Abstract
Nitroheterocyclic prodrugs have been used to treat trypanosomal diseases for more than 40 years. Recently, the key step involved in the activation of these compounds has been elucidated and shown to be catalyzed by a type I nitroreductase (NTR). This class of enzyme is normally associated with bacteria and is absent from most eukaryotes, with trypanosomes being a major exception. Here we exploit this difference by evaluating the trypanocidal activity of a library of nitrobenzylphosphoramide mustards against bloodstream-form Trypanosoma brucei parasites. Biochemical screening against the purified enzyme revealed that a subset of halogenated nitroaromatic compounds were effective substrates for T. brucei NTR (TbNTR), having apparent Kcat/Km values approximately 100 times greater than nifurtimox. When tested against T. brucei, cytotoxicity mirrored enzyme activity, with 50% inhibitory concentrations of the most potent substrates being less than 10 nM. T. brucei NTR plays a key role in parasite killing: heterozygous lines displayed resistance to the compounds, while parasites overexpressing the enzyme showed hypersensitivity. We also evaluated the cytotoxicities of substrates with the highest trypanocidal activities by using mammalian THP-1 cells. The relative toxicities of these newly identified compounds were much lower than that of nifurtimox. We conclude that halogenated nitrobenzylphosphoramide mustards represent a novel class of antitrypanosomal agents, and their efficacy validates the strategy of specifically targeting NTR activity to develop new therapeutics.
Over 10 million people are infected by the parasites Trypanosoma brucei and Trypanosoma cruzi, the causative agents of human African trypanosomiasis (HAT) and Chagas' disease, respectively. These organisms are responsible for more than 60,000 deaths per year (4). The primary route of transmission for both parasites is by the blood-sucking feeding habits of insect vectors. However, other important pathways have been reported, notably blood transfusion, organ transplantation, and illicit drug usage. Infections by these alternative routes have become a problem in the developed world. With no prospect of a vaccine, drugs are currently the viable option to treat these pathogens. The nitroheterocyclic drugs nifurtimox and benznidazole are the only drugs available to treat Chagas' disease. They are orally administered and are readily absorbed from the gastrointestinal tract. Additionally, nifurtimox can cross the blood-brain barrier, and recently, a nifurtimox-eflornithine combination therapy (NECT) for HAT has been added to the WHO essential medicines list (5, 25; www.dndi.org). This, in conjunction with reports that several new nitroheterocycles have trypanocidal activity, has restimulated interest in this previously neglected class of compounds (3, 28).
Nitroheterocyclic compounds encompass a range of molecules characterized by a nitro group linked to an aromatic ring (11). They include the broad-spectrum nitrofuran and nitroimidazole antibiotics, which are effective against a variety of urinary or digestive tract infections. In Europe and the United States, the use of nitrofuran-based compounds in food-producing animals has been discontinued, and their use against human infections is limited (13, 31). However, in light of emerging resistance to current therapies, there is a case for reinstating nitrofurans as a front-line treatment for urinary tract infections (2, 18). Elsewhere in the world, these drugs are commonly prescribed. Recently, there has been a renaissance of other nitroheterocycles. Several are currently undergoing evaluation for treatments of infectious organisms, including the nitric oxide-generating prodrug PA-824 targeting Mycobacterium tuberculosis and nitazoxanide against Giardia and Cryptosporidium (1, 29). Other compounds such as the dinitroaziridinylbenzamides, dinitrobenzamide mustards, and nitrobenzylcarbamates have shown promise as anticancer therapies (7, 12). All these nitroaromatic compounds function as prodrugs and must undergo activation before mediating their cytotoxic effects. The key step in this process involves reactions catalyzed by a group of oxidoreductases called nitroreductases (NTRs). Based on oxygen sensitivity, NTRs can be divided into two groups (24, 26). Type I NTRs are oxygen insensitive and contain flavin mononucleotide (FMN) as a cofactor. They are associated with bacteria and are absent from most eukaryotes, with a subset of protozoan parasites being major exceptions (23, 34). This difference in NTR distribution between the pathogens and human host forms the basis for the drug selectivity of nitroheterocyclic prodrugs. Type I NTRs mediate the sequential reduction of the nitro group via a series of 2-electron transfers from NAD(P)H through a nitroso intermediate to produce hydroxylamine derivatives. It was proposed previously that the hydroxylamine can generate nitrenium cations that promote DNA breakage (21, 30). Type II NTRs are ubiquitous oxygen-sensitive enzymes that contain flavin adenine dinucleotide and/or FMN as a cofactor. They function by mediating the 1-electron reduction of the nitro group that forms an unstable nitro-radical. In the presence of oxygen, this radical undergoes futile cycling to produce superoxide, with the subsequent regeneration of the parent nitro-compound (8, 22). Nitroaromatic drugs can undergo both activation events, but bacteria that are resistant to such agents invariably acquire mutations in their type I NTR complement, indicating that these enzymes mediate the major antimicrobial activation step (20, 33).
