Abstract
Altered pharmacokinetics of antituberculosis drugs may contribute to an increased risk of tuberculosis treatment failure for diabetic patients. We previously found that rifampin exposure was 2-fold lower in diabetic than in nondiabetic tuberculosis patients during the continuation phase of treatment. We now examined the influence of diabetes on the pharmacokinetics of antituberculosis drugs in the intensive phase of tuberculosis treatment, and we evaluated the effect of glycemic control. For this purpose, 18 diabetic and 18 gender- and body weight-matched nondiabetic tuberculosis patients were included in an Indonesian setting. Intensive pharmacokinetic sampling was performed for rifampin, pyrazinamide, and ethambutol at steady state. The bioavailability of rifampin was determined by comparing rifampin exposure after oral versus intravenous administration. Pharmacokinetic assessments were repeated for 10 diabetic tuberculosis patients after glycemic control. No differences in the areas under the concentration-time curves of the drugs in plasma from 0 to 24 h postdose (AUC0-24), the maximum concentrations of the drugs in plasma (Cmax), the times to Cmax (Tmax), and the half-lives of rifampin, pyrazinamide, and ethambutol were found between diabetic and nondiabetic tuberculosis patients in the intensive phase of tuberculosis treatment. For rifampin, oral bioavailability and metabolism were similar in diabetic and nondiabetic patients. The pharmacokinetic parameters of antituberculosis drugs were not correlated with blood glucose levels or glucose control. We conclude that diabetes does not alter the pharmacokinetics of antituberculosis drugs during the intensive phase of tuberculosis treatment. The reduced exposure to rifampin of diabetic patients in the continuation phase may be due to increased body weight and possible differences in hepatic induction. Further research is needed to determine the cause of increased tuberculosis treatment failure among diabetic patients.
Diabetes mellitus (DM) is a well-known risk factor for tuberculosis (TB) (1, 3, 7), with prevalence rates among TB patients ranging from 10 to 30% (1, 24, 25). There is a rapid increase in the global prevalence of DM, especially in developing countries, where TB is highly endemic. It is estimated that by the year 2030, 80% of DM patients will live in the high-burden countries for TB (28). As a result, the number of TB patients with DM will increase further (19).
Diabetes exerts a negative effect on TB treatment, especially among patients with poor glycemic control, with more treatment failure and more relapse than among TB patients in general (2, 3, 7, 24). One of the possible underlying mechanisms could be altered pharmacokinetics of anti-TB drugs. Lower concentrations of anti-TB drugs in plasma have been associated with clinical failure and acquired drug resistance (11, 23). Our previous study showed that the mean exposure to rifampin (expressed as the area under the concentration-time curve of the drug in plasma from 0 to 6 h postdose [AUC0-6]) and the mean peak concentration of the drug in plasma (Cmax) were 2-fold lower in Indonesian TB patients with DM than in those without DM (14). In multivariate analyses, a higher body weight (P < 0.001), the presence of DM (P = 0.06), and higher blood glucose levels (P = 0.016) contributed to lower plasma rifampin concentrations. These results suggested that heavier diabetic TB patients may need to be treated with a higher dose of rifampin and that glycemic control may increase drug concentrations.
In our previous study, TB patients with and without DM were not matched for weight (6, 15); only rifampin was measured; and limited sampling points were used to assess the pharmacokinetics of this drug. We have therefore performed an in-depth follow-up study in which TB patients with and without DM were matched for weight in order to enable disentangling of the effects of DM and weight on plasma drug concentrations. Furthermore, rifampin, pyrazinamide, and ethambutol were studied, and intensive pharmacokinetic sampling was performed. The first objective of the study was to compare the pharmacokinetics of rifampin, pyrazinamide, and ethambutol between weight-matched diabetic and nondiabetic TB patients. The second objective was to elaborate the possible mechanism of the alteration of pharmacokinetics of rifampin, and the third was to evaluate the effect of glycemic control on the pharmacokinetics of anti-TB drugs in diabetic TB patients.
MATERIALS AND METHODS
Study subjects.
Diabetic TB patients, who started with or were in the first 2 weeks of the intensive phase of TB treatment (arm 1), and nondiabetic TB patients, who were matched for gender and body weight (not more than 5 kg difference) as controls (arm 2), were recruited from an outpatient TB clinic in Bandung, Indonesia. The diagnosis of pulmonary TB was based on clinical symptoms, a chest X-ray examination, sputum microscopy, and M. tuberculosis culture. DM was diagnosed based on WHO criteria (31). In addition, patients were included if they had previously been diagnosed with DM and had poor glucose control at the time of recruitment, either with or without the use of antidiabetic drugs. Patients who were pregnant, below 18 or above 60 years of age, or taking any drug that is known to affect the pharmacokinetics of anti-TB drugs, and those with diarrhea, vomiting, or abnormal liver or kidney function were excluded. HIV status was assessed anonymously at the end of the study, and patients with positive results were excluded from further analysis, because HIV infection may affect the pharmacokinetics of TB drugs.
Study design.
