Abstract
Valacyclovir, the l-valyl ester prodrug of acyclovir (ACV), is widely prescribed to treat infections caused by varicella-zoster virus or herpes simplex virus. Rarely, treatment is complicated by reversible neuropsychiatric symptoms. By mechanisms not fully understood, this occurs more frequently in the setting of renal impairment. We characterized the steady-state pharmacokinetics of ACV and its metabolites 9-[(carboxymethoxy)methyl]guanine (CMMG) and 8-hydroxy-acyclovir (8-OH-ACV) in cerebrospinal fluid (CSF) and the systemic circulation. We administered multiple doses of high-dose valacyclovir to 6 subjects with normal renal function and 3 subjects with chronic renal impairment (creatinine clearance [CrCl], ∼15 to 30 ml/min). Dosages were 2,000 mg every 6 h and 1,500 mg every 12 h, respectively. Indwelling intrathecal catheters allowed serial CSF sampling throughout the dosing interval. The average steady-state concentrations of acyclovir, CMMG, and 8-OH-ACV were greater in both the systemic circulation and the CSF among subjects with impaired renal function than among subjects with normal renal function. However, the CSF penetration of each analyte, reflected by the CSF-to-plasma area under the concentration-time curve over the 6- or 12-h dosing interval (AUCτ) ratio, did not differ based on renal function. Renal impairment does not alter the propensity for ACV or its metabolites to distribute to the CSF, but the higher concentrations in the systemic circulation, as a result of reduced elimination, are associated with proportionally higher concentrations in CSF.
Valacyclovir, the l-valyl ester of the antiviral drug acyclovir (ACV), is widely prescribed for varicella-zoster virus and herpes simplex virus infections. Following oral administration, valacyclovir is rapidly and nearly completely hydrolyzed to acyclovir by first-pass intestinal and hepatic metabolism (13, 27). Acyclovir crosses the blood-brain barrier, a desirable quality for the treatment of herpes encephalitis, neonatal herpes simplex virus infections, and, possibly, multiple sclerosis (7, 28). Acute, reversible neuropsychiatric symptoms were first associated with acyclovir therapy in the early 1980s (26), and similar adverse effects have been reported for the use of valacyclovir (1, 2, 10, 12, 15, 21, 24). Symptoms include ataxia, involuntary movements, dysarthria, disturbed consciousness, hyperreflexia, and deranged cerebral functions (hallucinations, confusion, and lethargy). Neuropsychiatric symptoms with valacyclovir are infrequent, but the risk increases with acute or chronic renal dysfunction, concomitant neurotoxic drugs, and advanced age (5, 11). The underlying mechanism for these side effects is unknown, and attempts to relate central nervous system (CNS) symptoms and systemic concentrations of acyclovir have been inconsistent (3, 5, 6, 9), suggesting that a metabolite of acyclovir may be responsible or contributory (11).
The major route of acyclovir elimination is the renal excretion of unchanged drug. Urinary recovery data after acyclovir administration to healthy volunteers indicate that <15% and 1% of the acyclovir dose is metabolized to 9-[(carboxymethoxy)methyl]guanine (CMMG) and 8-hydroxy-acyclovir (8-OH-ACV), respectively (23). Sequential oxidation reactions by alcohol dehydrogenase and aldehyde dehydrogenase metabolize acyclovir to CMMG, and aldehyde oxidase metabolizes acyclovir to 8-OH-ACV (10, 23). To our knowledge, these metabolites have not been administered directly to humans; therefore, their toxicity relative to acyclovir toxicity is unknown. A suggestion that acyclovir metabolites (specifically CMMG) may contribute to toxicity in humans was derived from two retrospective studies: Helldén et al. previously identified a positive correlation between serum levels of CMMG and acyclovir-associated neuropsychiatric symptoms (11), and in a subsequent study, they detected CMMG in the cerebrospinal fluid (CSF) of symptomatic patients (8 of whom were being treated with acyclovir and 1 of whom was being treated with valacyclovir), but it was below the limit of detection in the CSF of asymptomatic patients (10).
A prospective simultaneous pharmacokinetic characterization of acyclovir and its metabolites CMMG and 8-OH-ACV in CSF and the systemic circulation has not been reported. In the present study, serial CSF and blood samples were collected after dosing to steady state with high-dose valacyclovir regimens. To assess the impact of renal dysfunction on concentration-time profiles and to characterize the CSF penetration of acyclovir and its metabolites, we studied a group of individuals with normal renal function and a group with moderate to severe renal impairment.
(These results were presented in part at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, September 2003 [8a]).
