Abstract
Patients with intra-abdominal infections differ with regard to the type of infection and the severity of illness. However, the impact of these factors, together with differences in drug exposure, on clinical response is not well understood. Using phase 2 and 3 data for patients with complicated intra-abdominal infections, the relative importance of tigecycline exposure, host factors, and disease factors, alone or in combination, for the probability of clinical response was examined. Patients with complicated intra-abdominal infections who received tigecycline intravenously as a 100-mg loading dose followed by 50 mg every 12 h for 5 to 14 days and who had adequate clinical, pharmacokinetic, and response data were evaluated. Multivariable logistic regression was used to identify factors associated with clinical response. A final multivariable logistic regression model demonstrated six factors based on 123 patients to be predictive of clinical success: a weight of <94 kg (P = 0.026), the absence of Pseudomonas aeruginosa in baseline cultures (P = 0.021), an APACHE II score of <13 (P = 0.029), non-Hispanic race (P = 0.005), complicated appendicitis or cholecystitis (P = 0.004), and a ratio of the area under the concentration-time curve (AUC) to the MIC (AUC/MIC ratio) of ≥3.1 (P = 0.003). The average model-predicted probability of clinical success when one unfavorable factor was present was 0.940. This probability was lower (0.855) when the AUC/MIC ratio was <3.1 and the remaining five factors were set to the favorable condition. The average model-predicted probability of clinical success in the presence of two unfavorable factors was 0.594. These findings demonstrated the impact of individual and multiple factors on clinical response in the context of drug exposure.
Unlike the situation in the early part of the 20th century, when the mortality rate associated with intra-abdominal infections approached 90%, today the vast majority (95%) of patients with these infections survive (26). This is due to improved diagnosis, modern surgical techniques, advances in critical care, and the advent of antimicrobial chemotherapy. Despite these advances, the risk of mortality remains far greater for those critically ill patients with organ dysfunction, especially those with multiple-organ failure (60%) and other comorbidities (7, 14, 19, 22, 24).
Although the polymicrobial aerobic and anaerobic nature of intra-abdominal infections had been elucidated previously (1), it was not until the early 1970s that the role of anaerobic microflora and the importance of broad-spectrum antimicrobial agents were appreciated (18). In addition to optimizing the spectrum of therapy, an understanding of the nature of exposure-response relationships for efficacy for the antimicrobial agent(s) selected provides the opportunity to ensure the use of appropriate dosing regimens. Exposure-response relationships, which have been identified previously for agents used to treat relatively homogenous patient populations with primarily monomicrobial infections (2), are more challenging to identify for intra-abdominal infections, where the presence of multiple pathogens may be a determinant of clinical response. In addition, patients with these infections may be immunocompromised or may have widely varying measures of severity of illness (e.g., APACHE II score) (15). Such variability in both the type and the severity of infection can result in greatly differing prognoses. The impact on clinical response of the factors described above, together with differences in drug exposure, is not well understood.
Given that so few clinical trials collect measurements of drug concentrations from patients, drug exposures for patient populations of interest are often not available for the purposes of characterizing exposure-response relationships for efficacy. In the analyses described herein, the exposure-response relationship for the efficacy of tigecycline was characterized using data from phase 2 and 3 studies for patients with complicated intra-abdominal infections in which blood samples for the assay of tigecycline were also collected. In addition to drug exposure, the influence of different host and disease factors, alone or in combination, on the probability of clinical response was examined.
MATERIALS AND METHODS
Patient population and clinical data.
Data were gathered from three clinical trials for hospitalized patients with complicated intra-abdominal infections who had received tigecycline intravenously as a 100-mg loading dose followed by 50 mg every 12 h for at least 5 days and not more than 14 days. The protocols for these studies were reviewed and approved by the institutional review board or ethical review committee at each participating center. Written informed consent was obtained from each patient or his or her guardian before the commencement of any study procedure according to the guidelines of each institution. The trials were conducted in accordance with the Declaration of Helsinki and its amendments. A complete description of these clinical trials has been presented elsewhere (4, 16).
Entry criteria.
Patients were eligible for study entry if they were ≥18 years old and required a surgical procedure to treat a complicated intra-abdominal infection. Patients presenting with intra-abdominal infections arising from one of the following conditions were eligible for enrollment: postsurgical intra-abdominal abscess (including liver and spleen) after receipt of standard antibacterial therapy (i.e., antibiotics for at least 2 days but not more than 5 days); appendicitis complicated by perforation and/or a periappendiceal abscess; perforated diverticulitis complicated by abscess formation or fecal contamination; complicated cholecystitis with evidence of perforation, empyema, or gangrene; perforation of a gastric or duodenal ulcer with symptoms exceeding 24 h in duration; purulent peritonitis or peritonitis associated with fecal contamination; or perforation of the large or small intestine with abscess or fecal contamination.
Exclusion criteria.