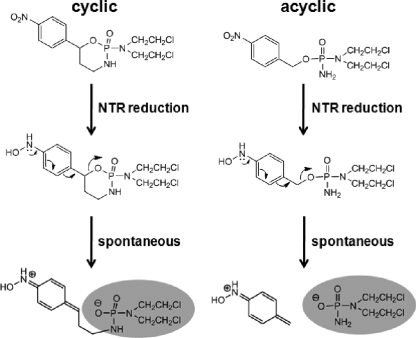
During the type I NTR-mediated conversion of the nitro group to the hydroxylamine derivative, a redistribution of electrons occurs within the nitroaromatic backbone (6). A class of compounds that exploit this property is the nitrobenzylphosphoramide mustards (NBPMs) (15-17, 19). These molecules consist of a nitrobenzyl group linked to a phosphoramide mustard moiety derived from the anticancer drug cyclophosphamide. The linkage between these two components is the key to their toxicity. After reduction by NTR, the NBPM hydroxylamine derivative donates electrons to the benzene ring, causing an electronic rearrangement. This promotes the cleavage of the benzylic C-O bond found in the para position with respect to the nitro group to produce two potent alkylating centers (Fig. 1): an aza quinine methide and the phosphoramide mustard. Initial studies have shown that the NBPMs have promise as anticancer agents in gene-directed prodrug therapies (15, 16, 19, 27).
FIG. 1.
Proposed mechanism for the activation of nitrobenzylphosphoramide mustards. The nitroreductase-mediated reduction of the nitro group (electron withdrawing) to hydroxylamine (electron donating) causes a rearrangement of electrons within the NBPM backbone. This promotes the cleavage of a C-O bond, activating a cytotoxic phosphoramide mustard moiety (shaded). It was previously proposed that this molecule triggers DNA damage by acting as an alkylating agent (15, 16). Two types of NBPM are shown. One form contains the mustard as part of a cyclic arrangement and is analogous to cyclophosphamide. After nitroreduction, the cyclophosphamide structure is predicted to undergo ring opening, exposing the cytotoxic mustard. In the second form, the mustard is part of a linear (acyclic) structure. Here, the NBPM is postulated to fragment, releasing the cytotoxic phosphoramide mustard.
Here, a comparison of 22 NBPMs was performed to determine whether there is a relationship between trypanosomal type I NTR activity and in vitro activity against bloodstream-form (BSF) parasites. Two of these compounds were highly active against T. brucei and displayed high selectivity toward the parasite. Compared to the existing therapies, NBPMs appear to be a promising new class of trypanocidal agent.
MATERIALS AND METHODS
Chemicals.
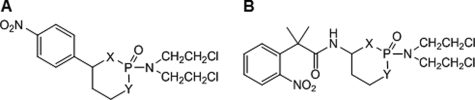
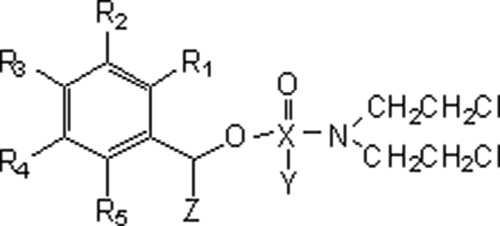
Nitrobenzylphosphoramide mustard structures are shown in Tables 1 and 2 (15-17). The synthesis of LH14 to 19, LH24, LH27, LH31 to LH34, and LH37 has yet to be reported (X. Wu and L. Hu, unpublished data). All compounds were fully characterized by using nuclear magnetic resonance (NMR) and mass spectrometry (MS), and their purity was judged to be >90%: most were >95% based on liquid chromatography (LC)-MS analysis.
TABLE 1.
Structures of cyclic nitrobenzylphosphoramide mustards
TABLE 2.
Structures of acyclic nitrobenzylphosphoramide mustards
Cell culturing.
T. brucei (MITat 427 strain; clone 221a) BSF trypomastigotes were grown at 37°C under a 5% CO2 atmosphere in modified Iscove's medium as previously described (14). Transformed parasite lines containing altered levels of T. brucei NTR (TbNTR) were maintained in this medium supplemented with either 2 μg ml−1 puromycin (for heterozygous NTR−/+ cells) or 2.5 μg ml−1 hygromycin-1 μg ml−1 phleomycin (for TbNTR-overexpressing cells) (34). Tetracycline-free fetal calf serum (Autogen Bioclear) was used in the growth medium.
A human acute monocytic leukemia cell line (THP-1) was grown at 37°C under a 5% CO2 atmosphere in RPMI 1640 medium supplemented with 10% tetracycline-free fetal calf serum, 20 mM HEPES (pH 8.0), 2 mM sodium glutamate, 2 mM sodium pyruvate, 2.5 U ml−1 penicillin, and 2.5 μg ml−1 streptomycin.
Antiproliferative assays.
T. brucei BSF parasites were seeded at 1 × 103 parasites ml−1 in 200 μl of growth medium containing different concentrations of NBPM. Where appropriate, induction was carried out by adding tetracycline (1 μg ml−1). After incubation at 37°C for 3 days, 20 μl of Alamar blue (Biosource UK Ltd.) was added to each well, and the plates were incubated for a further 16 h. The fluorescence of each culture was determined by using a Gemini fluorescent plate reader (Molecular Devices) at an excitation wavelength of 530 nm, an emission wavelength of 585 nm, and a filter cutoff at 550 nm. The color change resulting from the reduction of Alamar blue is proportional to the number of live cells. The 50% inhibitory concentration (IC50) for each compound was then established.