MATERIALS AND METHODS
Study population.
This multiple-dose, steady-state, open-label, pharmacokinetic study was designed to enroll 6 subjects with normal renal function (creatinine clearance [CrCl] rate of >75 ml/min, estimated by the Cockcroft-Gault equation) and 6 subjects with chronic kidney disease (CKD) (CrCl of ∼15 to 30 ml/min). The accrual of subjects with CKD was slower than anticipated, so we closed the study with 3 subjects in the CKD group. Eligible participants were clinically stable, as determined by medical history, physical examination, and laboratory studies. Subjects with CKD were required to have laboratory documentation of stable renal function for at least 3 months prior to screening. All subjects must have received stable dosages of concomitant medications for at least 1 week prior to screening and throughout the study. None of the concomitant medications received were known or expected to affect the pharmacokinetics of valacyclovir, acyclovir, or acyclovir metabolites. Screening occurred within 2 weeks prior to the first administration of valacyclovir. Exclusion criteria included pregnancy; known hypersensitivity to valacyclovir, acyclovir, famciclovir, or penciclovir; receipt of any investigational drug in the preceding 3 months; history of chronic or recurrent headaches; gastrointestinal disorders that may affect drug absorption; or unacceptable screening chemistry, hematology, or coagulation studies. None of the subjects required valacyclovir for the management of a herpesvirus infection. The Vanderbilt University Institutional Review Board approved the study, and all subjects provided written informed consent.
Study drugs and dosages.
Study subjects received multiple doses of high-dose valacyclovir to achieve steady state. The dosing regimens were similar to those used in clinical trials studying the efficacy of high-dose valacyclovir as prophylaxis against cytomegalovirus reactivation in high-risk transplant recipients (4, 16, 17). Subjects with normal renal function received 2,000 mg of valacyclovir (four 500-mg caplets) orally every 6 h for 5 doses, and subjects with CKD received 1,500 mg of valacyclovir (three 500-mg caplets) orally every 12 h for 5 doses. Study drug doses were either directly administered by study personnel or closely monitored by telephone contact.
Pharmacokinetic sampling of CSF and blood.
Samples of CSF, plasma, and serum for pharmacokinetic analyses were obtained after the fifth dose of valacyclovir, which was administered at 8 a.m. in the Vanderbilt Clinical Research Center. Subjects fasted from 8 h before until 2 h after the administration of this final dose.
CSF was collected through an 18-gauge indwelling lumbar intrathecal catheter as previously described (8). Intrathecal catheters were inserted within 1 h prior to the final dose of valacyclovir, and 2 ml of CSF was collected into polypropylene tubes on ice immediately before the dose (time zero) and at 0.5, 1, 1.5, 2, 3, 4, 6, and 8 h after the dose. An additional 12-h sample was obtained from subjects with CKD. At each time point, 6 ml of whole blood was collected through a heparinized, indwelling venous catheter into EDTA-containing tubes on ice and centrifuged at 4°C within 1 h. Plasma, serum, and CSF samples were promptly placed on dry ice and stored at −70°C until assayed.
Quantification of acyclovir and its metabolites.
Acyclovir was quantified in plasma and CSF by a validated scintillation proximity radioimmunoassay. The method was based on and successfully cross-validated against a method described previously by Tadepalli and Quinn (25) but was modified to an automated microtiter plate format and validated for use with CSF. The standard curve range for both matrices was 1.4 to 90 ng/ml (6.22 to 400 nM), with a lower limit of quantitation (LLQ) of 14.1 ng/ml (62.6 nM) at a 1:10 dilution. The accuracy (percent bias) and precision (percent coefficient of variation) of the validated method were <14%. The specificity of the monoclonal antibody to acyclovir showed less than 1% cross-reactivity to CMMG and 8-OH-ACV.
CMMG and 8-OH-ACV were quantified in serum and CSF by validated liquid chromatography/mass spectrometry (LC/MS) methods. Both metabolites, along with the stable isotopically labeled internal standards [15N313C]CMMG and [15N313C]8-OH-ACV, were extracted from serum and CSF by solid-phase extraction. The samples were subsequently analyzed by reversed-phase high-performance liquid chromatography (HPLC) using a Shimadzu HPLC system with a Waters Symmetry Shield RP18 3.5-μm column (50- by 2.1-mm internal diameter). An HPLC gradient with a flow rate of 0.35 ml/min was used with the following mobile phases: solvent A consisted of 3% methanol and 97% water with 0.025% acetic acid, and solvent B consisted of 30% methanol and 70% water with 0.025% acetic acid. Initial conditions were 100% solvent A for 1.1 min. Solvent B was increased to 100% from 1.1 min to 1.2 min and held until 2.8 min. The detection of CMMG, 8-OH-ACV, and their internal standards was performed by using Applied Biosystems API-3 and API-4000 mass spectrometers in the positive-ion mode with a TurboIon spray source and multiple-ion-reaction monitoring. Calibration curves of analyte/internal standard peak area ratio versus concentration were constructed, and a weighed 1/x2 linear regression was applied to the data for serum and CSF standards, quality control (QC) samples, and clinical samples.