A prospective, two-arm, three-period pharmacokinetic study was conducted. All study subjects received standard TB treatment consisting of 450 mg of rifampin (corresponding to 10 mg/kg of body weight for Indonesian patients, who have low body weights), 300 mg of isoniazid, 1,500 mg of pyrazinamide, and 750 mg of ethambutol daily for 2 months, followed by isoniazid (600 mg) and rifampin (450 mg) three times a week for 4 months, according to the Indonesian National Tuberculosis program. All patients received TB drugs from the same manufacturer, formulated in separate tablets. The bioequivalence of the rifampin tablets with an international reference standard has been established previously (26). Adherence to TB drugs was monitored by pill count and physician assessment methods (i.e., a physician assessing the patient if she/he forgot to take the drugs in the previous 2 weeks) every time patients attended for follow-up (22).
A first pharmacokinetic (PK) assessment was performed 2 weeks after the start of TB treatment, at steady state (Fig. 1). All TB drugs, including rifampin, were given orally (study objective 1). A second PK assessment was performed the following day with the same dose of rifampin administered intravenously, to enable adequate discrimination between the possible effects of DM on the absorption, metabolism, or elimination of rifampin (study objective 2). For intravenous infusion, 450 mg of rifampin (Rifadin; Bayer) was diluted in 250 ml of normal saline (0.9% NaCl) and administered over a period of 90 min (rate of infusion, 5 mg/min) (8).
FIG. 1.
Study design. R, rifampin at 450 mg daily; Z, pyrazinamide at 1,500 mg daily; E, ethambutol at 750 mg daily; p.o., per os; i.v., intravenous; PK, pharmacokinetic assessment. PK I took place 2 weeks after the start of TB treatment, and all TB drugs were administered per os. PK II was performed the day after PK I, and 450 mg of rifampin was administered intravenously by continuous infusion for 90 min. PK III was performed for 10 diabetic TB patients 3 weeks after normal blood glucose levels were achieved. For glycemic control, subcutaneous (s.c.) insulin injection was used, with the dose adjusted in order to normalize blood glucose within 2 to 3 weeks; all anti-TB drugs were administered per os.
In accordance with national guidelines, TB patients with DM were then treated with hypoglycemic agents starting after 2 to 4 weeks of TB treatment. For 10 selected patients, short-acting insulin (Humulin, 300 U; Novo Nordisk) was used, aiming at normal blood glucose levels within 2 to 3 weeks. Patients were educated about injecting insulin, recognizing hypoglycemia, and monitoring blood glucose at home. They were provided with glucometers, and the study physician made dose adjustments if necessary. A third PK assessment was performed after a normal blood glucose level was achieved (Fig. 1). In this PK assessment, all TB drugs were given orally (study objective 3). After the third PK assessment, glucose control was continued with oral antidiabetic drugs.
Based on the first objective and data from the previous study (20), it was calculated that a minimum number of 34 patients (17 in each arm) was required in order to demonstrate a difference of at least 25% in rifampin exposure between diabetic and nondiabetic TB patients by using the independent-samples t test with a significance level (α) of 5% and a power (1 − β) of 80%. Informed consent was obtained from all subjects, and the study was approved by the Independent Ethics Committee, Faculty of Medicine, University of Padjadjaran, Bandung, Indonesia.
Pharmacokinetic assessment and bioanalysis.
Patients refrained from the intake of any food or drugs from 11 pm on the day preceding the first or third PK assessment until 4 h after the intake of study medication. They took all TB drugs with 230 ml of still water. Serial venous blood samples were collected just prior to and, 1, 1, 2, 2, 3, 4, 6, 8, and 12 h after witnessed drug intake. Plasma was immediately separated, frozen at −20°C, transferred to −80°C within 72 h, and transported on dry ice to the Netherlands for bioanalysis. The stability of rifampin, its metabolite desacetylrifampin, pyrazinamide, and ethambutol under all these conditions has been validated before. The concentrations of rifampin, desacetylrifampin, pyrazinamide, and ethambutol in plasma were assessed with validated high-performance liquid chromatographic (HPLC) assays by methods described previously (21).
Pharmacokinetic analysis.
Noncompartmental analysis with WinNonLin, version 4.1 (Pharsight Corp., Mountain View, CA), was performed to compute the pharmacokinetic parameters of rifampin, desacetylrifampin, pyrazinamide, and ethambutol.
Cmax and the time to Cmax (Tmax) were determined directly from the plasma concentration-time data. The terminal, log-linear period (log concentration versus time) was defined by the last data points (n ≥ 3). The absolute value of the slope (−β/2.303, where β is the first-order elimination rate constant) was calculated by least-squares linear regression analysis. The elimination half-life (t1/2) was calculated from the expression 0.693/β. If the concentration at 12 h postdose (C12) was quantifiable, the concentration at 24 h (C24) was estimated as 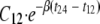 . AUC0-24 was assessed using the linear-log trapezoidal rule from zero up to the last concentration. Apparent clearance (CL/F, where F is bioavailability) was calculated by dividing the dose by AUC0-24, and the apparent volume of distribution (V/F) was calculated by dividing CL/F by β. The relative exposure of the metabolite desacetylrifampin compared to that of rifampin was expressed as the ratio of the metabolite to the parent drug. The oral bioavailability (F) of rifampin was calculated by dividing the oral AUC0-24 (PK assessment 1) by the intravenous AUC0-24 (PK assessment 2).