THP-1 cells were seeded at 1 × 104 cells ml−1 in 200 μl of growth medium containing different concentrations of compound. After incubation at 37°C for 6 days, 20 μl of Alamar blue (Biosource UK Ltd.) was added to each well, and the plates were incubated for a further 8 h. The cell density of each culture was determined as described above, and the IC50 was established.
Protein purification and enzyme assay.
A DNA fragment encoding the catalytic domain of T. brucei NTR (TbNTR) was amplified from genomic DNA with the primers ggatccTTGATGCATTTATACGTGTTG and gaattcTCAGAAGCGATTCCATCGGAC (lowercase type corresponds to restriction sites incorporated into the primers to facilitate cloning). The fragment was digested with BamHI/HindIII and then cloned into the corresponding sites of the vector pTrcHis-C (Invitrogen). A 2-liter Escherichia coli BL21(+) pTrcHis-TbNTR culture was grown at 37°C for 2 h with aeration. Protein expression was then induced by isopropyl-β-d-thiogalactopyranoside (IPTG), the culture was incubated overnight at 16°C, and the cells were harvested by centrifugation. His-tagged TbNTR was affinity purified on a Ni-nitrilotriacetic acid (NTA) column (Qiagen) and eluted with a solution containing 500 mM imidazole, 500 mM NaCl, and 50 mM NaHPO4 (pH 7.8). The elution steps were carried out in the presence of 0.5% Triton X-100 and protease inhibitors (Roche). Fractions were analyzed by SDS-PAGE, and protein concentrations were determined by the BCA protein assay system (Pierce).
Enzyme activity was measured by monitoring the change in absorbance at 340 nm due to NADH oxidation (34, 35). A reaction mixture (1 ml) containing 50 mM Tris-Cl (pH 7.0), 100 μM NADH, and NBPM (Tables 1 and 2) was incubated at room temperature for 5 min. The background rate of NADH oxidation was determined, and the reaction was initiated by the addition of 20 μg of TbNTR. The enzyme activity was calculated by using an ɛ of 6,220 M−1 cm−1. The absorbance spectrum (320 to 600 nm) for each NBPM was determined previously. At the concentration ranges used, no significant signal was detected at 340 nm for any of the drugs tested.
RESULTS
Metabolism of nitrobenzylphosphoramide mustards by trypanosomal NTR.
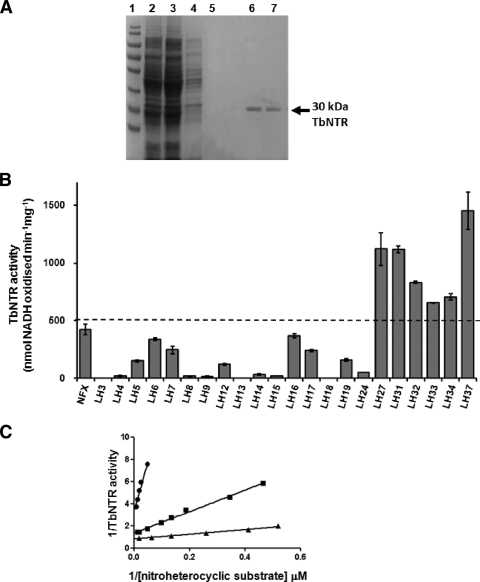
The activation of the nitroheterocyclic prodrugs nifurtimox and benznidazole by trypanosomes is mediated by a type I NTR (34). As an initial screening strategy, we determined whether recombinant T. brucei NTR displayed activity toward the library of NBPMs described in Tables 1 and 2. The region of the T. brucei NTR gene encoding the catalytic domain was expressed in E. coli cells (see Materials and Methods): attempts to express the full-length protein failed to generate soluble enzyme. In the system used, the recombinant enzyme was tagged at its amino terminus with a histidine-rich sequence and an epitope detectable with the anti-Xpress monoclonal antibody (Invitrogen). A band of 30 kDa was detectable in bacterial lysates by Western blotting (data not shown). The native protein could be purified by one round of affinity chromatography on a Ni-NTA column (Fig. 2A).
FIG. 2.
Activity of TbNTR toward different nitrobenzylphosphoramide mustards. (A) SDS-PAGE gel (10%) stained with Coomassie blue. Lane 1, size standards; lane 2, crude E. coli extract loaded onto a Ni-NTA column; lane 3, flowthrough. The column was washed extensively with 50 mM imidazole (lane 4) and 100 mM imidazole (lane 5). Recombinant protein was eluted with 500 mM imidazole containing 0.5% Triton X-100 (lanes 6 and 7). (B) The activity of purified His-tagged TbNTR was assessed by using various NBPMs as substrates at a fixed concentration of 100 μM NADH and 100 μM NBPM. The values shown are the means of data from three experiments ± standard deviations. TbNTR activity was deemed to be high if it was >500 nmol NADH oxidized min−1 mg−1 (dotted line). The activity obtained when using nifurtimox (NFX) as a substrate is also shown. (C) TbNTR activity was assayed by monitoring the oxidation of NADH (100 μM) in the presence of TbNTR (20 μg ml−1) and nitroheterocyclic substrate (2 to 75 μM). The substrates used were nifurtimox (•), LH34 (▪), or LH37 (▴). All reactions were initiated by the addition of the recombinant enzyme. TbNTR activities are expressed as nmol NADH oxidized min−1 mg−1 of enzyme.