The validated range of the assay for serum CMMG was 16.4 to 5,000 ng/ml (68.6 to 20,921 nM), and that for serum 8-OH-ACV was 1.5 to 1,600 ng/ml (6.23 to 6,640 nM). The validated range for CSF CMMG was 1.82 to 200 ng/ml (7.61 to 837 nM), and that for 8-OH-ACV was 1.0 to 200 ng/ml (4.15 to 830 nM). QC samples were prepared at 3 different concentrations over the assay range for both analytes in both serum and CSF and were analyzed with each batch of samples against separately prepared calibration standards. All analytical runs in the study met the predefined acceptance criteria of <15% for precision and accuracy. Although concentrations of acyclovir and its metabolites in the systemic circulation were measured in plasma and serum, respectively, the term “plasma” is used throughout for simplicity.
Pharmacokinetic analysis.
Pharmacokinetic analysis was conducted by standard noncompartmental methods using WinNonlin software (Pharsight Corp., Mountain View, CA) to estimate maximum concentration (Cmax), time to maximum concentration (tmax), and area under the concentration-time curve over the 6- or 12-h dosing interval (AUCτ) for acyclovir and metabolites in both CSF and plasma and for apparent oral clearance (CL/F) of acyclovir in plasma for each subject.
CSF penetration of acyclovir and its metabolites was defined for each analyte as the ratio of AUCτ in CSF to the corresponding AUCτ in plasma. To characterize metabolite formation and disposition, the AUCτ and Cmax values were calculated for each metabolite relative to that of the parent acyclovir in both the CSF and plasma.
Statistical analysis.
Descriptive summary statistics for each analyte and the CSF-to-plasma ratios are provided. Because of the small number of subjects and the differences in dosing regimens between groups, we compared only the CSF-to-plasma AUCτ values. We did not assume a normal distribution; therefore, comparisons were made by using the Wilcoxon rank-sum test (Prism v4.03; GraphPad, La Jolla, CA).
RESULTS
Subject characteristics.
Participants enrolled into the study between July 2000 and November 2002. The six participants with normal renal function ranged in age from 19 to 46 years, all were white, 4 were female, and the mean CrCl ± standard deviation (SD) (range) was 111 ± 20 ml/min (91 to 144 ml/min). The three participants with CKD ranged in age from 31 to 65 years, 2 were white, 1 was African-American, 2 were female, and their creatinine clearance rates were 17, 30, and 31 ml/min. All subjects tolerated their assigned regimen. None experienced signs or symptoms of neurotoxicity or worsened renal impairment, although 5 subjects (3 in the normal renal function group and 2 in the CKD group) experienced headache.
Plasma exposure for acyclovir and its metabolites.
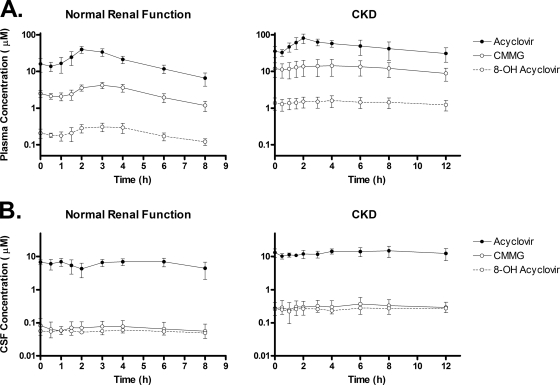
At every time point and for every study participant, plasma acyclovir concentrations exceeded those of CMMG, which exceeded those of 8-OH-ACV. Summary plasma concentration-time profiles for each group are presented in Fig. 1A. Selected pharmacokinetic parameters are presented in Table 1.
FIG. 1.
Plasma and CSF concentration-time profiles of acyclovir and its metabolites. Participants with normal renal function were studied after the fifth dose of 2,000 mg valacyclovir orally every 6 h. Participants with chronic kidney disease were studied after the fifth dose of 1,500 mg valacyclovir orally every 12 h. Mean ± SD values are shown.