. AUC0-24 was assessed using the linear-log trapezoidal rule from zero up to the last concentration. Apparent clearance (CL/F, where F is bioavailability) was calculated by dividing the dose by AUC0-24, and the apparent volume of distribution (V/F) was calculated by dividing CL/F by β. The relative exposure of the metabolite desacetylrifampin compared to that of rifampin was expressed as the ratio of the metabolite to the parent drug. The oral bioavailability (F) of rifampin was calculated by dividing the oral AUC0-24 (PK assessment 1) by the intravenous AUC0-24 (PK assessment 2).
Statistical analysis.
Differences in AUC0-24, Cmax, t1/2, CL/F, and V/F between diabetic and nondiabetic TB patients (study objective 1) were tested by the independent-samples t test, and a geometric mean ratio plus 95% confidence interval was calculated for every comparison. To enable statistical testing using parametric tests, pharmacokinetic parameters that were not normally distributed were log transformed, so that the data became normally distributed. Values for Tmax were not transformed and were compared using the Wilcoxon rank-sum test. Proportions of diabetic and nondiabetic patients with concentrations of TB drugs in plasma above reference values were compared using the chi-square test. Reference concentrations in plasma are defined as the usual drug concentrations found in patients as well as in healthy volunteers taking a standard dose of TB drugs: > 8 mg/liter for rifampin, >20 mg/liter for pyrazinamide, and >2 mg/liter for ethambutol (18). The independent-samples t test was used to examine the difference in the oral bioavailability of rifampin between diabetic and nondiabetic TB patients (study objective 2), while the paired t test was used to assess the within-subject effect of glucose control on the pharmacokinetics of TB drugs (study objective 3), and the Wilcoxon signed-rank test was used to compare the Tmax values. All statistical evaluations were performed with SPSS for Windows, version 16.0.1 (SPSS Inc., Chicago, IL). P values less than 0.05 were considered statistically significant in all analyses.
RESULTS
Patients' baseline characteristics.
Thirty-six pulmonary TB patients were included in this study. Sixty percent of the diabetic TB patients knew they had diabetes, but no information on the date of diagnosis was recorded. The remaining 40% of diabetic TB patients included in this study did not know that they had diabetes; diabetes was diagnosed only through screening. Patient characteristics were similar in the two arms, except for age; diabetic patients were older than nondiabetic patients (Table 1). Since patients were matched for body weight, there was no difference between the two arms in the distribution of patients based on body weight, body mass index (BMI), or drug dose per kilogram. None of the diabetic TB patients used antidiabetic drugs at the time of the pharmacokinetic assessments. As would be expected, diabetic patients had higher blood glucose levels (Table 1). None of the patients were HIV positive. Before and during all pharmacokinetic assessments, no patient had diarrhea or vomiting that might have affected the pharmacokinetic profiles that were recorded. No comedication was recorded during the pharmacokinetic assessments.
TABLE 1.
Patient characteristics before the start of treatment
| Patient characteristic | Value for groupa |
P | |
|---|---|---|---|
| TB-DM (n = 18) | TB (n = 18) | ||
| No. (%) male | 7 (39) | 8 (44) | 1.00b |
| Age (yr) | 47 (8) | 35 (10) | 0.00c |
| Body wt (kg) | 47.3 (7.0) | 47.3 (7.2) | 0.85c |
| BMI (kg/m2) | 20.3 (3.5) | 19.6 (3.0) | 0.49c |
| Drug dose (mg/kg of body wt) | |||
| Rifampin | 9.7 (1.6) | 9.6 (1.6) | 0.82c |
| Ethambutol | 16.2 (2.7) | 16.1 (2.6) | 0.86c |
| Pyrazinamide | 32.5 (5.5) | 32.2 (5.2) | 0.86c |
| Fasting blood glucose (mmol/liter) | 16.6 (3.3) | 5.6 (1.6) | 0.00c |
| HbA1c (%) | 11.1 (2.2) | ||
| No. (%) with the following clinical manifestation: | |||
| Cough | 18 (100) | 18 (100) | |
| Hemoptysis | 8 (44) | 4 (22) | |
| Shortness of breath | 15 (83) | 17 (94) | |
| Fever | 15 (83) | 14 (79) | |
| Loss of wt | 16 (89) | 13 (72) | |
| Positive sputum smear | 16 (89) | 18 (100) | |
| Positive M. tuberculosis culture | 16 (89) | 18 (100) | |
Unless otherwise specified, data are given as means (standard deviations).
By Pearson's chi-square test.
By an independent t test.
Pharmacokinetics of TB drugs in diabetic and nondiabetic TB patients.