The activity of TbNTR toward NBPMs was followed by monitoring the change in absorbance at 340 nm, corresponding to NADH oxidation (Fig. 2B). In total, 22 compounds were screened: 8 structures where the nitrobenzyl group is attached to cyclophosphamide directly or through a carbamate linker (Table 1) and 14 structures where phosphoramide mustard is linked to the nitrobenzyl group with various substituents (Table 2). The cyclophosphamide analogues were poor substrates for TbNTR, but six of the linear compounds (LH27, LH31 to LH34, and LH37) were shown to be “good” NTR substrates, generating an activity of >500 nmol NADH oxidized min−1 mg−1 (nifurtimox yielded an activity of 423 ± 45 nmol NADH oxidized min−1 mg−1). Further analysis with LH27 was discontinued after it was shown to lack trypanocidal activity (see below). Of the remaining 5 structures, all contained at least one halogen linked to the nitro-substituted benzene ring. Kinetic studies were carried out to investigate the interaction of TbNTR with the NBPM compounds. Double-reciprocal plots of 1/TbNTR activity against 1/[NBPM] were linear for substrate concentrations of up to 75 μM (Fig. 2C). Extrapolation of the slopes allowed apparent kinetic constants for each substrate to be calculated (Table 3). TbNTR exhibited a higher affinity for and activity toward all halogenated NBPM compounds than nifurtimox, as judged by their lower apparent Km and higher apparent Vmax and catalytic efficiency values.
TABLE 3.
Activity of TbNTR toward different nitrobenzylphosphoramide mustardsa
| Substrate | Mean Km (μM) ± SD | Mean Vmax (nmol NADH oxidized min−1 mg−1) ± SD | Kcat/Km (M−1 s−1) |
|---|---|---|---|
| Nifurtimox | 53.1 ± 15.2 | 423.3 ± 45 | 3.4 × 103 |
| LH7 | 80.3 ± 24.5 | 245.0 ± 34.5 | 1.3 × 103 |
| LH32 | 6.9 ± 0.5 | 832 ± 9 | 5.2 × 104 |
| LH33 | 2.4 ± 0.3 | 654 ± 14 | 1.2 × 105 |
| LH34 | 8.4 ± 0.6 | 706 ± 27 | 4.3 × 104 |
| LH37 | 2.8 ± 0.4 | 1238 ± 48 | 1.8 × 105 |
The enzyme activity (Vmax) was calculated using an ε value of 6.22 mM−1. Kcat assumes one catalytic site per 30-kDa monomer.
Trypanocidal activity of nitrobenzylphosphoramide mustards.
To determine whether there was a correlation between biochemical activity and parasite killing, all NBPMs were initially screened for trypanocidal activity against T. brucei BSF parasites (see Materials and Methods). Out of the 22 compounds, 15 had no effect on parasite growth at concentrations of up to 10 μM (Table 4). These were not analyzed further. For the remaining 7 compounds, growth inhibition assays were performed to determine their IC50 values (Table 4). All of these compounds displayed an appreciable trypanocidal activity (<5 μM), with 4 compounds having an IC50 lower than 500 nM. Two of these compounds (LH34 and LH37) had IC50s of <10 nM, more than 2 orders of magnitude lower than that of nifurtimox. The 4 compounds generating the lowest IC50s correspond to structures previously designated “good” TbNTR substrates (Table 3).
TABLE 4.
Susceptibilities of bloodstream-form T. brucei and mammalian cells to nitrobenzylphosphoramide mustardsa
| Compound(s) | Mean IC50 (μM) ± SD |
Therapeutic index | |
|---|---|---|---|
| T. brucei | THP1 | ||
| Nifurtimox | 1.800 ± 0.4 | 64.8 ± 1.5 | 36 |
| LH3-LH6; LH8; LH9, LH12-16; LH18; LH19; LH24; LH27 | >10 | ND | ND |
| LH7 | 3.400 ± 0.300 | 66.0 ± 0.8 | 19 |
| LH17 | 1.200 ± 0.100 | 33.5 ± 5.8 | 28 |
| LH31 | 3.200 ± 0.200 | 17.6 ± 1.5 | 6 |
| LH32 | 0.268 ± 0.008 | 20.3 ± 1.8 | 76 |
| LH33 | 0.149 ± 0.020 | 19.0 ± 1.9 | 128 |
| LH34 | 0.008 ± 0.001 | 10.0 ± 1.2 | 1,250 |
| LH37 | 0.007 ± 0.001 | 6.9 ± 1.3 | 986 |
ND, not determined.