TABLE 1.
Pharmacokinetic parameters
| Analyte | Parametera | Mean value ± SD (range) for group |
|||
|---|---|---|---|---|---|
| Normal renal function (n = 6) |
Chronic kidney disease (n = 3) |
||||
| Systemic | CSF | Systemic | CSF | ||
| Acyclovir | Cmax (μM) | 42 ± 6.8 (33-49) | 7.8 ± 1.5 (4.9-8.7) | 82 ± 24 (55-101) | 16 ± 4.9 (11-21) |
| tmax (h) | 2.1 ± 0.49 | 2.3 ± 2.2 | 2.0 ± 0 | 5.3 ± 2.3 | |
| AUCτ (h·μM) | 137 ± 18 (118-161) | 37 ± 7.5 (25-44) | 577 ± 195 (376-765) | 159 ± 46 (119-210) | |
| CL/F (ml/min) | 759 ± 100 (638-873) | 145 ± 54 (101-205) | |||
| CMMG | Cmax (μM) | 4.1 ± 1.1 (2.5-5.1) | 0.095 ± 0.046 (0.034-0.16) | 15 ± 7.0 (7.1-21) | 0.37 ± 0.20 (0.17-0.58) |
| tmax (h) | 3.3 ± 0.52 | 1.8 ± 2.0 | 4.3 ± 1.5 | 6.7 ± 1.2 | |
| AUCτ (h·μM) | 17 ± 4.1 (11-22) | 0.42 ± 0.21 (0.17-0.79) | 148 ± 66 (78-207) | 3.8 ± 2.0 (1.8-5.8) | |
| 8-OH-ACV | Cmax (μM) | 0.32 ± 0.10 (0.22-0.46) | 0.062 ± 0.014 (0.050-0.089) | 1.6 ± 0.57 (0.97-2.1) | 0.29 ± 0.080 (0.21-0.38) |
| tmax (h) | 3.5 ± 0.55 | 2.4 ± 1.5 | 4.0 ± 0 | 7.0 ± 4.6 | |
| AUCτ (h·μM) | 1.4 ± 0.38 (0.96-1.9) | 0.34 ± 0.070 (0.27-0.48) | 17 ± 5.6 (11-22) | 3.1 ± 0.94 (2.2-4.1) | |
τ is the dosing interval (0 to 6 h for normal renal function and 0 to 12 h for renal impairment).
Plasma concentrations of acyclovir peaked approximately 2 h after an oral dose for both subjects with normal renal function and those with CKD. Concentrations of the acyclovir metabolites CMMG and 8-OH-ACV peaked approximately 1 to 2 h later. Among subjects with normal renal function, the average steady-state concentrations (Cavg = AUCτ/τ) of acyclovir, CMMG, and 8-OH-ACV were 23 μM, 2.9 μM, and 0.24 μM, respectively; of the total combined AUC for this group, ∼88% was attributable to acyclovir, 11% was attributable to CMMG, and 1% was attributable to 8-OH-ACV. Among subjects with CKD, the Cavg values of acyclovir, CMMG, and 8-OH-ACV were 48 μM, 12 μM, and 1.4 μM, respectively; of the total combined AUC for this group, 78% was attributable to acyclovir, 20% was attributable to CMMG, and 2% was attributable to 8-OH-ACV.
CSF exposure to acyclovir and metabolites.
CSF concentration-time profiles of acyclovir and its metabolites are summarized in Fig. 1B. Selected pharmacokinetic parameters are presented in Table 1.
Similar to the systemic circulation, at every time point and for every study participant, CSF acyclovir concentrations exceeded those of CMMG, which exceeded those of 8-OH-ACV. In contrast to the systemic circulation, concentrations of acyclovir, CMMG, and 8-OH-ACV were remarkably stable in CSF throughout the dosing interval. This finding is consistent with relatively slow equilibration of these compounds between CSF and plasma. Among subjects with normal renal function, the Cavg values of acyclovir, CMMG, and 8-OH-ACV in the CSF were 6.25 μM, 0.07 μM, and 0.06 μM, respectively; of the total combined AUC for this group, ∼98% was attributable to acyclovir and ∼1% each was attributed to CMMG and 8-OH-ACV. Among subjects with CKD, the Cavg values of acyclovir, CMMG, and 8-OH-ACV were 13.3 μM, 0.31 μM, and 0.26 μM, respectively; of the total combined AUC for this group, ∼96% was attributable to acyclovir and ∼2% each was attributed to CMMG and 8-OH-ACV. Thus, acyclovir metabolites in CSF comprise a far smaller proportion of the total AUC (acyclovir plus metabolites) than they do in the systemic circulation.
