconazole (PSC) regimens with significant reductions in log10 CFU/g compared to untreated controls (*, P < 0.05).
When the infecting inocula were further lowered (7.7 × 104 to 7.8 × 104 conidia/animal), improvements in median survival (>15 days) and percent survival (64%) were observed for those animals in the posaconazole 50-mg/kg BID dosage group compared to untreated controls (8 days and 18%, respectively; P ≤ 0.005). In addition, a significant reduction in kidney fungal burden was also observed in mice that received this regimen. However, it should also be noted that in the control group, the median survival was increased and tissue fungal burden was lower than in the corresponding untreated control group in the mid-inoculum level. Although the median survival times in the posaconazole 6.25-, 12.5-, and 25-mg/kg BID groups were longer than those of untreated controls (12.5, 10, and 11 days, respectively), these differences were not significant, nor were the percentages of animals that survived to the end of the study period (42, 25, and 38%, respectively). However, posaconazole at 25 mg/kg BID did significantly reduce fungal burden within the kidneys.
Prophylaxis.
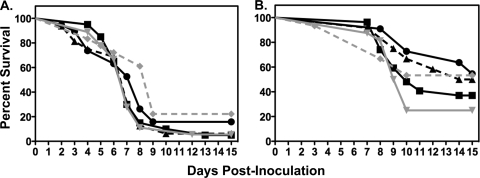
Due to the poor overall survival among the treatment groups and untreated controls at the highest inoculum studied, only the mid- and low inoculum ranges were evaluated in the prophylaxis studies. In the mid-inoculum study (6.5 × 105 conidia/animal), the median survival (9 days) was significantly improved in animals that received posaconazole at 50 mg/kg BID compared to untreated controls (7 days; P ≤ 0.002; Fig. 4). In addition, tissue fungal burden was significantly reduced within the kidneys at this dosage level compared to untreated controls (Table 2). However, no improvements in survival or reductions in tissue fungal burden were observed with the other posaconazole regimens. As with the treatment studies, no differences in tissue fungal burden were observed between untreated controls that succumbed early to infection and those that survived longer.
FIG. 4.
Kaplan-Meier survival curves for the different posaconazole prophylaxis dosage groups at the mid-inoculum (6.5 × 105 conidia/animal) (A) and low inoculum (7.5 × 104 conidia/animal) (B). Twice-daily oral dosing with posaconazole (black circles, 6.25 mg/kg; black triangles, 12.5 mg/kg; gray inverted triangles, 25 mg/kg; gray diamonds, 50 mg/kg) was initiated 2 days prior to intravenous inoculation in neutropenic mice, and values were compared to those for untreated controls (black squares).
TABLE 2.
Fusarium solani kidney tissue fungal burden in mice that received posaconazole as prophylaxis beginning 2 days prior to intravenous inoculationa
| Inoculum (no. of conidia/animal; no. of animals/group) | Median log10 CFU/g of kidneys (range) for group: |
||||
|---|---|---|---|---|---|
| Control | PSC at mg/kg: |
||||
| 6.25 | 12.5 | 25 | 50 | ||
| Middle (6.5 × 105; >10) | 5.8 (1.3-6.5) | 5.6 (4.0-6.2) | 5.5 (3.2-6.2) | 5.5 (3.2-6.3) | 4.7 (1.3-6.2)* |
| Low (7.5 × 104; >10) | 4.4 (3.3-6.1) | 2.8 (1.6-6.0) | 4.4 (1.3-5.9) | 4.5 (1.8-6.1) | 2.9 (1.3-5.1)** |
Median log10 CFU/g values and ranges are reported for each dosage group. P values are reported for posaconazole (PSC) regimens with significant reductions in log10 CFU/g compared to untreated controls (*, P = 0.02; **, P = 0.03).