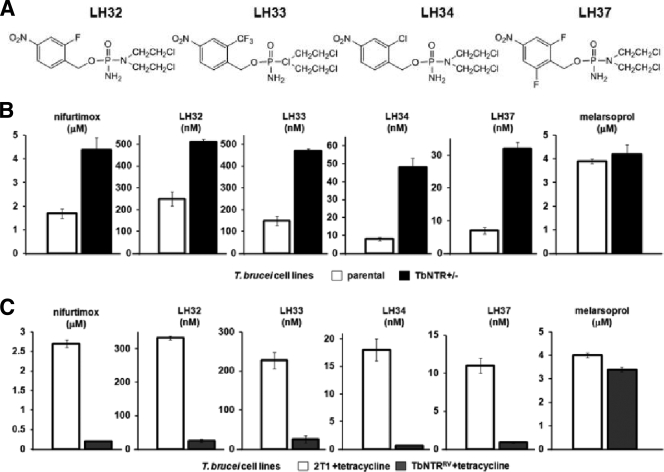
To confirm that trypanosomal NTRs play a key role in the activation of NBPM prodrugs, we used T. brucei BSF cells where the level of the enzyme had been genetically altered (34). The heterozygous and overexpressing cell lines were grown with different concentrations of LH32, LH33, LH34, or LH37 (Fig. 3A), and the IC50s were determined. Cells containing a single copy of the TbNTR gene (NTR+/−) were up to 6-fold-more resistant to the nitroaromatic structures than controls (Fig. 3B). In contrast, parasites with elevated levels of TbNTR were shown to be 10-fold-more sensitive to the mustard compounds than controls (Fig. 3C). This was shown to be NTR specific, as all parasite lines, when treated with melarsoprol, a nonnitroaromatic drug control, displayed similar drug sensitivities (IC50 approximately 4 μM).
FIG. 3.
Susceptibility of bloodstream-form T. brucei with altered levels of NTR to nitrobenzylphosphoramide mustards. (A) Structures of the NBPMs with the highest trypanocidal activities (Table 3). (B) Growth-inhibitory effect of LH32, LH33, LH34, and LH37 on T. brucei NTR heterozygote (NTR−/+) cells as judged by the IC50. (B) Overexpression of TbNTR (TbNTRRV) confers hypersensitivity to NBPMs. Data in A and B are means of data from 4 experiments ± SD, and the differences in susceptibility were statistically significant (P < 0.01), as assessed by a Student's t test. Melarsoprol and nifurtimox were used as drug controls.
Cytotoxicity against mammalian cells.
The 7 compounds identified as having appreciable trypanocidal activity were assayed for cytotoxicity against THP-1 cells (Table 4). The therapeutic index (TI) (IC50 against the mammalian line/IC50 against the parasite) for each compound was then determined. In all cases, the agents displayed selective toxicity toward the parasite. For the parent, nonhalogenated compound (LH7) and 2 other mustards (LH17 and LH31), a TI equivalent to that determined for nifurtimox was obtained. For the 4 halogenated compounds identified as being preferred TbNTR substrates and having the highest trypanocidal activity (LH32, LH33, LH34, and LH37), higher TIs were observed. For 2 compounds, LH34 and LH37, their TIs were 1,250 and 986, respectively, 35- and 27-fold higher than that of nifurtimox. Thus, the relative toxicities of 2 of these newly identified compounds are much lower than that of nifurtimox.
DISCUSSION
Nitrobenzylphosphoramide mustards are a new class of phosphoramide mustard analogues currently under investigation as anticancer agents (15-17). In these molecules, a nitroaromatic group has been incorporated as the trigger for reductive activation. The reduction of the nitro group to the hydroxylamine derivative, in a reaction catalyzed by a type I NTR, leads to the fragmentation of the structure, releasing toxic moieties (6). However, most eukaryotic organisms lack type I NTR activity. Trypanosomes are one of the few eukaryotes to express a type I NTR. Exploiting this activity, we performed a structure-activity-relationship study on a library of NBPMs using both biochemical and trypanocidal screens (Fig. 2B and Table 4). Several classes of NBPMs are now available, differing in the nature of the linkage between the nitrobenzyl group and the phosphoramide mustard (15-17). In one set of compounds, the phosphoramide is part of a cyclic structure analogous to cyclophosphamide (Table 1), while in the second group, the phosphoramide chain has been linearized, with the latter compounds referred to as acyclic (Table 2). TbNTR was shown to metabolize some of the cyclic phosphoramide analogues albeit at a low rate (Fig. 2B). However, none of them killed BSF parasites in the concentration range tested (Table 4). The acyclic NBPM (LH7) was shown to be reduced by TbNTR at a rate similar to that of the cyclic analogues, but in contrast, this translated into a trypanocidal activity (Fig. 2B and Table 4). Based on this, only derivatives of the acyclic NBPM were evaluated further.
