Abstract
Peroxidic antimalarials such as the semisynthetic artemisinins are critically important in the treatment of drug-resistant malaria. Nevertheless, their peroxide bond-dependent mode of action is still not well understood. Using combination experiments with cultured Plasmodium falciparum cells, we investigated the interactions of the nitroxide radical spin trap, 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), and four of its analogs with artemisinin and the ozonide drug development candidate OZ277. The antagonism observed for combinations of artemisinin or OZ277 with the TEMPO analogs supports the hypothesis that the formation of carbon-centered radicals is critical for the activity of these two antimalarial peroxides. The TEMPO analogs showed a trend toward greater antagonism with artemisinin than they did with OZ277, an observation that can be explained by the greater tendency of artemisinin-derived carbon-centered radicals to undergo internal self-quenching reactions, resulting in a lower proportion of radicals available for subsequent chemical reactions such as the alkylation of heme and parasite proteins. In a further mechanistic experiment, we tested both artemisinin and OZ277 in combination with their nonperoxidic analogs. The latter had no effect on the antimalarial activities of the former. These data indicate that the antimalarial properties of peroxides do not derive from reversible interactions with parasite targets.
The semisynthetic artemisinins are critically important antimalarials in the treatment of drug-resistant malaria and are recommended for use in combination with other antimalarial drugs (32) to increase efficacy and preclude or delay drug resistance. The discovery of artemisinin led to an investigation of diverse classes of synthetic peroxides as potential antimalarial agents (17, 27). One such peroxide, the ozonide OZ277 (arterolane) (31), has now entered phase III clinical trials in the form of an arterolane maleate-piperaquine phosphate combination (22). A working hypothesis (16, 19, 23) put forth to account for the antimalarial specificity (18) of natural-product and synthetic peroxides is that the pharmacophoric peroxide bond undergoes reductive activation by heme released by parasite hemoglobin digestion (9, 11). The irreversible redox reaction between antimalarial peroxides and heme produces carbon-centered radicals or carbocations that alkylate heme (5, 20, 24, 25) and proteins (2, 3, 8, 33), leading to the perturbation of lipid components of the parasite digestive vacuole (6, 13). Although artemisinin and OZ277 are nearly equipotent inhibitors of Plasmodium falciparum growth in vitro (18), their very different 50% inhibitory concentrations (IC50s) (79 and 7,700 nM) (30) against one putative target enzyme, the Sarcoendoplasmic reticulum Ca2+-ATPase PfATP6 (8, 12), reveal that the precise mechanism of action of antimalarial peroxides is still not well understood.
In this study, we report the results of two different types of in vitro P. falciparum combination experiments with artemisinin and OZ277 designed to better understand the mechanisms of action of these two drugs. In the first set of experiments, we assessed if the nitroxide free radical 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and several of its analogs (21) could antagonize the action of either artemisinin or OZ277 (Fig. 1). Nitroxide free radicals such as TEMPO are very efficient carbon-centered radical spin traps (21). In the second set of experiments, we used combinations of either artemisinin or OZ277 and their nonperoxidic counterparts deoxyartemisinin (4) and carbaOZ277 (18) (Fig. 1) to assess if reversible binding interactions with parasite targets play a role in the antimalarial properties of peroxides such as artemisinin and OZ277.
FIG. 1.
Structures of artemisinin, deoxyartemisinin, OZ277, carbaOZ277, and TEMPO derivatives.
MATERIALS AND METHODS
Materials.
Deoxyartemisinin, OZ277, and carbaOZ277 were synthesized as previously described (4, 18, 31). The TEMPO analogs were purchased from Sigma-Aldrich, [8-3H]hypoxanthine was purchased from ANAWA Trading SA, and artemisinin was purchased from Saokim Pharma. The test compounds were dissolved in dimethyl sulfoxide (DMSO) at 10 mg/ml or, where required, at 50 mg/ml. The stock solutions were kept at 4°C for less than 6 months.
Parasite assays.