Similar to that observed in the treatment studies, an inoculum effect was observed for survival in animals administered posaconazole prophylaxis. As shown in Fig. 4, the median survival in all prophylaxis groups and untreated controls at the low inoculum was higher than that observed at the mid-inoculum. However, only animals that received posaconazole at 50 mg/kg BID had a median survival (>15 days) that was significantly greater than that of untreated controls (10 days; P = 0.02), and only this dosage resulted in significant reductions in tissue burden within the kidneys (Table 2).
DISCUSSION
Invasive infections caused by Fusarium species are difficult to treat and are often associated with poor clinical outcomes. The availability of advanced expanded-spectrum triazoles (i.e., voriconazole and posaconazole) has resulted in a modest improvement in treatment response. In a multicenter, open-label study of voriconazole as salvage therapy, a satisfactory global response was reported in 5 of 11 patients (45%) with fusariosis (17). However, it is important to remember that in this study stable disease was considered part of the global response. This is in contrast to a recent report of 21 patients who received posaconazole salvage therapy for proven or probable invasive fusariosis (18). In this retrospective analysis of three independent, multicenter open-label trials, which defined a successful outcome as complete or partial response and classified those with stable disease as failures, the overall response rate was 48%.
One potential reason for the poor response rates in clinical studies of invasive fusariosis is the limited activity of antifungal agents against Fusarium species (8, 10, 16). However, modifications to testing parameters used in determining the in vitro activity can affect these results. Paphitou et al. noted that an increased inoculum size and lengthened duration of incubation resulted in higher azole MICs (16). Interestingly, one of the F. solani isolates reported by these authors (24-h MIC, 2 μg/ml) was previously shown to be responsive in vivo to posaconazole at 100 mg/kg despite minimal in vitro activity of this azole (48-h MIC, >16 μg/ml) (10, 16). A recent study also reported improved survival in mice dosed with posaconazole at 100 mg/kg following infection with a Fusarium oxysporum isolate with an MIC of >16 μg/ml (20).
The results of our study are consistent with these previous reports. Survival was improved and tissue fungal burden within the kidneys was significantly reduced in mice that received escalating dosages of posaconazole. This was consistently found with the highest-dose regimen of posaconazole evaluated (50 mg/kg BID). We also found discrepancies between the in vitro activities of this azole when measured after 24 and 48 h and observed that the survival and tissue burden results were also related to the infecting inoculum sizes. This may be relevant as the lowest inoculum size used in vitro was similar to the lowest in vivo inoculum range where the greatest improvements in survival and reductions in tissue burden were observed. We also measured serum concentrations and found an association between levels achieved 4 h after administration of posaconazole and efficacy. Animals in the 50-mg/kg posaconazole BID group had the highest serum concentrations as well as the greatest improvements in survival and reductions in tissue burden. The median plasma concentration achieved in this group (5.3 μg/ml) was similar to the in vitro IC90 value after 24 h of incubation at the lower starting inoculum (4.1 μg/ml) but was less than that for the higher inoculum (7.2 μg/ml). These data suggest that the in vivo efficacy was also related to the starting inoculum, which is in agreement with the results of Paphitou et al. (16), as well as clinical data demonstrating improved outcomes with higher posaconazole concentrations (6, 7, 22).
Although our results are consistent with those reported by others, several differences must be considered. The types of immunosuppression used, if any, were different among the studies, as were the species of Fusarium tested (F. oxysporum and F. solani), and the inoculum sizes used to establish infection. In addition, we did not evaluate the efficacy of combination therapy, which was recently shown to be effective (20). Finally, the serum concentrations measured at the highest-dose posaconazole regimen used in our study are much higher than can currently be achieved clinically (5-7, 21). A more detailed analysis comparing a larger number of Fusarium isolates along with a more rigorous pharmacokinetic/pharmacodynamic analysis is needed to clarify the relationships between in vitro potency, in vivo efficacy, and bloodstream concentrations.
Acknowledgments
This study was funded by Schering-Plough, Inc.