Patients were excluded from enrollment for any one of the following reasons: preoperative suspicion of a diagnosis of spontaneous bacterial peritonitis; simple cholecystitis; gangrenous cholecystitis without rupture; simple appendicitis; acute suppurative cholangitis; pancreatic abscess, or infected necrotizing pancreatitis; an APACHE II score of >30; active or treated leukemia or systemic malignancy within the prior 3 months or a metastatic malignancy to the abdomen within the prior 6 months; known AIDS; presence of any uncontrolled central nervous system (CNS) disease; pregnancy or breastfeeding; known or suspected hypersensitivity either to the study drug or to related compounds; concomitant ganciclovir therapy; significant hepatic disease (i.e., an aspartate aminotransferase or alanine aminotransferase level >10 times the upper limit of normal or a total bilirubin value >3 times the upper limit of normal) or acute hepatic failure or acute decompensation of chronic hepatic failure; significant renal disease (i.e., calculated creatinine clearance of <41 ml/min/1.73 m2 after adequate hydration); neutropenia with an absolute neutrophil count of <1,000 cells/mm3, although counts as low as 500 cells/mm3 were permitted if they were a result of the acute infectious process; current intra-abdominal infection known to be caused by ≥1 bacterial isolate not susceptible to study drug; a surgical procedure requiring that fascia or deep muscular layers be left open or expectation of planned abdominal reexploration either in or out of the operating room; and administration of intraoperative antibacterial irrigants or peritoneal antibacterial agents (e.g., irrigants or antibiotic-impregnated sponges).
Outcome evaluation.
Clinical response (as determined by the investigator) was categorized as either cure, failure, or indeterminate at the test-of-cure visit (12 to 42 days after the end of therapy). Cure was defined as the resolution of the infectious process after tigecycline therapy plus the initial surgical intervention. Failure was defined as the need for additional antibacterial therapy other than the study drug or additional surgical or radiological intervention to treat the infection; death due to infection after 2 days of therapy; receipt of more than 120% of the planned treatment schedule; or study discontinuation secondary to an adverse drug event. Patients were classified as indeterminate if they were lost to follow-up, died within 48 h after the first dose of the study drug for any reason, or died after 48 h because of non-infection-related reasons.
Microbiological susceptibility testing.
As described previously (8), all pathogens isolated at baseline from the primary intra-abdominal site of infection were identified and tested for susceptibility to tigecycline by the broth microdilution (aerobic organisms) and standard agar dilution (anaerobic organisms) methods in accordance with the guidelines of the Clinical and Laboratory Standards Institute (10, 17).
Determination of drug exposure.
Venous blood samples for tigecycline concentration analysis were collected from patients on the day before discharge or the day of discharge. Samples were drawn at the following times: just prior to the dose, at the end of the infusion (30 min or 1 h, depending on the study), and 3 and 6 h after the start of the infusion.
Given that preclinical data have demonstrated that the ratio of the area under the concentration-time curve (AUC) to the MIC (AUC/MIC ratio) is the pharmacokinetic-pharmacodynamic (PK-PD) measure most predictive of efficacy (11), measures of AUC were estimated using a previously described population pharmacokinetic model (25), which was constructed using drug concentrations from noninfected subjects enrolled in a phase 1 tissue penetration study and from the patients enrolled in the phase 2 and 3 studies described here in. Individual posthoc estimates of clearance based on this model, which was a two-compartment model with zero-order intravenous input and first-order elimination, were used in conjunction with the dose to obtain a total-drug steady-state AUC value over 24 h for serum, expressed in mg·h/liter, for each patient, by equation 1:
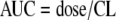 |
(1) |
where CL is the serum clearance in liters per hour.
The steady-state AUC values described above were normalized by the MIC to derive the total-drug AUC/MIC ratio. Given the polymicrobial nature of the disease, multiple pathogens per patient were anticipated. The highest MIC from among the pathogens isolated from the infection site at baseline was chosen for each patient in order to calculate the AUC/MIC ratio. In a separate analysis, the AUC/MIC ratio was also calculated for those patients who had Enterobacteriaceae isolated at baseline using the pathogen-specific MIC. If more than one Enterobacteriaceae isolate was recovered at baseline, the highest MIC was chosen.
Statistical analyses.
Univariable analyses, which consisted of a chi-square test or Fisher's exact test for categorical independent variables and logistic regression for continuous independent variables, were used to identify factors associated with clinical response. Independent variables considered included the following: the AUC/MIC ratio; patient age, weight, and race; the baseline APACHE II score; isolation of Pseudomonas aeruginosa or anaerobes in baseline cultures; and intra-abdominal infection diagnosis category. Continuous independent variables (age, weight, baseline APACHE II score, and AUC/MIC ratio) were also evaluated as categorical variables after breakpoint values were identified for continuous variables by classification and regression tree (CART) analysis using SYSTAT 11 (27).