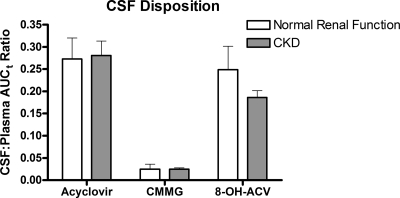
CSF disposition of acyclovir and metabolites.
The primary measure of CSF penetration for each analyte was the ratio of the CSF AUCτ to the systemic AUCτ (Fig. 2). Regardless of renal function, the CSF-to-plasma AUCτ ratios of acyclovir and 8-OH-ACV were similar (∼0.25). CMMG penetrated into CSF to a far lesser extent, with CSF-to-plasma AUCτ ratios of ∼0.025. Impaired renal function did not affect CSF-to-plasma ratios for acyclovir, 8-OH-ACV, or CMMG. These data suggest that renal impairment does not alter the propensity for acyclovir or its metabolites to distribute to the CSF. Higher concentrations of these compounds in the systemic circulation, as a result of reduced elimination, are associated with proportionally higher concentrations in the CSF.
FIG. 2.
CSF-to-plasma AUCτ ratios for acyclovir and its metabolites. Participants with normal renal function were studied after the fifth dose of 2,000 mg of valacyclovir orally every 6 h. Participants with chronic kidney disease were studied after the fifth dose of 1,500 mg valacyclovir orally every 12 h. Mean ± SD values are shown. (Comparing normal renal function to chronic kidney disease, P = 0.71 for acyclovir, P = 0.71 for CMMG, and P = 0.17 for 8-OH-ACV, by Wilcoxon rank-sum test.)
The CSF-to-plasma Cmax ratios of acyclovir and its metabolites were similar to the CSF-to-plasma AUCτ ratios. Specifically, among subjects with normal renal function, mean CSF-to-plasma Cmax ratios for acyclovir, CMMG, and 8-OH-ACV were 0.19 ± 0.05, 0.025 ± 0.012, and 0.20 ± 0.04, respectively; among subjects with CKD, the corresponding ratios were 0.20 ± 0.02, 0.025 ± 0.002, and 0.19 ± 0.03, respectively.
DISCUSSION
Valacyclovir is a preferred drug for treating infections caused by varicella-zoster virus and herpes simplex virus, but it infrequently causes neuropsychiatric symptoms, particularly for patients with acute or chronic renal insufficiency. These symptoms resolve when the drug is stopped or removed with dialysis. Because the simultaneous sampling of CSF and plasma over an entire dosing interval provides the most precise description of drug exposure in CSF and facilitates the study of drug transit across the blood-CSF barrier, we applied this technique to describe the pharmacokinetics and disposition of acyclovir and its metabolites CMMG and 8-OH-ACV. We found that renal dysfunction increases systemic and CSF exposure to acyclovir and its metabolites considerably. Furthermore, to our knowledge, this study is the first to report the pharmacokinetics of 8-OH-ACV, which we detected in CSF at concentrations similar to those of CMMG despite its far-lower concentration in plasma.
The systemic pharmacokinetics of acyclovir from high-dose valacyclovir in subjects with normal renal function described in this study are similar to previously published parameters (14, 27) but also indicate that a greater proportion of acyclovir elimination results from metabolism with renal impairment. This conclusion is based on the use of the metabolite-to-parent Cavg (or AUC) ratio at steady state to estimate the fraction of parent drug converted to metabolite (22). By using the Cavg data observed in this study, the fractions of ACV converted to CMMG and 8-OH-ACV were 12.6% and 1.0%, respectively, among those with normal renal function and 25.0% and 2.9%, respectively, among those with renal impairment.
The relatively flat concentration-time profiles of acyclovir, CMMG, and 8-OH-ACV in the CSF are consistent with CSF being a slowly equilibrating compartment relative to plasma. Lycke et al. previously sampled CSF twice (2 h and 8 h postdose) for 10 patients with multiple sclerosis after 6 days of treatment with 1,000 mg valacyclovir three times daily (19). They measured similar concentrations of acyclovir at both time points and assumed a flat concentration-time profile based on their previous experience (18) and based on a pilot study in which two subjects underwent 8 h of sampling through an intradural catheter. Our results robustly confirm this description of acyclovir in the CSF and extend the observation to its metabolites CMMG and 8-OH-ACV.