N.P.W. has received research support from Schering-Plough, Pfizer, Merck, Basilea, and CyDex. T.F.P. has received research support from Merck, Basilea, Pfizer, Schering-Plough, and Nektar Therapeutics; has received speaker fees from Merck and Pfizer; and has served as a consultant for Basilea, Merck, Pfizer, and Toyama.
Footnotes
Published ahead of print on 11 January 2010.
REFERENCES
- 1.Boutati, E. I., and E. J. Anaissie. 1997. Fusarium, a significant emerging pathogen in patients with hematologic malignancy: ten years' experience at a cancer center and implications for management. Blood 90:999-1008. [PubMed] [Google Scholar]
- 2.Dignani, M. C., and E. Anaissie. 2004. Human fusariosis. Clin. Microbiol. Infect. 10(Suppl. 1):67-75. [DOI] [PubMed] [Google Scholar]
- 3.Gonzalez, G. M., A. W. Fothergill, D. A. Sutton, M. G. Rinaldi, and D. Loebenberg. 2005. In vitro activities of new and established triazoles against opportunistic filamentous and dimorphic fungi. Med. Mycol. 43:281-284. [DOI] [PubMed] [Google Scholar]
- 4.Graybill, J. R., L. K. Najvar, G. M. Gonzalez, S. Hernandez, and R. Bocanegra. 2003. Improving the mouse model for studying the efficacy of voriconazole. J. Antimicrob. Chemother. 51:1373-1376. [DOI] [PubMed] [Google Scholar]
- 5.Gubbins, P. O., G. Krishna, A. Sansone-Parsons, S. R. Penzak, L. Dong, M. Martinho, and E. J. Anaissie. 2006. Pharmacokinetics and safety of oral posaconazole in neutropenic stem cell transplant recipients. Antimicrob. Agents Chemother. 50:1993-1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krishna, G., M. AbuTarif, F. Xuan, M. Martinho, D. Angulo, and O. A. Cornely. 2008. Pharmacokinetics of oral posaconazole in neutropenic patients receiving chemotherapy for acute myelogenous leukemia or myelodysplastic syndrome. Pharmacotherapy 28:1223-1232. [DOI] [PubMed] [Google Scholar]
- 7.Krishna, G., M. Martinho, P. Chandrasekar, A. J. Ullmann, and H. Patino. 2007. Pharmacokinetics of oral posaconazole in allogeneic hematopoietic stem cell transplant recipients with graft-versus-host disease. Pharmacotherapy 27:1627-1636. [DOI] [PubMed] [Google Scholar]
- 8.Lewis, R. E., N. P. Wiederhold, and M. E. Klepser. 2005. In vitro pharmacodynamics of amphotericin B, itraconazole, and voriconazole against Aspergillus, Fusarium, and Scedosporium spp. Antimicrob. Agents Chemother. 49:945-951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lionakis, M. S., R. E. Lewis, G. S. May, N. P. Wiederhold, N. D. Albert, G. Halder, and D. P. Kontoyiannis. 2005. Toll-deficient Drosophila flies as a fast, high-throughput model for the study of antifungal drug efficacy against invasive aspergillosis and Aspergillus virulence. J. Infect. Dis. 191:1188-1195. [DOI] [PubMed] [Google Scholar]
- 10.Lozano-Chiu, M., S. Arikan, V. L. Paetznick, E. J. Anaissie, D. Loebenberg, and J. H. Rex. 1999. Treatment of murine fusariosis with SCH 56592. Antimicrob. Agents Chemother. 43:589-591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, J. P. Donnelly, and P. E. Verweij. 2001. Colorimetric assay for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:3402-3408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meletiadis, J., J. W. Mouton, J. F. Meis, B. A. Bouman, P. J. Donnelly, and P. E. Verweij. 2001. Comparison of spectrophotometric and visual readings of NCCLS method and evaluation of a colorimetric method based on reduction of a soluble tetrazolium salt, 2,3-bis[2-methoxy-4-nitro-5-[(sulfenylamino)carbonyl]-2H-tetrazolium-hydroxide], for antifungal susceptibility testing of Aspergillus species. J. Clin. Microbiol. 39:4256-4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi; accepted standard M38-A. National Committee for Clinical Laboratory Standards, Wayne, PA.