A second series of acyclic compounds was then evaluated. Initially, the positional effect of the nitro group on the benzyl ring in relation to the phosphoramide mustard was examined. This demonstrated that compounds with the nitro group in the 2- or 4-arrangement functioned as TbNTR substrates but that only the 4-NBPM (LH7) displayed trypanocidal activity (Fig. 2B and Table 4): no TbNTR or trypanocidal activity was detected when the nitro group was in the 3-position. All subsequent compounds contained the nitro group in the 4-position. The addition of a methyl group on the benzylic carbon or benzyl ring using the LH7 structure as a template resulted in compounds with reduced enzymatic activity and parasite killing. Interestingly, a compound (LH27) where the phosphorous on the phosphoramide chain had been replaced with a carbamate was readily metabolized by TbNTR. However, for reasons that are unclear, this derivative had no effect against BSF trypanosomes. It may be inefficiently transported into the cell or unable to access the mitochondrion, the subcellular location where TbNTR is found. Alternatively, nitroreduction may not result in the cleavage of this particular carbamate analogue and the subsequent elimination of the nitrogen mustard. However, this result does illustrate the importance of phosphoramide at this position and highlights its presence as an essential requirement in this class of compounds.
With acyclic 4-NBPM (LH7) as a lead structure, the effects of substitutions that alter the electronic characteristics of the aromatic ring and the benzylic carbon were examined. The addition of an electron-donating methoxy group in the 2-position produced a substrate (LH17) with TbNTR activity equivalent to that of the lead compound but with a slightly increased trypanocidal activity (Table 4). In contrast, an NBPM containing a 3-methoxy group (LH18) was not metabolized by TbNTR and did not kill trypanosomes in the concentration range tested. The incorporation of electron-withdrawing groups such as fluoro (LH32), trifluoromethyl (LH33), and chloro (LH34) at the 2-position dramatically increased TbNTR activity and considerably improved the trypanocidal properties. LH32 and LH33 had IC50s of 268 ± 8 nM and 149 ± 20 nM, respectively, while LH34 had an IC50 of 8 ± 1 nM. The latter value is 225-fold lower than that calculated for nifurtimox. To evaluate whether the increase in enzymatic and trypanocidal activities was due specifically to halogenation at the 2-position, a 3-fluorinated nitrobenzyl compound was examined (LH31). This molecule was efficiently metabolized by TbNTR but had a parasite-killing activity on par with that of LH7 (Tables 3 and 4). Therefore, although halogenation at any position on the phenyl ring is sufficient to stimulate TbNTR activity, only 2-halogenated compounds have increased trypanocidal activity. Analysis of a difluorinated (2,6-difluroro) compound (LH37) showed that it had the highest TbNTR activity and lowest IC50 of any of the NBPMs tested. To summarize, in this study we have determined that the optimal NTR-activated structures are acyclic 2-halogenated 4-nitrobenzyl phosphoramides that can be additionally halogenated in their 6-position (Fig. 3A).
One possible explanation for the increased enzymatic and trypanocidal activities of the halogenated NBPMs could be the electronic inductive and resonance properties displayed by chlorine and fluorine. Prior to NTR-mediated activation, the phenyl ring contains two electron-withdrawing substituents, a nitro group and a halogen. The electron-withdrawing inductive effect of the halogen increases the potential of the nitro group being reduced by NTR. NTR reduction converts the electron-withdrawing nitro group to an electron-donating hydroxylamine. This pushes electrons on the aromatic ring to the para benzylic carbon, promoting the cleavage of the benzylic C-O bonding and the release of the phosphoramide mustard. This cleavage is further facilitated by the electron-donating resonance effect of a halogen substitution at the 2-position. The combined electronic effect of hydroxylamine at the 4-position and halogen at the 2-position causes a faster flux of electrons around the aromatic ring through to the benzylic C-O bond. This should increase the rate at which the compound fragments, thus releasing the cytotoxic products.
The NBPMs metabolized in vitro by TbNTR are generally potent trypanocidal agents. To conclusively demonstrate this correlation, the susceptibilities of T. brucei cell lines with reduced or elevated levels of NTR to the most effective compounds (LH32, LH33, LH34, and LH37) were investigated (Fig. 3B and C) (34). In this context, trypanosomes with lower levels of NTR displayed relative resistance to all 4 compounds, whereas cells overexpressing the enzyme exhibited hypersensitivity. This is in agreement with the phenotype shown by these parasite cell lines toward other nitroheterocyclic compounds (34). As mammalian cells lack type I NTR, they should be less susceptible to agents that rely on this mechanism of activation. When we compared the relative toxicities of the most potent NBPMs against T. brucei and the THP-1 mammalian line, we observed that the 2 most effective trypanocidal agents were 1,250-fold (LH34) and 986-fold (LH37) more toxic to the parasite (Table 4). This difference is greater than that exhibited by nifurtimox (36-fold), a drug which is a key component of the recently sanctioned HAT treatment, NECT. However, it is important to stress that the in vitro toxicity data generated here were against only one mammalian cell line. Therefore, it is imperative that the pharmacokinetic properties of the trypanocidal NBPMs be determined to establish whether they do have potential as antiparasitic therapies.
By exploiting the trypanosomal type I NTR system, we have identified several molecules with antitrypanosomal activities. As this was achieved by using a small focused compound library, the strategy of specifically targeting this novel activity in the development of new therapeutics has been validated. It is feasible to extend this approach to develop other trypanocidal agents, possibly by tagging different cytotoxic ligands onto various nitroaromatic rings. The ligands selected could target any biochemical pathways within the parasite. Therefore, as the type I NTR activation system is absent from the mammalian host and a number of trypanosome-specific systems have already been characterized, it may be possible to combine these features to develop new, safer, and cheap treatments against this debilitating disease.