The chloroquine-sensitive NF54 isolate and the chloroquine- and pyrimethamine-resistant K1 isolate of P. falciparum, provided by F. Hoffmann-La Roche Ltd. (Basel, Switzerland), were used for the in vitro assays. Parasite cultivation in RPMI 1640 medium (10.44 g/liter) supplemented with HEPES (5.94 g/liter), Albumax II (5 g/liter), hypoxanthine (50 mg/liter), sodium bicarbonate (2.1 g/liter), and neomycin (100 mg/liter) was performed according to a method described previously by Trager and Jensen (29), in an atmosphere of 93% N2, 4% CO2, and 3% O2 at 37°C. P. falciparum growth was assessed by measuring the incorporation of the nucleic acid precursor [3H]hypoxanthine (7). In detail, compounds were dissolved in DMSO (10 mg/ml) and diluted in hypoxanthine-free culture medium. Infected erythrocytes (100 μl per well with 2.5% hematocrit and 0.3% parasitemia) were added to each drug titrated in 100-μl duplicates over a 64-fold range. After 48 h of incubation, 0.5 μCi of [3H]hypoxanthine in 50 μl of medium was added, and plates were incubated for an additional 24 h. Parasites were harvested onto glass-fiber filters, and radioactivity was counted by using a Beta-Plate liquid scintillation counter (Wallac, Zurich, Switzerland). The results were recorded as counts per minute per well at each drug concentration and expressed as a percentage of the untreated controls. Fifty-percent inhibitory concentrations (IC50) were estimated by linear interpolation (15). The experimental DMSO concentrations had no inhibitory effect on parasite growth.
Combination experiments.
The fixed-ratio method (26) uses serial dilutions (1:2) of fixed ratios of two compounds. Initially, the IC50s of the test compounds alone were determined. Subsequently, compound solutions were diluted with hypoxanthine-free culture medium to initial concentrations of at least 1.5 times the predetermined IC50s. The solutions were combined in IC50 ratios of 1:1, 1:3, and 3:1. Single and combination compound solutions were then introduced into 96-well plates to give duplicative rows. The IC50s of the compounds in combination were expressed as fractions of the IC50s of the compounds alone and were recorded as fractional inhibitory concentrations (FICs) for compounds A and B, respectively. These data were expressed numerically as the sum FICs (ΣFICs) of FICA and FICB. ΣFIC values indicate the nature of the interactions as follows: ΣFIC of <0.8 is synergism, ΣFIC of 0.8 to 1.4 is additive, and ΣFIC of >1.4 is antagonism. Cutoff values of 0.8 for synergism and 1.4 for antagonism were determined by mixing the same compound at various ratios and accounting for experimental variation. Statistical significance was determined by using the Student t test. Compound interactions were also assessed by using a checkerboard combination assay (10) in which one compound was kept at a constant subinhibitory concentration and the other compound was serially diluted (1:2). To assess dose-response relationships, the subinhibitory concentrations were increased in four steps from 0 to approximately 0.5 times the respective IC50s.
RESULTS
Table 1 shows the IC50s of all tested compounds. Against P. falciparum strain NF54, the IC50s of artemisinin (2.7 ± 1.2 ng/ml) and OZ277 (0.54 ± 0.29 ng/ml) were approximately 10,000-fold lower than the IC50s of their respective nonperoxidic analogs, deoxyartemisinin (27,000 ± 3,200 ng/ml) and carbaOZ277 (5,200 ± 380 ng/ml). The IC50s of these four compounds against the K1 strain of P. falciparum varied by no more than 2.3-fold from those against the NF54 strain. The IC50s for TEMPO and the functionalized TEMPO derivatives against the NF54 strain of P. falciparum ranged from 10,000 to 35,000 ng/ml; the most potent of these was 4-amino-TEMPO (Table 1). The K1 strain of P. falciparum was similarly affected by all TEMPO derivatives.
TABLE 1.
IC50s against the NF54 and K1 strains of P. falciparuma
| Compound | Mean IC50 (ng/ml) ± SD |
|
|---|---|---|
| NF54 | K1 | |
| Artemisinin | 2.7 ± 1.2 | 1.6 ± 0.84 |
| Deoxyartemisinin | 27,000 ± 3,200 | 19,000 ± 2,500 |
| OZ277 | 0.54 ± 0.29 | 0.57 ± 0.35 |
| CarbaOZ277 | 5,200 ± 380 | 2,200 ± 190 |
| TEMPO | 25,000 ± 8,900 | 16,000 ± 4,000 |
| 4-Oxo-TEMPO | 28,000 ± 5100 | 12,000 ± 5,100 |
| 4-Hydroxy-TEMPO | 34,000 ± 2,800 | 36,000 ± 4,300 |
| 4-Carboxy-TEMPO | 33,000 ± 4,400 | 34,000 ± 5,800 |
| 4-Amino-TEMPO | 10,000 ± 2,900 | 8,200 ± 770 |
Values are the means and standard deviations from ≥5 experiments.