- 14.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 15.Nucci, M., E. J. Anaissie, F. Queiroz-Telles, C. A. Martins, P. Trabasso, C. Solza, C. Mangini, B. P. Simoes, A. L. Colombo, J. Vaz, C. E. Levy, S. Costa, V. A. Moreira, J. S. Oliveira, N. Paraguay, G. Duboc, J. C. Voltarelli, A. Maiolino, R. Pasquini, and C. A. Souza. 2003. Outcome predictors of 84 patients with hematologic malignancies and Fusarium infection. Cancer 98:315-319. [DOI] [PubMed] [Google Scholar]
- 16.Paphitou, N. I., L. Ostrosky-Zeichner, V. L. Paetznick, J. R. Rodriguez, E. Chen, and J. H. Rex. 2002. In vitro activities of investigational triazoles against Fusarium species: effects of inoculum size and incubation time on broth microdilution susceptibility test results. Antimicrob. Agents Chemother. 46:3298-3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perfect, J. R., K. A. Marr, T. J. Walsh, R. N. Greenberg, B. DuPont, J. de la Torre-Cisneros, G. Just-Nubling, H. T. Schlamm, I. Lutsar, A. Espinel-Ingroff, and E. Johnson. 2003. Voriconazole treatment for less-common, emerging, or refractory fungal infections. Clin. Infect. Dis. 36:1122-1131. [DOI] [PubMed] [Google Scholar]
- 18.Raad, I. I., R. Y. Hachem, R. Herbrecht, J. R. Graybill, R. Hare, G. Corcoran, and D. P. Kontoyiannis. 2006. Posaconazole as salvage treatment for invasive fusariosis in patients with underlying hematologic malignancy and other conditions. Clin. Infect. Dis. 42:1398-1403. [DOI] [PubMed] [Google Scholar]
- 19.Rex, J. H., L. H. Hanson, M. A. Amantea, D. A. Stevens, and J. E. Bennett. 1991. Standardization of a fluconazole bioassay and correlation of results with those obtained by high-pressure liquid chromatography. Antimicrob. Agents Chemother. 35:846-850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruiz-Cendoya, M., M. Marine, M. M. Rodriguez, and J. Guarro. 2009. Interactions between triazoles and amphotericin B in treatment of disseminated murine infection by Fusarium oxysporum. Antimicrob. Agents Chemother. 53:1705-1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ullmann, A. J., O. A. Cornely, A. Burchardt, R. Hachem, D. P. Kontoyiannis, K. Topelt, R. Courtney, D. Wexler, G. Krishna, M. Martinho, G. Corcoran, and I. Raad. 2006. Pharmacokinetics, safety, and efficacy of posaconazole in patients with persistent febrile neutropenia or refractory invasive fungal infection. Antimicrob. Agents Chemother. 50:658-666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walsh, T. J., I. Raad, T. F. Patterson, P. Chandrasekar, G. R. Donowitz, R. Graybill, R. E. Greene, R. Hachem, S. Hadley, R. Herbrecht, A. Langston, A. Louie, P. Ribaud, B. H. Segal, D. A. Stevens, J. A. van Burik, C. S. White, G. Corcoran, J. Gogate, G. Krishna, L. Pedicone, C. Hardalo, and J. R. Perfect. 2007. Treatment of invasive aspergillosis with posaconazole in patients who are refractory to or intolerant of conventional therapy: an externally controlled trial. Clin. Infect. Dis. 44:2-12. [DOI] [PubMed] [Google Scholar]