Acknowledgments
We thank John Kelly for valuable discussions and for comments on the manuscript.
S.R.W. acknowledges the financial support of the Wellcome Trust, and L.H. acknowledges the financial support from the Elsa U. Pardee Foundation.
Footnotes
Published ahead of print on 22 December 2009.
REFERENCES
- 1.Adagu, I. S., D. Nolder, D. C. Warhurst, and J. F. Rossignol. 2002. In vitro activity of nitazoxanide and related compounds against isolates of Giardia intestinalis, Entamoeba histolytica and Trichomonas vaginalis. J. Antimicrob. Chemother. 49:103-111. [DOI] [PubMed] [Google Scholar]
- 2.Arya, S. C., and N. Agarwal. 2009. Nitrofurantoin: the return of an old friend in the wake of growing resistance. BJU Int. 103:994-995. [DOI] [PubMed] [Google Scholar]
- 3.Baliani, A., G. J. Bueno, M. L. Stewart, V. Yardley, R. Brun, M. P. Barrett, and I. H. Gilbert. 2005. Design and synthesis of a series of melamine-based nitroheterocycles with activity against trypanosomatid parasites. J. Med. Chem. 48:5570-5579. [DOI] [PubMed] [Google Scholar]
- 4.Barrett, M. P., R. J. Burchmore, A. Stich, J. O. Lazzari, A. C. Frasch, J. J. Cazzulo, and S. Krishna. 2003. The trypanosomiases. Lancet 362:1469-1480. [DOI] [PubMed] [Google Scholar]
- 5.Checchi, F., P. Piola, H. Ayikoru, F. Thomas, D. Legros, and G. Priotto. 2007. Nifurtimox plus eflornithine for late-stage sleeping sickness in Uganda: a case series. PLoS Negl. Trop. Dis. 1:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen, Y., and L. Hu. 2009. Design of anticancer prodrugs for reductive activation. Med. Res. Rev. 29:29-64. [DOI] [PubMed] [Google Scholar]
- 7.Denny, W. A. 2003. Prodrugs for gene-directed enzyme-prodrug therapy (suicide gene therapy). J. Biomed. Biotechnol. 2003:48-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Docampo, R., R. P. Mason, C. Mottley, and R. P. Muniz. 1981. Generation of free radicals induced by nifurtimox in mammalian tissues. J. Biol. Chem. 256:10930-10933. [PubMed] [Google Scholar]
- 9.Reference deleted.
- 10.Reference deleted.
- 11.Grunberg, E., and E. H. Titsworth. 1973. Chemotherapeutic properties of heterocyclic compounds: monocyclic compounds with five-membered rings. Annu. Rev. Microbiol. 27:317-346. [DOI] [PubMed] [Google Scholar]
- 12.Hay, M. P., R. F. Anderson, D. M. Ferry, W. R. Wilson, and W. A. Denny. 2003. Synthesis and evaluation of nitroheterocyclic carbamate prodrugs for use with nitroreductase-mediated gene-directed enzyme prodrug therapy. J. Med. Chem. 46:5533-5545. [DOI] [PubMed] [Google Scholar]
- 13.Hiraku, Y., A. Sekine, H. Nabeshi, K. Midorikawa, M. Murata, Y. Kumagai, and S. Kawanishi. 2004. Mechanism of carcinogenesis induced by a veterinary antimicrobial drug, nitrofurazone, via oxidative DNA damage and cell proliferation. Cancer Lett. 215:141-150. [DOI] [PubMed] [Google Scholar]
- 14.Hirumi, H., and K. Hirumi. 1989. Continuous cultivation of Trypanosoma brucei blood stream forms in a medium containing a low concentration of serum protein without feeder cell layers. J. Parasitol. 75:985-989. [PubMed] [Google Scholar]
- 15.Hu, L., C. Yu, Y. Jiang, J. Han, Z. Li, P. Browne, P. R. Race, R. J. Knox, P. F. Searle, and E. I. Hyde. 2003. Nitroaryl phosphoramides as novel prodrugs for E. coli nitroreductase activation in enzyme prodrug therapy. J. Med. Chem. 46:4818-4821. [DOI] [PubMed] [Google Scholar]
- 16.Jiang, Y., J. Han, C. Yu, S. O. Vass, P. F. Searle, P. Browne, R. J. Knox, and L. Hu. 2006. Design, synthesis, and biological evaluation of cyclic and acyclic nitrobenzylphosphoramide mustards for E. coli nitroreductase activation. J. Med. Chem. 49:4333-4343. [DOI] [PubMed] [Google Scholar]
- 17.Jiang, Y., and L. Hu. 2008. N-(2,2-Dimethyl-2-(2-nitrophenyl)acetyl)-4-aminocyclophosphamide as a potential bioreductively activated prodrug of phosphoramide mustard. Bioorg. Med. Chem. Lett. 18:4059-4063. [DOI] [PubMed] [Google Scholar]
- 18.Kashanian, J., P. Hakimian, M. Blute, Jr., J. Wong, H. Khanna, G. Wise, and R. Shabsigh. 2008. Nitrofurantoin: the return of an old friend in the wake of growing resistance. BJU Int. 102:1634-1637. [DOI] [PubMed] [Google Scholar]