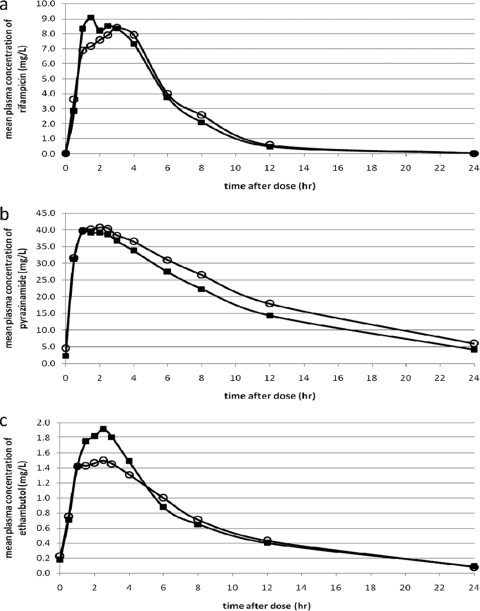
For comparison of the pharmacokinetic parameters of TB drugs between diabetic and nondiabetic TB patients (study objective 1), data were available for 17, 18, and 17 pairs of patients for rifampin, pyrazinamide and ethambutol, respectively. There were no differences in exposure to rifampin, pyrazinamide, or ethambutol between diabetic and nondiabetic TB patients (Tables 2 and 3; Fig. 2). Other pharmacokinetic parameters of these three drugs were similar for the two groups (Tables 2 and 3). An alternative test for analysis of the data, i.e., the paired t test on the pharmacokinetic parameters of the pairs of matched patients, did not reveal any difference, either. The proportions of patients reaching the reference values of rifampin, pyrazinamide, and ethambutol showed no significant differences between the two arms (Table 2 and 3). There were no significant correlations between fasting blood glucose levels and the rifampin AUC0-24 (Pearson correlation coefficient, −0.026; P = 0.884) or the rifampin Cmax (correlation coefficient, 0.094; P = 0.598), and the same applied for pyrazinamide and ethambutol. Age, which was higher in diabetic patients, did not display a significant correlation with the rifampin AUC0-24 or Cmax, or with the pharmacokinetics of pyrazinamide or ethambutol (data not shown). Male gender was associated with a lower rifampin AUC0-24 (P = 0.037) and Cmax (P = 0.057), but this did not confound a possible relationship between DM and rifampin exposure (data not shown). No association was found between gender and the pharmacokinetics of pyrazinamide and ethambutol.
TABLE 2.
Pharmacokinetic parameters of rifampin and desacetylrifampin in diabetic and nondiabetic tuberculosis patients
| Parameter | Value for groupa |
Ratio of TB-DM to TB value | P | |
|---|---|---|---|---|
| TB-DM | TB | |||
| Rifampinb (n, 17 per group) | ||||
| AUC0-24 (mg·h/liter) | 49.0 (40.9-58.7) | 50.6 (42.9-59.8) | 0.97 (0.77-1.23) | 0.81c |
| Cmax (mg/liter) | 10.5 (9.0-12.3) | 9.6 (8.4-11.0) | 1.09 (0.90-1.31) | 0.81c |
| No. with Cmax of >8 mg/liter/total no. (%) | 9/16 (56.3) | 8/16 (50%) | 1.00d | |
| Median (range) Tmax (h) | 2 (0.5-4) | 2.5 (1-4) | 0.28e | |
| t1/2 (h) | 2.1 (1.8-2.4) | 2.2 (1.9-2.5) | 0.94 (0.78-1.12) | 0.64c |
| CL/F (liters/h) | 9.2 (7.7-11.0) | 8.9 (7.5-10.5) | 1.04 (0.83-1.31) | 0.81c |
| V/F (liters) | 27.4 (23.7-31.6) | 27.6 (23.6-32.4) | 0.98 (0.80-1.19) | 0.81c |
| F (%) | 69 | 74 | 0.41c | |
| Desacetylrifampinb (n, 9 per group) | ||||
| AUC0-24 (mg·h/liter) | 5.7 (3.9-8.4) | 8.3 (5.6-12.1) | 0.69 (0.42-1.15) | 0.14c |
| Cmax (mg/liter) | 1.1 (0.85-1.5) | 1.4 (1.1-2.0) | 0.78 (0.53-1.15) | 0.20c |
| Median (range) Tmax (h) | 3.0 (2.0-4.0) | 4.0 (1.5-4.0) | 0.81e | |
| t1/2 (h) | 2.3 (1.9-2.8) | 2.4 (2.1-2.8) | 0.94 (0.76-1.17) | 0.57c |
| Desacetylrifampin/rifampin ratio | ||||
| AUC0-24 | 0.11 (0.09-0.13) | 0.13 (0.10-0.17) | 0.83 (0.62-1.12) | 0.20c |
| Cmax | 0.12 (0.10-0.15) | 0.16 (0.12-0.19) | 0.80 (0.61-1.05) | 0.10c |
Data are presented as geometric means (95% confidence intervals) unless stated otherwise.