The three individuals with CKD in our study had considerably greater Cmax and AUCτ values of acyclovir, CMMG, and 8-OH-ACV, both systemically and in the CSF, despite a dose reduction from 8 g/day to 3 g/day. Furthermore, the percentage of the total AUC attributable to the metabolites, especially CMMG, was higher in subjects with CKD. The CSF-to-plasma Cmax and AUC ratios, however, were similar regardless of renal function; therefore, our data do not support an enhanced uptake or reduced efflux of acyclovir or its metabolites as a result of renal insufficiency-associated changes in the blood-CSF barrier. We observed no unexpected safety findings, including signs or symptoms of neurotoxicity, or worsening renal function.
The present study had several limitations. We studied only three individuals with CKD. Although the pharmacokinetic results were remarkably consistent among these individuals, a larger sample size would provide more robust and better estimates of central tendency and interindividual variability. We also did not study subjects with end-stage renal disease. This study was not designed to confirm or refute the involvement of acyclovir metabolites in the pathophysiology of acyclovir-associated neurotoxicity. Helldén et al. (10) reported CMMG concentrations of 0.6 to 7 μM in the CSF among a heterogeneous population of nine symptomatic subjects (CrCl from 5 to 32 ml/min for 8 subjects; normal renal function for 1 subject) who were on various regimens of intravenous or oral acyclovir (n = 8) or valacyclovir (n = 1), several of whom clearly received higher-than-recommended doses. These concentrations, which were measured at various times from 6 to <24 h postdose, are much higher than the range of concentrations observed in the present study (Cmax values of 0.034 to 0.160 μM among those with normal renal function and 0.17 to 0.58 μM among those with renal impairment). Interestingly, the range of acyclovir concentrations in the CSF of subjects in the present study (Cmax of 4.9 to 8.7 μM among subjects with normal renal function and 11 to 21 μM among those with renal impairment), none of whom experienced central nervous system symptoms, is more similar to values observed by Helldén et al. among symptomatic subjects (3.5 to 28.6 μM) than among asymptomatic subjects (1.1 to 5.1 μM). This suggests that intrathecal exposure to increased concentrations of acyclovir and/or its metabolites may not entirely explain the occurrence of central nervous system symptoms.
Although renal impairment is associated with higher levels of acyclovir and its metabolites, the etiology of neurotoxicity associated with acyclovir or valacyclovir may be multifactorial. The underlying disease warranting treatment (e.g., HSV encephalitis) may predispose an individual to drug-related neurotoxicity by unknown mechanisms. Renal insufficiency may also lead to the accumulation of uremic toxins or neurotransmitter metabolites, which could promote neurological symptoms. Furthermore, competition between uremic toxins and acyclovir, or its metabolites, for organic anion efflux transport at the blood-brain barrier may lead to neurotoxicity. In this regard, Ohtsuki et al. previously demonstrated using a Xenopus oocyte expression system that 1 mM acyclovir inhibits organic anion transporter 3 (OAT3)-mediated transport of 2 μM indoxyl sulfate, a uremic toxin, and that other uremic toxins share this brain-to-blood efflux mechanism (20). This suggests the possibility that acyclovir may enhance the accumulation of uremic toxins in the brain.
The pharmacokinetics and disposition of 8-OH-ACV in the systemic circulation or in CSF have not been previously described. This metabolite distributes into the CSF in a proportion similar to that of acyclovir and an order of magnitude greater than that of CMMG. As a result, despite the far-lower systemic exposure of 8-OH-ACV than of CMMG, the exposures of these two metabolites in the CSF are similar. Furthermore, like acyclovir and CMMG, renal impairment significantly increases systemic and CSF concentrations of 8-OH-ACV. Therefore, this metabolite cannot be excluded as a possible contributor to acyclovir-associated central nervous system symptoms.
In conclusion, this study describes the simultaneous pharmacokinetics of acyclovir and its metabolites CMMG and 8-OH-ACV in the CSF and systemic circulation. Renal impairment significantly increases systemic and CSF exposure to all three compounds but does not alter their propensity to distribute into CSF.
Acknowledgments
Funding was provided by GlaxoSmithKline (GSK study HS240016). This study was also supported in part by RR024975 (Vanderbilt CTSA grant from the NCRR, NIH) and NIH grants 5T32GM07569-32 (J.P.S.) and MH071205 (D.W.H.).
All of the authors had access to the raw data. We gratefully acknowledge Kathy O'Mara, John Dunn, and Michael O'Mara for valuable bioanalytical support.