Univariable analyses were then followed by multivariable logistic regression to identify factors associated with clinical response. The base model included the independent variable with the highest log likelihood. Model expansion was conducted by evaluating the remaining independent variables using the likelihood ratio test. The inclusion of an independent variable in the model was based on determining twice the log-likelihood difference between the expanded versus base models and comparing this against a χ2 distribution with the appropriate number of degrees of freedom (significance level for inclusion [α], 0.05). This process was repeated until no further model expansion could be justified.
RESULTS
A total of 123 patients with complicated intra-abdominal infections had adequate clinical, microbiological, pharmacokinetic, and response data and were included in these analyses. The mean (standard deviation) age of this group of patients was 45 (18) years, and the median (range) age was 43 (18, 85) years. A summary of additional demographic characteristics for these patients can be found in Table 1. Complicated appendicitis represented the most frequent type of infection (57.7%), while complicated cholecystitis, peritonitis due to perforation of the small/large intestine, or intra-abdominal, hepatic, or splenic abscesses occurred with lower frequencies (8.9 to 12.2%). Of the 123 patients, 53% had more than one pathogen isolated at baseline. Anaerobes were present in baseline cultures for approximately 31.7% of patients. A successful clinical response was observed for 87.1% of patients.
TABLE 1.
Patient characteristics (n = 123)
| Characteristic | Valuea |
|---|---|
| Age (yr) | 42.6 (18, 85) |
| Sex (% male) | 63.4 |
| Ethnic origin (%) | |
| White | 74.0 |
| Hispanic | 20.3 |
| Black | 4.1 |
| Other | 1.6 |
| Wt (kg) | 75 (45, 138) |
| Baseline APACHE II scoreb | 6 (0, 25) |
| Diagnosis (% of total) | |
| Complicated appendicitis | 57.7 |
| Complicated cholecystitis | 12.2 |
| Peritonitis due to perforation of small/large | |
| intestine | 8.9 |
| Intra-abdominal, hepatic, or splenic abscess | 10.6 |
| Other | 10.6 |
| Presence of P. aeruginosa in baseline cultures (%) | 8.1 |
| Presence of anaerobes in baseline cultures (%) | 31.7 |
Expressed as the percentage of the group with the given characteristic or as the median value (range).
Maximum score permitted, 30.
The median (range) estimated tigecycline clearance and steady-state AUC values were 16.1 (1.02, 25.6) liters/h and 6.19 (3.91, 97.6) mg·h/liter, respectively. AUC/MIC ratios, which were calculated by dividing the steady-state AUC values by the highest MIC when more than one pathogen was isolated at baseline, were based primarily on Enterobacteriaceae isolates (69.1%) which included Escherichia coli (48.0%), Klebsiella species (11.4%), and Enterobacter species (5.7%). Anaerobic isolates accounted for 21.1% of MICs. MICs for the baseline pathogens with the highest MICs ranged from 0.004 to 4 mg/liter. The MICs at which 50% and 90% of isolates were inhibited (MIC50 and MIC90, respectively) based on this distribution were 0.5 and 1.0 mg/liter. The median (range) AUC/MIC ratio based on these AUCs and MICs was 19.5 (0.976, 1815). Figure 1 shows the distribution of AUC/MIC ratios using a log10 scale. Among the patients with at least one Enterobacteriaceae isolated at baseline, by using the highest MIC for these organisms, the MIC ranged from 0.06 to 1 mg/liter, with a MIC50 and MIC90 of 0.25 and 1.0 mg/liter, respectively. The median (range) AUC/MIC ratio for this group was 21.8 (4.99, 390).
FIG. 1.
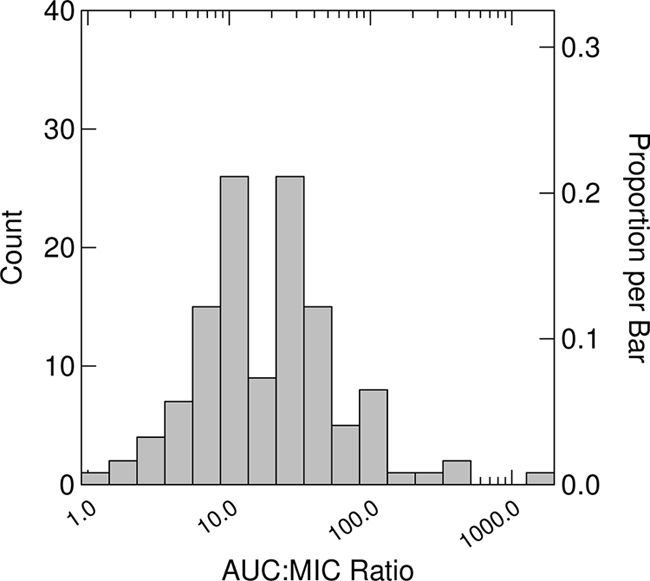
Distribution of AUC/MIC ratios using a log10 scale (n = 123). AUC/MIC ratios were based on the pathogen with the highest MIC.