- 19.Li, Z., J. Han, Y. Jiang, P. Browne, R. J. Knox, and L. Hu. 2003. Nitrobenzocyclophosphamides as potential prodrugs for bioreductive activation: synthesis, stability, enzymatic reduction, and antiproliferative activity in cell culture. Bioorg. Med. Chem. 11:4171-4178. [DOI] [PubMed] [Google Scholar]
- 20.McCalla, D. R., C. Kaiser, and M. H. Green. 1978. Genetics of nitrofurazone resistance in Escherichia coli. J. Bacteriol. 133:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCalla, D. R., A. Reuvers, and C. Kaiser. 1971. Breakage of bacterial DNA by nitrofuran derivatives. Cancer Res. 31:2184-2188. [PubMed] [Google Scholar]
- 22.Moreno, S. N., R. P. Mason, and R. Docampo. 1984. Reduction of nifurtimox and nitrofurantoin to free radical metabolites by rat liver mitochondria. Evidence of an outer membrane-located nitroreductase. J. Biol. Chem. 259:6298-6305. [PubMed] [Google Scholar]
- 23.Muller, J., J. Wastling, S. Sanderson, N. Muller, and A. Hemphill. 2007. A novel Giardia lamblia nitroreductase, GlNR1, interacts with nitazoxanide and other thiazolides. Antimicrob. Agents Chemother. 51:1979-1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Peterson, F. J., R. P. Mason, J. Hovsepian, and J. L. Holtzman. 1979. Oxygen-sensitive and -insensitive nitroreduction by Escherichia coli and rat hepatic microsomes. J. Biol. Chem. 254:4009-4014. [PubMed] [Google Scholar]
- 25.Priotto, G., C. Fogg, M. Balasegaram, O. Erphas, A. Louga, F. Checchi, S. Ghabri, and P. Piola. 2006. Three drug combinations for late-stage Trypanosoma brucei gambiense sleeping sickness: a randomized clinical trial in Uganda. PLoS Clin. Trials 1:e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roldan, M. D., E. Perez-Reinado, F. Castillo, and C. Moreno-Vivian. 2008. Reduction of polynitroaromatic compounds: the bacterial nitroreductases. FEMS Microbiol. Rev. 32:474-500. [DOI] [PubMed] [Google Scholar]
- 27.Searle, P. F., M. J. Chen, L. Hu, P. R. Race, A. L. Lovering, J. I. Grove, C. Guise, M. Jaberipour, N. D. James, V. Mautner, L. S. Young, D. J. Kerr, A. Mountain, S. A. White, and E. I. Hyde. 2004. Nitroreductase: a prodrug-activating enzyme for cancer gene therapy. Clin. Exp. Pharmacol. Physiol. 31:811-816. [DOI] [PubMed] [Google Scholar]
- 28.Stewart, M. L., G. J. Bueno, A. Baliani, B. Klenke, R. Brun, J. M. Brock, I. H. Gilbert, and M. P. Barrett. 2004. Trypanocidal activity of melamine-based nitroheterocycles. Antimicrob. Agents Chemother. 48:1733-1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stover, C. K., P. Warrener, D. R. VanDevanter, D. R. Sherman, T. M. Arain, M. H. Langhorne, S. W. Anderson, J. A. Towell, Y. Yuan, D. N. McMurray, B. N. Kreiswirth, C. E. Barry, and W. R. Baker. 2000. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature 405:962-966. [DOI] [PubMed] [Google Scholar]
- 30.Streeter, A. J., and B. A. Hoener. 1988. Evidence for the involvement of a nitrenium ion in the covalent binding of nitrofurazone to DNA. Pharm. Res. 5:434-436. [DOI] [PubMed] [Google Scholar]
- 31.Takahashi, M., S. Iizuka, T. Watanabe, M. Yoshida, J. Ando, K. Wakabayashi, and A. Maekawa. 2000. Possible mechanisms underlying mammary carcinogenesis in female Wistar rats by nitrofurazone. Cancer Lett. 156:177-184. [DOI] [PubMed] [Google Scholar]
- 32.Reference deleted.
- 33.Whiteway, J., P. Koziarz, J. Veall, N. Sandhu, P. Kumar, B. Hoecher, and I. B. Lambert. 1998. Oxygen-insensitive nitroreductases: analysis of the roles of nfsA and nfsB in development of resistance to 5-nitrofuran derivatives in Escherichia coli. J. Bacteriol. 180:5529-5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wilkinson, S. R., M. C. Taylor, D. Horn, J. M. Kelly, and I. Cheeseman. 2008. A mechanism for cross-resistance to nifurtimox and benznidazole in trypanosomes. Proc. Natl. Acad. Sci. U. S. A. 105:5022-5027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zenno, S., H. Koike, A. N. Kumar, R. Jayaraman, M. Tanokura, and K. Saigo. 1996. Biochemical characterization of NfsA, the Escherichia coli major nitroreductase exhibiting a high amino acid sequence homology to Frp, a Vibrio harveyi flavin oxidoreductase. J. Bacteriol. 178:4508-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]