D.W.H. has received research grants from Bavarian Nordic, Boehringer-Ingelheim, Bristol-Myers Squibb, Gilead Sciences, Merck, Tanox, and Tibotec. He is on scientific advisory boards for GlaxoSmithKline and Tibotec. S.W. is an employee of GlaxoSmithKline.
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Almond, M. K. 2003. Aciclovir and valaciclovir neurotoxicity in patients with renal failure. Nephrol. Dial. Transplant. 18:2680. [DOI] [PubMed] [Google Scholar]
- 2.Carlon, R., C. Possamai, and U. Corbanese. 2005. Acute renal failure and severe neurotoxicity following valacyclovir. Intensive Care Med. 31:1593. [DOI] [PubMed] [Google Scholar]
- 3.de Knegt, R. J., H. van der Pijl, and L. A. van Es. 1995. Acyclovir-associated encephalopathy, lack of relationship between acyclovir levels and symptoms. Nephrol. Dial. Transplant. 10:1775-1777. [PubMed] [Google Scholar]
- 4.Egan, J. J., K. B. Carroll, N. Yonan, A. Woodcock, and A. Crisp. 2002. Valacyclovir prevention of cytomegalovirus reactivation after heart transplantation: a randomized trial. J. Heart Lung Transplant. 21:460-466. [DOI] [PubMed] [Google Scholar]
- 5.Ernst, M. E., and R. J. Franey. 1998. Acyclovir- and ganciclovir-induced neurotoxicity. Ann. Pharmacother. 32:111-113. [DOI] [PubMed] [Google Scholar]
- 6.Feldman, S., J. Rodman, and B. Gregory. 1988. Excessive serum concentrations of acyclovir and neurotoxicity. J. Infect. Dis. 157:385-388. [DOI] [PubMed] [Google Scholar]
- 7.Friedman, J. E., J. B. Zabriskie, C. Plank, D. Ablashi, J. Whitman, B. Shahan, R. Edgell, M. Shieh, O. Rapalino, R. Zimmerman, and D. Sheng. 2005. A randomized clinical trial of valacyclovir in multiple sclerosis. Mult. Scler. 11:286-295. [DOI] [PubMed] [Google Scholar]
- 8.Haas, D. W., J. Stone, L. A. Clough, B. Johnson, P. Spearman, V. L. Harris, J. Nicotera, R. H. Johnson, S. Raffanti, L. Zhong, P. Bergqwist, S. Chamberlin, V. Hoagland, and W. D. Ju. 2000. Steady-state pharmacokinetics of indinavir in cerebrospinal fluid and plasma among adults with human immunodeficiency virus type 1 infection. Clin. Pharmacol. Ther. 68:367-374. [DOI] [PubMed] [Google Scholar]
- 8a.Haas, D. W., S. Weller, B. Johnson, J. Nicotera, J. M. Luther, V. Broumand, K. Good, and D. S. Stein. 2003. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-1796.
- 9.Haefeli, W. E., R. A. Schoenenberger, P. Weiss, and R. F. Ritz. 1993. Acyclovir-induced neurotoxicity: concentration-side effect relationship in acyclovir overdose. Am. J. Med. 94:212-215. [DOI] [PubMed] [Google Scholar]
- 10.Helldén, A., J. Lycke, T. Vander, J. O. Svensson, I. Odar-Cederlof, and L. Stahle. 2006. The aciclovir metabolite CMMG is detectable in the CSF of subjects with neuropsychiatric symptoms during aciclovir and valaciclovir treatment. J. Antimicrob. Chemother. 57:945-949. [DOI] [PubMed] [Google Scholar]
- 11.Helldén, A., I. Odar-Cederlof, P. Diener, L. Barkholt, C. Medin, J. O. Svensson, J. Sawe, and L. Stahle. 2003. High serum concentrations of the acyclovir main metabolite 9-carboxymethoxymethylguanine in renal failure patients with acyclovir-related neuropsychiatric side effects: an observational study. Nephrol. Dial. Transplant. 18:1135-1141. [DOI] [PubMed] [Google Scholar]
- 12.Izzedine, H., L. Mercadal, G. Aymard, V. Launay-Vacher, V. Martinez, B. Issad, and G. Deray. 2001. Neurotoxicity of valacyclovir in peritoneal dialysis: a pharmacokinetic study. Am. J. Nephrol. 21:162-164. [DOI] [PubMed] [Google Scholar]
- 13.Jacobson, M. A. 1993. Valaciclovir (BW256U87): the L-valyl ester of acyclovir. J. Med. Virol. Suppl. 1:150-153. [DOI] [PubMed] [Google Scholar]