Given at a dose of 450 mg (10 mg/kg) in the intensive phase of TB treatment.
By an independent t test on log-transformed data.
By Pearson's chi-square test.
By the Wilcoxon rank-sum test.
TABLE 3.
Pharmacokinetic parameters of pyrazinamide and ethambutol in diabetic and nondiabetic tuberculosis patients
| Parameter | Value for groupa |
Ratio of TB-DM to TB value | P | |
|---|---|---|---|---|
| TB-DM | TB | |||
| Pyrazinamideb (n, 18 per group) | ||||
| AUC0-24 (mg·h/liter) | 409 (369-455) | 468 (422-519) | 0.88 (0.76-1.01) | 0.07c |
| Cmax (mg/liter) | 45.5 (41.6-49.3) | 47.0 (44.1-50.1) | 0.96 (0.87-1.07) | 0.47c |
| No. with Cmax of >20 mg/liter/total no. (%) | 18/18 (100) | 18/18 (100) | ||
| Median (range) Tmax (h) | 1.0 (0.5-4.0) | 1.5 (0.5-6.0) | 0.61d | |
| Ethambutole (n, 17 per group) | ||||
| AUC0-24 (mg·h/liter) | 13.8 (12.0-15.9) | 13.5 (12.0-15.1) | 1.02 (0.86-1.22) | 0.77c |
| Cmax (mg/liter) | 2.2 (1.8-2.7) | 1.95 (1.6-2.4) | 1.12 (0.86-1.47) | 0.39c |
| No. with a Cmax of >2 mg/liter/total no. (%) | 10/17 (58.8) | 8/17 (47.1) | 0.37f | |
| Median (range) Tmax (h) | 3 (1.0-4.0) | 3 (1.0-6.0) | 0.93d | |
Unless stated otherwise, data are presented as geometric means (95% confidence intervals).
Given at a dose of 1,500 mg (30 mg/kg) in the intensive phase of TB treatment.
By an independent t test on log-transformed data.
By the Wilcoxon rank-sum test.
Given at a dose of 750 mg (15 mg/kg) in the intensive phase of TB treatment.
By Pearson's chi-square test.
FIG. 2.
Mean steady-state plasma concentration-time profiles of antituberculosis drugs. Mean steady-state plasma concentration-time profiles of rifampin (n = 17) (a), pyrazinamide (n = 18) (b), and ethambutol (n = 17) (c) in TB patients with (▪) and without (○) DM are shown.
Absorption, metabolism, and elimination of antituberculosis drugs.
Comparison of pharmacokinetic assessments following oral and intravenous administration of rifampin showed that oral bioavailability was similar in the two groups (Table 2). There was also no delayed absorption in diabetic TB patients, as shown by the similar Tmax values for diabetic and nondiabetic TB patients (Table 2). Rifampin clearance in the two groups was also similar (Table 2). Due to interferences in the plasma samples, desacetylrifampin could not be measured accurately in all samples; nine data pairs were available for statistical analysis. The mean AUC0-24 and Cmax values of desacetylrifampin did not differ between the two groups, but the numbers may be too small to find a significant difference. In addition, the desacetylrifampin/rifampin ratios for AUC0-24 and Cmax were similar in the two groups (Table 2). Like rifampin, pyrazinamide and ethambutol showed no differences in absorption, metabolism, and clearance between TB patients with and without DM (Table 3).
Effect of blood glucose control on the pharmacokinetics of tuberculosis drugs.
Ten patients received subcutaneous insulin to help them achieve and maintain normal blood glucose levels. After 5 to 6 weeks, they had decreases in the mean fasting blood glucose levels (16.3 to 7.9 mmol/liter; P = 0.000) and HbA1c (10.6 to 7.1%; P = 0.001) and a significant (11%) weight gain. Repeated pharmacokinetic assessment did not show a significant increase in drug exposure. The ratio of the AUC0-24 after glycemic control to that before glycemic control was 1.15 for rifampin, 0.94 for pyrazinamide, and 0.87 for ethambutol (not significant [NS]). None of the other pharmacokinetic parameters was significantly different after blood glucose control (data not shown).
DISCUSSION
This study showed that there were no differences in the pharmacokinetics of rifampin, pyrazinamide, and ethambutol in the intensive phase of TB treatment between Indonesian TB patients with and without DM who were matched for gender and body weight. Exposure to anti-TB drugs and the maximal concentrations of these drugs were not correlated with blood glucose level or glucose control, and the oral bioavailability, absorption, metabolism, and clearance of rifampin, pyrazinamide, and ethambutol were similar in the two groups. This is important information, especially for highly active anti-TB drugs, such as rifampin and pyrazinamide. Ethambutol is a weak anti-TB drug; the concentrations achieved in serum with 15 mg per dosing are barely at or above the MIC.