A summary of univariable relationships between independent variables of interest and clinical response can be found in Table 2. Patients with complicated appendicitis or cholecystitis had higher probabilities of clinical success than patients with other diagnoses (0.919 versus 0.757; P = 0.02). Patients without P. aeruginosa in baseline cultures had higher probabilities of clinical success than patients with this pathogen at baseline (0.894 versus 0.6; P = 0.025). Hispanic race compared to other races was associated with significantly lower probabilities of clinical response (0.68 versus 0.918; P = 0.004). Relationships between both APACHE II score and weight, evaluated both continuously and categorically, and clinical response were significant. As determined using CART, patients with a baseline APACHE II score of <13, a weight of <94 kg, or an AUC/MIC ratio of ≥3.1 had higher probabilities of clinical success than patients with an APACHE II score of ≥13, a weight of ≥94 kg, and an AUC/MIC ratio of <3.1. Probabilities of clinical success for patients with APACHE II scores of <13 and ≥13 were 0.888 and 0.5 (P = 0.029), respectively. Similarly, for weights of <94 and ≥94 kg, these probabilities were 0.904 and 0.684 (P = 0.018), respectively. No CART-derived breakpoint was identified for age.
TABLE 2.
Univariable analyses for factors predictive of clinical success
| Independent variable | P |
|---|---|
| AUC/MIC ratio, continuous | 0.208 |
| AUC:MIC ratio, ≥3.1a | 0.029 |
| APACHE II score, continuous | 0.025 |
| APACHE II score, <13b | 0.029 |
| Wt, continuous | 0.008 |
| Wt, <94 kgc | 0.018 |
| Age, continuous | 0.322 |
| Absence of P. aeruginosad in baseline cultures | 0.025 |
| Absence of anaerobese in baseline cultures | 0.819 |
| Non-Hispanic racef | 0.004 |
| Diagnosis of complicated appendicitis or cholecystitisg | 0.020 |
The reference group included patients with AUC/MIC ratios of <3.1 (n = 6).
The reference group included patients with APACHE II scores of ≥13 (n = 6).
The reference group included patients weighing ≥94 kg (n = 19).
The reference group included patients with P. aeruginosa in baseline cultures (n = 10).
The reference group included patients with anaerobic organisms growing in baseline cultures (n = 43).
The reference group included patients of Hispanic race (n = 25).
The reference group included diagnoses of peritonitis due to perforation of the small/large intestine; intra-abdominal, hepatic, or splenic abscess; or other (n = 37).
Probabilities of clinical success for patients with AUC/MIC ratios of ≥3.1 and <3.1 were 0.889 and 0.5, respectively (P = 0.029). In the analysis among those patients with at least one Enterobacteriaceae isolated at baseline (n = 97), a significant relationship was also found between clinical response and AUC/MIC ratio when evaluated as a categorical variable. Probabilities of clinical success for patients with AUC/MIC ratios of ≥12.96 and <12.96 were 0.923 and 0.75, respectively (P = 0.027).
As shown in Table 3, six factors remained in the final multivariable logistic regression model for clinical success. These factors were a weight of <94 versus ≥94 kg, the absence of P. aeruginosa in baseline cultures, an APACHE II score of <13 versus ≥13, non-Hispanic versus Hispanic race, complicated appendicitis or cholecystitis versus all other diagnoses, and an AUC/MIC ratio of ≥3.1 versus <3.1. As evidenced by the examination of the odds ratios for each of the variables, an AUC/MIC ratio of >3.1 had the largest impact on the probability of clinical success (odds ratio, 33.0; 95% confidence interval [CI], 3.27 to 333). In contrast, a weight of ≥94 kg had the smallest impact on the probability of clinical success (odds ratio, 6.35; 95% CI, 1.25 to 32.4).
TABLE 3.
Final multivariable logistic regression model for factors predictive of clinical successa
| Independent variable | Estimate | Odds ratio (95% CI) | P |
|---|---|---|---|
| Intercept | −9.831 | <0.001 | |
| Wt, <94 kgb | 1.849 | 6.35 (1.25, 32.4) | 0.026 |
| Absence of P. aeruginosac in baseline cultures | 2.317 | 10.1 (1.43, 72.0) | 0.021 |
| APACHE II score, <13d | 2.390 | 10.9 (1.28, 93.3) | 0.029 |
| Non-Hispanic racee | 2.503 | 12.2 (2.12, 70.6) | 0.005 |
| Diagnosis of complicated appendicitis or cholecystitisf | 2.545 | 12.7 (2.27, 71.5) | 0.004 |
| AUC/MIC ratio, ≥3.1g | 3.497 | 33.0 (3.27, 333) | 0.003 |
McFadden's ρ2 = 0.416.
The reference group included patients weighing ≥ 94 kg (n = 19).
The reference group included patients with P. aeruginosa in baseline cultures (n = 10).