- 14.Jacobson, M. A., J. Gallant, L. H. Wang, D. Coakley, S. Weller, D. Gary, L. Squires, M. L. Smiley, M. R. Blum, and J. Feinberg. 1994. Phase I trial of valaciclovir, the L-valyl ester of acyclovir, in patients with advanced human immunodeficiency virus disease. Antimicrob. Agents Chemother. 38:1534-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Linssen-Schuurmans, C. D., E. J. van Kan, G. W. Feith, and D. R. Uges. 1998. Neurotoxicity caused by valacyclovir in a patient on hemodialysis. Ther. Drug Monit. 20:385-386. [DOI] [PubMed] [Google Scholar]
- 16.Ljungman, P., R. de La Camara, N. Milpied, L. Volin, C. A. Russell, A. Crisp, and A. Webster. 2002. Randomized study of valacyclovir as prophylaxis against cytomegalovirus reactivation in recipients of allogeneic bone marrow transplants. Blood 99:3050-3056. [DOI] [PubMed] [Google Scholar]
- 17.Lowance, D., H. H. Neumayer, C. M. Legendre, J. P. Squifflet, J. Kovarik, P. J. Brennan, D. Norman, R. Mendez, M. R. Keating, G. L. Coggon, A. Crisp, and I. C. Lee. 1999. Valacyclovir for the prevention of cytomegalovirus disease after renal transplantation. International Valacyclovir Cytomegalovirus Prophylaxis Transplantation Study Group. N. Engl. J. Med. 340:1462-1470. [DOI] [PubMed] [Google Scholar]
- 18.Lycke, J., O. Andersen, B. Svennerholm, L. Appelgren, and C. Dahlof. 1989. Acyclovir concentrations in serum and cerebrospinal fluid at steady state. J. Antimicrob. Chemother. 24:947-954. [DOI] [PubMed] [Google Scholar]
- 19.Lycke, J., C. Malmestrom, and L. Stahle. 2003. Acyclovir levels in serum and cerebrospinal fluid after oral administration of valacyclovir. Antimicrob. Agents Chemother. 47:2438-2441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ohtsuki, S., H. Asaba, H. Takanaga, T. Deguchi, K. Hosoya, M. Otagiri, and T. Terasaki. 2002. Role of blood-brain barrier organic anion transporter 3 (OAT3) in the efflux of indoxyl sulfate, a uremic toxin: its involvement in neurotransmitter metabolite clearance from the brain. J. Neurochem. 83:57-66. [DOI] [PubMed] [Google Scholar]
- 21.Okada, T., T. Nakao, H. Matsumoto, Y. Nagaoka, H. Iwasawa, K. Nanri, and T. Yamazaki. 2002. Valacyclovir neurotoxicity in a patient with end-stage renal disease treated with continuous ambulatory peritoneal dialysis. Clin. Nephrol. 58:168-170. [DOI] [PubMed] [Google Scholar]
- 22.Rowland, M., and T. N. Tozer. 1980. Clinical pharmacokinetics: concepts and applications. Lea & Febiger, Philadelphia, PA.
- 23.Soul-Lawton, J., E. Seaber, N. On, R. Wootton, P. Rolan, and J. Posner. 1995. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob. Agents Chemother. 39:2759-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strumia, S., P. De Mitri, and E. Bionda. 2004. Neurotoxicity of acyclovir and valacyclovir in a hemodialyzed patient. Eur. J. Neurol. 11:68-69. [DOI] [PubMed] [Google Scholar]
- 25.Tadepalli, S. M., and R. P. Quinn. 1996. Scintillation proximity radioimmunoassay for the measurement of acyclovir. J. Pharm. Biomed. Anal. 15:157-163. [DOI] [PubMed] [Google Scholar]
- 26.Wade, J. C., and J. D. Meyers. 1983. Neurologic symptoms associated with parenteral acyclovir treatment after marrow transplantation. Ann. Intern. Med. 98:921-925. [DOI] [PubMed] [Google Scholar]
- 27.Weller, S., M. R. Blum, M. Doucette, T. Burnette, D. M. Cederberg, P. de Miranda, and M. L. Smiley. 1993. Pharmacokinetics of the acyclovir pro-drug valaciclovir after escalating single- and multiple-dose administration to normal volunteers. Clin. Pharmacol. Ther. 54:595-605. [DOI] [PubMed] [Google Scholar]
- 28.Whitley, R. J. 1988. Herpes simplex virus infections of the central nervous system. A review. Am. J. Med. 85:61-67. [PubMed] [Google Scholar]