The results of this study are different from those of our previous pharmacokinetic study of rifampin in diabetic TB patients (14). In that study we found that diabetic patients, especially those with poor glucose control, had strongly reduced exposure to rifampin. What factors can explain the contrasting results of the present and previous studies? It is unlikely that the study setting plays a significant role. The patients in the present study came from a different clinic, but they have been shown to be clinically, ethnically, and genetically homogenous (12). The severity of DM is not an explanation, either, since the fasting blood glucose and HbA1c levels were higher in the present study (15.6 versus 9.3 mmol/liter and 11.2 versus 9.85%, respectively). None of the diabetic patients in the current study, versus 71% in the previous study, took antidiabetic drugs before pharmacokinetic assessment (14), but there is no evidence that antidiabetic drugs affect the pharmacokinetics of antituberculosis drugs (27).
The difference was also not due to more-intensive sampling in the present study; limited time point analysis (AUC0-6) from the present data led to similar results (data not shown). The two studies used drugs from the same manufacturer, and samples were processed, transferred, and analyzed by the same validated methods. The pharmacokinetic data of TB drugs for nondiabetic TB patients in this study were very similar to the data for patients (taking a similar dose of rifampin) in a previous study in the same setting (21). Therefore, of all the possible explanations, we feel that only two factors may have had a significant role: matching for differences in body weight and the timing of sampling (intensive versus continuation phase of TB treatment).
Body weight is likely to have affected the results. In the previous study, diabetic TB patients had a 20% higher body weight than nondiabetic TB patients, and a higher body weight results in a more than dose-proportional decrease in the mean AUC0-24, consistent with the nonlinear pharmacokinetics of rifampin (4, 17). Other studies have shown the effect of body weight (29, 30, 32). The higher body weight of diabetic patients in the previous study may have explained their lower rifampin exposure, although regression analysis showed that DM and blood glucose level had independent effects on plasma rifampin exposure (14). To exclude possible confounding by body weight, in the present study we matched patients for body weight (and gender).
The timing of pharmacokinetic sampling may also have affected the results, because the current study was performed in the intensive phase and the previous study in the continuation phase of treatment, with rifampin taken three times a week without pyrazinamide and ethambutol. Our studies of Indonesian patients have shown lower plasma rifampin concentrations during the continuation phase (14, 16) than during the intensive phase (20, 21) of treatment. On the one hand, this seems counterintuitive, because higher induction of liver cytochromes with daily use of rifampin (4, 13) and coadministration of pyrazinamide (9, 10) might lower rifampin concentrations in the intensive phase. On the other hand, a higher body weight might decrease rifampin concentrations during the continuation phase.
The current and previous studies suggest that DM does not alter the pharmacokinetics of TB drugs during the intensive phase of TB treatment but that it possibly reduces rifampin exposure during the continuation phase. This is supported by the fact that DM was strongly associated with a positive sputum culture after the continuation phase, but not after the intensive phase, of TB treatment (2).
We hypothesize that the differential effect of DM on the pharmacokinetics of TB drugs during the intensive and continuation phases of treatment is due to differences in rifampin induction. In the intensive phase, with daily administration of rifampin, the activity of liver enzymes and transport pumps would be controlled completely by the very strong rifampin induction (13). No drug or disease state would be able to overcome this. In contrast, thrice weekly dosing in the continuation phase may be associated with less induction of liver enzymes and transport mechanisms, and the possible effect of DM might become manifest. In a 1996 study by Fromm et al. (5) on the induction of prehepatic and hepatic metabolism of verapamil by rifampin, it was found that the half-life of enzyme induction is 1.5 to 2.1 days (5). Clearly, this issue needs further investigation.
To summarize, we have examined the effect of DM on the pharmacokinetics of TB drugs in Indonesian diabetic TB patients in the intensive phase of TB treatment. Our data suggest that DM per se is not associated with altered pharmacokinetics of TB drugs in the intensive phase. It is likely that the higher body weight of diabetic TB patients, especially in the continuation phase, plays a role in the alteration of the pharmacokinetics of TB drugs that might lead to a negative effect of TB treatment. Further study is needed to confirm these findings and to examine the concentration-effect relationship (pharmacodynamics) of TB treatment. Also, more research is needed to examine why DM puts TB patients at risk for treatment failure and whether diabetic TB patients should receive prolonged or dose-adjusted TB treatment.
Acknowledgments
We thank the patients for their participation in this study. The staff at the outpatient clinic of Balai Besar Kesehatan Paru Masyarakat (BBKPM) and the staff at Hasan Sadikin Hospital Bandung, in particular Lika Apriyani and Mutia Sesunan, are warmly thanked for their efforts. The technicians of the Department of Clinical Pharmacy, Nijmegen, especially Alexander Kempers and Noor van Ewijk-Beneken Kolmer, are acknowledged for the analysis of the plasma samples.
We thank Novo Nordisk Indonesia for support in providing insulin injection and glucometers. This study was supported by the Royal Academy of Arts and Sciences (KNAW) of the Netherlands and by a grant from PRIOR, a research network supported by the Netherlands Foundation for Advancement of Tropical Research (NWO-WOTRO). R. Ruslami has a DC fellowship from NWO-WOTRO (WB98-158). R. van Crevel has a fellowship from the Netherlands Organization for Health Research and Development (ZonMw; 907-00-100).