The reference group included patients with APACHE II scores ≥ 13 (n = 6).
The reference group included patients of Hispanic race (n = 25).
The reference group included diagnoses of peritonitis due to perforation of the small/large intestine; intra-abdominal, hepatic, or splenic abscess; or other (n = 37).
The reference group included patients with AUC/MIC ratios of <3.1 (n = 6).
Model-predicted probabilities of clinical success, as a function of one of the six unfavorable factors, are presented in Table 4. An AUC/MIC ratio of <3.1, with the remaining factors set to the favorable condition, was associated with a probability of clinical success of 0.855. When other single factors were set to the unfavorable condition (the presence of P. aeruginosa in baseline cultures, Hispanic race, an APACHE II score of ≥13, a weight of ≥94 kg, or a diagnosis of intra-abdominal, hepatic, or splenic abscess, peritonitis due to perforation of the small/large intestine, or other), probabilities of clinical success ranged from 0.938 (diagnosis category) to 0.968 (weight, ≥94 kg).
TABLE 4.
Probability of clinical success in the presence of one unfavorable factor
| Factora | Probability |
|---|---|
| Wt, ≥94 kg | 0.968 |
| Presence of P. aeruginosa in baseline cultures | 0.950 |
| APACHE II score, ≥13 | 0.947 |
| Hispanic race | 0.941 |
| Diagnosis of abscess, peritonitis due to perforation, or other | 0.938 |
| AUC/MIC ratio, <3.1 | 0.855 |
The remaining factors were set to the conditions favoring clinical response. The following conditions represented the most favorable for optimizing clinical response: weight of <94 kg, absence of P. aeruginosa in baseline cultures, APACHE II score of <13, non-Hispanic race, diagnosis of complicated appendicitis or cholecystitis, and an AUC/MIC ratio of ≥3.1.
As shown in Table 5, the impact of two unfavorable factors on the probability of clinical response was magnified. Probabilities of clinical success in the presence of two unfavorable factors ranged from 0.316 to 0.751. An AUC/MIC ratio of <3.1, in combination with another unfavorable factor, was associated with the lowest probabilities of clinical success, ranging from 0.316 (lower AUC/MIC ratio plus diagnosis category) to 0.481 (lower AUC/MIC ratio and weight of ≥94 kg).
TABLE 5.
Probability of clinical success in the presence of two unfavorable factorsa
| Factor 1 | Factor 2b | Probability |
|---|---|---|
| Wt, ≥94 kg | Presence of P. aeruginosa in baseline cultures | 0.751 |
| APACHE II score, ≥13 | 0.737 | |
| Hispanic race | 0.714 | |
| Diagnosis, perforation | 0.706 | |
| AUC/MIC ratio, <3.1 | 0.481 | |
| Presence of P. aeruginosa in baseline cultures | APACHE II score, ≥13 | 0.637 |
| Hispanic race | 0.610 | |
| Diagnosis of perforation | 0.600 | |
| AUC/MIC ratio, <3.1 | 0.367 | |
| APACHE II score, ≥13 | Hispanic race | 0.593 |
| Diagnosis of perforation | 0.583 | |
| AUC/MIC ratio, <3.1 | 0.350 | |
| Hispanic race | Diagnosis of perforation | 0.555 |
| AUC/MIC ratio, <3.1 | 0.324 | |
| Diagnosis of abscess or peritonitis due to perforation | AUC/MIC ratio, <3.1 | 0.316 |
Each pair of unfavorable factors is shown only once.
The remaining factors were set to the condition favoring clinical response. The following conditions represented the most favorable for optimizing clinical response: a weight of <94 kg, the absence of P. aeruginosa in baseline cultures, an APACHE II score of <13, non-Hispanic race, a diagnosis of complicated appendicitis or cholecystitis, and an AUC/MIC ratio of ≥3.1.
Observed and model-predicted probabilities of clinical success based on the final multivariable logistic regression model were evaluated in the instances where one or two of the six factors predictive of clinical success were unfavorable. When sample sizes for a given cohort of patients were equal to six or more, observed and model-predicted probabilities of clinical success were in close agreement for the evaluation of one unfavorable factor. Observed and model-predicted probabilities of clinical success were 1.0 and 0.968, 0.917 and 0.941, and 0.955 and 0.938 for a weight of ≥94 kg, Hispanic race, and diagnosis of peritonitis or intra-abdominal abscess, respectively. The average observed and model-predicted probabilities of clinical success when one unfavorable factor was present were 0.909 and 0.940, respectively. For the evaluation of two unfavorable factors, samples sizes for individual cohorts of patients did not exceed five. Despite the small sizes of these cohorts, the average observed and model-predicted probabilities of clinical success when two unfavorable factors were present were in reasonably close agreement (0.682 and 0.594, respectively). For the evaluation of one or two unfavorable factors, good agreement was evident between the average observed and model-predicted probabilities of clinical success (0.833 and 0.825, respectively). The model-predicted probability of clinical success in the presence of no unfavorable factors was 0.995, which was expected given that the observed probability of clinical success was 1.0 in the 52 cases with no unfavorable factors.