All authors declare no conflict of interest.
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Alisjahbana, B., R. van Crevel, E. Sahiratmadja, M. Heijer, A. Maya, E. Istriana, H. Danusantoso, T. H. Ottenhoff, R. H. Nelwan, and J. W. van der Meer. 2006. Diabetes mellitus is strongly associated with tuberculosis in Indonesia. Int. J. Tuberc. Lung Dis. 10:696-699. [PubMed] [Google Scholar]
- 2.Alisjahbana, B., E. Sahiratmadja, E. J. Nelwan, A. M. Purwa, Y. Ahmad, T. H. Ottenhoff, R. H. Nelwan, I. Parwati, J. W. van der Meer, and R. van Crevel. 2007. The effect of type 2 diabetes mellitus on the presentation and treatment response of pulmonary tuberculosis. Clin. Infect. Dis. 45:428-435. [DOI] [PubMed] [Google Scholar]
- 3.Boucot, K. R. 1957. Diabetes mellitus and pulmonary tuberculosis. J. Chronic Dis. 6:256-279. [DOI] [PubMed] [Google Scholar]
- 4.Burman, W. J., K. Gallicano, and C. A. Peloquin. 2001. Comparative pharmacokinetics and pharmacodynamics of the rifamycin antibacterials. Clin. Pharmacokinet. 40:327-341. [DOI] [PubMed] [Google Scholar]
- 5.Fromm, M. F., D. Busse, H. K. Kroemer, and M. Eichelbaum. 1996. Differential induction of prehepatic and hepatic metabolism of verapamil by rifampin. Hepatology 24:796-801. [DOI] [PubMed] [Google Scholar]
- 6.Gadkowski, L. B., and J. E. Stout. 2007. Pharmacokinetics of rifampicin. Clin. Infect. Dis. 44:618-619. (Letter to the editor.) [DOI] [PubMed] [Google Scholar]
- 7.Guptan, A., and A. Shah. 2000. Tuberculosis and diabetes: an appraisal. Indian J. Tuberc. 47:3-8. [Google Scholar]
- 8.Houin, G., A. Beucler, S. Richelet, R. Brioude, C. Lafaix, and J. P. Tillement. 1983. Pharmacokinetics of rifampicin and desacetylrifampicin in tuberculous patients after different rates of infusion. Ther. Drug Monit. 5:67-72. [DOI] [PubMed] [Google Scholar]
- 9.Immanuel, C., P. Gurumurthy, G. Ramachandran, P. Venkatesan, V. Chandrasekaran, and R. Prabhakar. 2003. Bioavailability of rifampicin following concomitant administration of ethambutol or isoniazid or pyrazinamide or combination of three drugs. Indian J. Med. Res. 118:109-114. [PubMed] [Google Scholar]
- 10.Jain, A., V. L. Mehta, and S. Kulshrestha. 1993. Effect of pyrazinamide on rifampicin kinetics in patients with tuberculosis. Tuber. Lung Dis. 74:87-90. [DOI] [PubMed] [Google Scholar]
- 11.Kimerling, M. E., P. Phillips, P. Patterson, M. Hall, C. A. Robinson, and N. E. Dunlap. 1998. Low serum antimycobacterial drug levels in non-HIV-infected tuberculosis patients. Chest 113:1178-1183. [DOI] [PubMed] [Google Scholar]
- 12.Nejentsev, S., T. Thye, J. S. Szeszko, H. Stevens, Y. Balabanova, A. M. Chinbuah, M. Hibberd, E. van de Vosse, B. Alisjahbana, R. van Crevel, T. H. Ottenhoff, E. Png, F. Drobniewski, J. A. Todd, M. Seielstad, and R. D. Horstmann. 2008. Analysis of association of the TIRAP (MAL) S180L variant and tuberculosis in three populations. Nat. Genet. 40:261-262. [DOI] [PubMed] [Google Scholar]
- 13.Niemi, M., J. T. Backman, M. F. Fromm, P. J. Neuvomen, and K. T. Kivisto. 2003. Pharmacokinetic interactions with rifampicin: clinical relevance. Clin. Pharmacokinet. 42:819-850. [DOI] [PubMed] [Google Scholar]
- 14.Nijland, H. M. J., R. Ruslami, J. E. Stalenhoef, E. J. Nelwan, B. Alisjahbana, R. H. Nelwan, A. van der Ven, H. Danusantoso, R. E. Aarnoutse, and R. van Crevel. 2006. Exposure to rifampicin is strongly reduced in tuberculosis patients with type 2 diabetes. Clin. Infect. Dis. 43:848-854. [DOI] [PubMed] [Google Scholar]
- 15.Nijland, H. M. J., R. E. Aarnoutse, R. Ruslami, and R. van Crevel. 2007. Reply to Gadkowski and Stout. Clin. Infect. Dis. 44:619.17243073 [Google Scholar]
- 16.Nijland, H. M. J., R. Ruslami, A. J. Suroto, D. M. Burger, B. Alisjahbana, R. van Crevel, and R. E. Aarnoutse. 2007. Rifampicin reduces plasma concentrations of moxifloxacin in patients with tuberculosis. Clin. Infect. Dis. 45:1001-1007. [DOI] [PubMed] [Google Scholar]
- 17.Pargal, A., and S. Rani. 2001. Non-linear pharmacokinetics of rifampicin in healthy Asian Indian volunteers. Int. J. Tuberc. Lung Dis. 5:70-79. [PubMed] [Google Scholar]
- 18.Peloquin, C. A. 2002. Therapeutic drug monitoring in the treatment of tuberculosis. Drugs 62:2169-2183. [DOI] [PubMed] [Google Scholar]
- 19.Restrepo, B. I. 2007. Convergence of the tuberculosis and diabetes epidemics: renewal of old acquaintances. Clin. Infect. Dis. 45:436-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ruslami, R., H. Nijland, R. Aarnoutse, B. Alisjahbana, A. Y. Soeroto, S. Ewalds, and R. van Crevel. 2006. Evaluation of high- versus standard-dose rifampin in Indonesian patients with pulmonary tuberculosis. Antimicrob. Agents Chemother. 50:822-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruslami, R., H. J. Nijland, B. Alisjahbana, I. Parwati, R. van Crevel, and R. E. Aarnoutse. 2007. Pharmacokinetics and tolerability of a higher rifampicin dose versus the standard dose in pulmonary tuberculosis patients. Antimicrob. Agents Chemother. 51:2546-2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ruslami, R., R. van Crevel, E. van de Berge, B. Alisjahbana, and R. E. Aarnoutse. 2008. A step-wise approach to find a valid and feasible method to detect non-adherence to tuberculosis drugs. Southeast Asian J. Trop. Med. Public Health 39:1083-1087. [PubMed] [Google Scholar]
- 23.Sahai, J., K. Gallicano, L. Swick, S. Tailor, G. Garber, I. Seguin, L. Oliveras, S. Walker, A. Rachlis, and D. W. Cameron. 1997. Reduced plasma concentrations of antituberculosis drugs in patients with HIV infection. Ann. Intern. Med. 127:289-293. [DOI] [PubMed] [Google Scholar]
- 24.Singla, R., N. Khan, N. Al-Sharif, M. O. Al-Sayegh, M. A. Shaikh, and M. M. Osman. 2006. Influence of diabetes on manifestations and treatment outcome of pulmonary TB patients. Int. J. Tuberc. Lung Dis. 10:74-79. [PubMed] [Google Scholar]
- 25.Stevenson, C. R., N. G. Forouhi, G. Roglic, B. G. Williams, J. A. Lauer, C. Dye, and N. Unwin. 2007. Diabetes and tuberculosis: the impact of diabetes epidemic on tuberculosis incidence. BMC Public Health 7:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Crevel, R., R. H. Nelwan, F. Borst, E. Sahiratmadja, J. Cox, W. van der Meij, M. de Graaff, B. Alisjahbana, W. C. de Lange, and D. Burger. 2004. Bioavailability of rifampicin in Indonesian subjects: a comparison of different local drug manufacturers. Int. J. Tuberc. Lung Dis. 8:500-503. [PubMed] [Google Scholar]
- 27.Venkatesan, K. 1992. Pharmacokinetic drug interactions with rifampicin. Clin. Pharmacokinet. 22:47-65. [DOI] [PubMed] [Google Scholar]
- 28.Wild, S., G. Roglic, A. Green, R. Sicree, and H. King. 2004. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care 27:1047-1053. [DOI] [PubMed] [Google Scholar]
- 29.Wilkins, J. J., G. Langdon, H. McIlleronn, G. C. Pillai, P. J. Smith, and U. S. H. Simonsson. 2006. Variability in the population pharmacokinetics of pyrazinamide in South African tuberculosis patients. Eur. J. Clin. Pharmacol. 62:727-735. [DOI] [PubMed] [Google Scholar]
- 30.Wilkins, J. J., R. M. Savic, M. O. Karlson, G. Langdon, H. McIlleron, G. Pillai, P. J. Smith, and U. S. H. Simonsson. 2008. Population pharmacokinetics of rifampin in pulmonary tuberculosis patients, including a semimechanistic model to describe variable absorption. Antimicrob. Agents Chemother. 52:2138-2148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. 1999. Definition, diagnosis and classification of diabetes mellitus and its complications. World Health Organization, Geneva, Switzerland.
- 32.Zhu, M., W. J. Burman, J. R. Starke, J. J. Stambaugh, P. Steiner, A. E. Bulpitt, D. Ashkin, B. Auclair, S. E. Berning, R. W. Jelliffe, G. S. Jaresko, and C. A. Peloquin. 2004. Pharmacokinetics of ethambutol in children and adults with tuberculosis. Int. J. Tuberc. Lung Dis. 8:1360-1367. [PubMed] [Google Scholar]