DISCUSSION
In these analyses, we examined both phase 2 and phase 3 data from patients with complicated intra-abdominal infections treated with tigecycline, and we demonstrated six factors that independently had a significant impact on the probability of clinical response. Among the factors evaluated, APACHE II score, patient weight, race, intra-abdominal infection diagnosis, the presence of P. aeruginosa in baseline cultures, and AUC/MIC ratio were found to be predictive of clinical response.
Previous studies have shown the importance of several of these host and disease factors on the outcome of intra-abdominal infections. It has been demonstrated repeatedly that a high APACHE II score can markedly increase the probability of failure (9, 12, 20, 28). Other data have also shown that obese patients undergoing intra-abdominal surgeries have a decreased probability of obtaining a successful clinical response (5). These investigators observed that the performance of a left colectomy for obese patients was associated with a significantly higher rate of secondary intra-abdominal infections. Among patients undergoing proctectomies, there was a significantly higher risk of death for obese patients. The type of intra-abdominal infection has also been known to influence patient outcome: patients suffering from diffuse peritonitis and those with intra-abdominal abscesses have higher failure rates (6). In addition to the type of infection, the recovery of P. aeruginosa from the primary infection site has been demonstrated to have an independent adverse impact on the outcome of these infections (13, 24, 29). Finally, in this analysis, Hispanic race was also associated with a significantly lower probability of clinical success. We have no explanation for this finding but speculate that it may be explained by socioeconomic status or access to health care.
In the therapy of patients with infectious diseases, there are factors that the clinician can and cannot control. In the case of intra-abdominal infections, the physician cannot control the infection locus, the pathogen(s) or its antimicrobial susceptibility, or the severity of illness. One of the few factors the clinician can control is the choice of antimicrobial therapy and dosage regimen.
Of the six significant factors identified, an AUC/MIC ratio of >3.1 was found to be the most important factor in determining outcome. This was also the only factor that is amenable to intervention by the clinician. Given the classical mixed-organism nature of intra-abdominal infections, evaluations of exposure-response relationships for efficacy among patients with such infections can be challenging. As demonstrated by Onderdonk et al. (21), both aerobic and anaerobic organisms represent important components of the intra-abdominal infection process; Enterobacteriaceae isolates are more likely to generate diffuse peritonitis and bacteremia, and anaerobic isolates are more likely to generate intra-abdominal abscesses. When all pathogens were considered and the AUC/MIC ratio was based on the highest MIC, an AUC/MIC ratio of ≥3.1 was found to be predictive of higher probabilities of clinical success. Such PK-PD target thresholds, when identified, will be useful to support dose selection decisions for specific disease states. When the analysis was performed for patients from whom at least one Enterobacteriaceae was isolated at baseline and using the highest MIC for these organisms, the AUC/MIC ratio predictive of clinical success increased from 3.1 to a value in excess of 12. Such data supporting a higher PK-PD target may be useful for supporting susceptibility breakpoint decisions, which are typically based on exposure-response relationships and PK-PD targets for homogenous groups of pathogens (e.g., those belonging to the same family and/or genus).
These findings, which show the impact of individual and multiple unfavorable factors on clinical response and the nonlinear nature of these relationships, served to demonstrate the complex relationship among host and disease factors and drug exposure, and the probability of a successful clinical response. While any one unfavorable factor was associated with probabilities for clinical success ranging from 0.855 to 0.968, probabilities dropped dramatically when two unfavorable factors were present (0.316 to 0.751). However, given the limited number of patients evaluated and the limited size of samples resulting from the examination of cohorts of patients with any two unfavorable factors, it was difficult to examine the observed versus predicted proportion of successful responses and thus to assess the stability of the model for predictions based on the presence of more than one unfavorable factor. Additional studies will be necessary to validate these observations.
It has been shown previously that clinical studies that include the collection of pharmacokinetic information are useful for understanding the impact of drug exposure on therapeutic outcome (2). Using clinical and pharmacokinetic data from well-controlled clinical trials involving a very heterogeneous patient population, the impact of tigecycline exposure on clinical outcome was demonstrated. In these analyses, exposure-response relationships were explored considering the MICs for Gram-negative, Gram-positive, and anaerobic organisms, the pathogens encountered in a mixed intra-abdominal infection. Thus, the highest MIC from among all the pathogens isolated at baseline was considered to be more predictive of clinical response relative to drug exposure than the actual bacterial species of the pathogen. Given this approach, the AUC/MIC ratio breakpoint of 3.1 identified for tigecycline based on the analyses described herein represented a disease-state PK-PD breakpoint for the treatment of intra-abdominal infections with this agent. In a recent analysis conducted using the same data set (23), a CART-derived AUC/MIC ratio breakpoint of 6.96 was identified to be predictive of microbiologic success among the cohort of patients with at least one Enterobacteriaceae plus or minus one anaerobic pathogen isolated at baseline (n = 71). As discussed in the context of the CART-derived breakpoint for the AUC/MIC ratio for patients with Enterobacteriaceae described herein (12.96), such breakpoints, which are determined for patients with similar infecting pathogens, are well suited for setting susceptibility breakpoints for different antibacterial agents against specific bacterial species or groups of similar organisms (3).
Through the analyses conducted here, the influence of a number of factors, including adequate antibacterial therapy, on the clinical response of patients with intra-abdominal infections was demonstrated. As newer antibacterial agents are studied, it will be critical to perform such analyses in order for clinicians to understand the probability of clinical response for those factors that can be controlled, such as exposure to adequate antibacterial therapy, and those factors that cannot be controlled, such as underlying medical illness.
Acknowledgments
We acknowledge Jeffrey P. Hammel and Kim Charpentier at the Institute for Clinical Pharmacodynamics, Ordway Research Institute, Latham, NY, for assistance in the statistical review and the preparation of the manuscript, respectively. We also gratefully acknowledge Wyeth Pharmaceuticals for scientific review of the manuscript.
This analysis was supported in part by funding from Wyeth Pharmaceuticals.
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Altemeier, W. A. 1938. The bacterial flora of acute perforated appendicitis with peritonitis: a bacteriologic study based upon one hundred cases. Ann. Surg. 107:517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambrose, P. G., S. M. Bhavnani, C. M. Rubino, A. Louie, T. Gumbo, A. Forrest, and G. L. Drusano. 2007. Pharmacokinetics-pharmacodynamics of antimicrobial therapy: it's not just for mice anymore. Clin. Infect. Dis. 44:79-86. [DOI] [PubMed] [Google Scholar]
- 3.Ambrose, P. G., A. K. Meagher, J. A. Passarell, S. A. Van Wart, B. B. Cirincione, C. M. Rubino, J. M. Korth-Bradley, T. Babinchak, and E. Ellis-Grosse. 2009. Use of a clinically derived exposure-response relationship to evaluate potential tigecycline-Enterobacteriaceae susceptibility breakpoints. Diagn. Microbiol. Infect. Dis. 63:38-42. [DOI] [PubMed] [Google Scholar]
- 4.Babinchak, T., E. Ellis-Grosse, N. Dartois, G. M. Rose, and E. Loh. 2005. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin. Infect. Dis. 41(Suppl. 5):S354-S367. [DOI] [PubMed] [Google Scholar]
- 5.Benoist, S., Y. Panis, A. Alves, and P. Valleur. 2000. Impact of obesity on surgical outcomes after colorectal resection. Am. J. Surg. 179:275-281. [DOI] [PubMed] [Google Scholar]
- 6.Berne, T. V., A. W. Yellin, M. D. Appleman, and P. N. Heseltine. 1982. Antibiotic management of surgically treated gangrenous or perforated appendicitis. Comparison of gentamicin and clindamycin versus cefamandole versus cefoperazone. Am. J. Surg. 144:8-13. [DOI] [PubMed] [Google Scholar]
- 7.Bohnen, J., M. Boulanger, J. L. Meakins, and A. P. McLean. 1983. Prognosis in generalized peritonitis. Relation to cause and risk factors. Arch. Surg. 118:285-290. [DOI] [PubMed] [Google Scholar]
- 8.Bradford, P. A., D. T. Weaver-Sands, and P. J. Petersen. 2005. In vitro activity of tigecycline against isolates from patients enrolled in Phase 3 clinical trials of treatment for complicated skin and skin-structure infections and complicated intra-abdominal infections. Clin. Infect. Dis. 41(Suppl. 5):S315-S332. [DOI] [PubMed] [Google Scholar]
- 9.Christou, N. V., P. S. Barie, E. P. Dellinger, J. P. Waymack, and H. H. Stone. 1993. Surgical Infection Society intra-abdominal infection study. Prospective evaluation of management techniques and outcome. Arch. Surg. 128:193-198. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing. Fifteenth informational supplement, M100-S15. CLSI, Wayne, PA.
- 11.Craig, W. A. 2007. Pharmacodynamics of antimicrobials: general concepts and applications, p. 1-19. In C. H. Nightingale, P. G. Ambrose, G. L. Drusano, and T. Murakawa (ed.), Antimicrobial pharmacodynamics in theory and clinical practice, 2nd ed., Informa Healthcare USA, Inc., New York, NY.
- 12.Dellinger, E. P., M. J. Wertz, J. L. Meakins, J. S. Solomkin, M. D. Allo, R. J. Howard, and R. L. Simmons. 1985. Surgical infection stratification system for intra-abdominal infection. Multicenter trial. Arch. Surg. 120:21-29. [DOI] [PubMed] [Google Scholar]
- 13.Heseltine, P. N., A. E. Yellin, M. D. Appleman, M. A. Gill, F. C. Chenella, J. W. Kern, and T. V. Berne. 1983. Perforated and gangrenous appendicitis: an analysis of antibiotic failures. J. Infect. Dis. 148:322-329. [DOI] [PubMed] [Google Scholar]
- 14.Koperna, T., and F. Schulz. 1996. Prognosis and treatment of peritonitis. Do we need new scoring systems? Arch. Surg. 131:180-186. [DOI] [PubMed] [Google Scholar]
- 15.Levison, M. E., and L. M. Bush. 2000. Peritonitis and other intra-abdominal infections, p. 823. In G. L. Mandell, R. G. Douglas, J. E. Bennett, and R. Dolin (ed.), Principles and practice of infectious diseases, 5th ed. Churchill Livingstone, Philadelphia, PA.
- 16.Murray, J., S. Wilson, S. Klein, A. Yellin, and E. Loh. 2003. The clinical response to tigecycline in the treatment of complicated intra-abdominal infections in hospitalized patients, a phase 2 clinical trial, abstr. L-739. Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 17.National Committee for Clinical and Laboratory Standards. 2004. Methods for antimicrobial susceptibility testing of anaerobic bacteria. Approved standard M11-A6. NCCLS, Wayne, PA.
- 18.Nichols, R. L. 1995. Surgical antibiotic prophylaxis. Med. Clin. North Am. 79:509-522. [DOI] [PubMed] [Google Scholar]
- 19.Nystrom, P. O., R. Bax, E. P. Dellinger, L. Dominioni, W. A. Knaus, J. L. Meakins, C. Ohmann, J. S. Solomkin, H. Wacha, and D. H. Wittmann. 1990. Proposed definitions for diagnosis, severity scoring, stratification, and outcome for trials on intraabdominal infection. Joint Working Party of SIS North America and Europe. World J. Surg. 14:148-158. [DOI] [PubMed] [Google Scholar]
- 20.Ohmann, C., D. H. Wittmann, and H. Wacha. 1993. Prospective evaluation of prognostic scoring systems in peritonitis. Peritonitis Study Group. Eur. J. Surg. 159:267-274. [PubMed] [Google Scholar]
- 21.Onderdonk, A. B., J. G. Bartlett, T. Louie, N. Sullivan-Seigler, and S. L. Gorbach. 1976. Microbial synergy in experimental intra-abdominal abscess. Infect. Immun. 13:22-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pacelli, F., G. B. Doglietto, S. Alfieri, E. Piccioni, A. Sgadari, D. Gui, and F. Crucitti. 1996. Prognosis in intra-abdominal infections. Multivariate analysis on 604 patients. Arch. Surg. 131:641-645. [DOI] [PubMed] [Google Scholar]
- 23.Passarell, J. A., A. K. Meagher, K. Liolios, B. B. Cirincione, S. A. Van Wart, T. Babinchak, E. J. Ellis-Grosse, and P. G. Ambrose. 2008. Exposure-response analyses of tigecycline efficacy in patients with complicated intra-abdominal infections. Antimicrob. Agents Chemother. 52:204-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pine, R. W., M. J. Wertz, E. S. Lennard, E. P. Dellinger, C. J. Carrico, and B. H. Minshew. 1983. Determinants of organ malfunction or death in patients with intra-abdominal sepsis. A discriminant analysis. Arch. Surg. 118:242-249. [DOI] [PubMed] [Google Scholar]
- 25.Rubino, C. M., L. Ma, S. M. Bhavnani, J. Korth-Bradley, J. Speth, E. Ellis-Grosse, K. R. Rodvold, P. G. Ambrose, and G. L. Drusano. 2007. Evaluation of tigecycline penetration into colon wall tissue and epithelial lining fluid using a population pharmacokinetic model and Monte Carlo simulation. Antimicrob. Agents Chemother. 51:4085-4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salomone, J. P., and R. L. Nichols. 1998. Intra-abdominal surgical infections, p. 461-488. In B. A. Cunha (ed.), Infectious diseases in critical care medicine. Marcel Dekker, Inc., New York, NY.
- 27.Systat Software, Inc. 2004. Systat 11. Statistics II, p. 657. Systat Software, Inc., Richmond, CA.
- 28.Wacha, H., T. Hau, R. Dittmer, and C. Ohmann. 1999. Risk factors associated with intraabdominal infections: a prospective multicenter study. Peritonitis Study Group. Langenbecks Arch. Surg. 384:24-32. [DOI] [PubMed] [Google Scholar]
- 29.Yellin, A. E., P. N. Heseltine, T. V. Berne, M. D. Appleman, M. A. Gill, C. E. Riggio, and F. C. Chenella. 1985. The role of Pseudomonas species in patients treated with ampicillin and sulbactam for gangrenous and perforated appendicitis. Surg. Gynecol. Obstet. 161:303-307. [PubMed] [Google Scholar]