The ΣFIC values (NF54 strain) for artemisinin and OZ277 in combination with the TEMPO analogs are shown in Table 2. For artemisinin and OZ277, an antagonistic tendency was evident for 4/5 and 2/5 of the TEMPO analogs, respectively. However, antagonism with artemisinin was statistically significant only for 4-oxo-TEMPO and 4-amino-TEMPO, and antagonism with OZ277 was statistically significant only for 4-amino-TEMPO. ΣFIC data (not shown) obtained for the K1 strain were very similar to the NF54 data shown in Table 2.
TABLE 2.
In vitro interactions of artemisinin and OZ277 plus TEMPO drug combinations against the NF54 strain of P. falciparuma
| Drug combination | Mean ΣFIC50 ± SD at a ratio ofb: |
Mean ΣFIC50 ± SD | Interaction | ||
|---|---|---|---|---|---|
| 1:3 | 1:1 | 3:1 | |||
| Artemisinin + TEMPO | 1.3 ± 0.6 | 2.1 ± 1.3 | 2.2 ± 1.2 | 1.9 ± 0.4 | Antagonism |
| Artemisinin + 4-oxo-TEMPO | 2.3 ± 1.2c | 2.6 ± 1.0c | 2.0 ± 0.6c | 2.3 ± 0.2c | Antagonism |
| Artemisinin + 4-hydroxy-TEMPO | 1.8 ± 0.6 | 4.0 ± 2.2d | 2.5 ± 0.6 | 2.8 ± 0.9 | Antagonism |
| Artemisinin + 4-carboxy-TEMPO | 1.4 ± 0.1 | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | Addition |
| Artemisinin + 4-amino-TEMPO | 1.3 ± 0.1 | 1.9 ± 0.2c | 2.8 ± 0.4c | 2.0 ± 0.6 | Antagonism |
| OZ277 + TEMPO | 1.8 ± 0.2 | 1.5 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.2 | Antagonism |
| OZ277 + 4-oxo-TEMPO | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.1 ± 0.1 | 1.3 ± 0.1 | Addition |
| OZ277 + 4-hydroxy-TEMPO | 1.5 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | Addition |
| OZ277 + 4-carboxy-TEMPO | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.0 | 1.3 ± 0.1 | Addition |
| OZ277 + 4-amino-TEMPO | 2.3 ± 0.3c | 2.4 ± 0.2c | 1.7 ± 0.0c | 2.1 ± 0.3e | Antagonism |
Values are the means and standard deviations from ≥3 experiments.
The 1:1, 1:3, and 3:1 ratios refer to fixed-concentration ratios of compound A to compound B.
Significant (P < 0.05) based on a cutoff value for antagonism of 1.4.
Even with six independent repetitions of the experiment, we obtained only two values; in four of the experiments, the 1:1 combination was not active enough, precluding the calculation of an IC50.
P = 0.052 based on a cutoff value for antagonism of 1.4.
Representative combinations were also tested with the checkerboard assay, in which the performance of an active compound is evaluated in the presence of an inactive compound. The concentrations used were 0, 750, 1,500, and 3,000 ng/ml for 4-amino-TEMPO and 0, 5,000, 10,000, and 20,000 ng/ml for 4-hydroxy-TEMPO. The IC50 of artemisinin increased with increasing concentrations of the TEMPO analogs. This is in accordance with the results from the fixed-ratio assay, where we also observed antagonism between artemisinin and the TEMPO analogs. However, contrary to the results from the fixed-ratio assay, in which we observed antagonism between OZ277 and 4-amino-TEMPO, we observed no antagonistic effect of 4-amino-TEMPO in the checkerboard assay (Fig. 2). Data for the K1 strain (not shown) paralleled those for the NF54 strain.
FIG. 2.
In vitro P. falciparum IC50s of artemisinin and OZ277 in the presence of increasing 4-amino-TEMPO concentrations representative of the interactions of the peroxides and TEMPO analogs tested. IC50s of artemisinin are the means from 4 independent experiments, and those of OZ277 are the means from 2 independent experiments.
The ΣFIC values (NF54 strain) from combination experiments with either artemisinin or OZ277 and its nonperoxidic analogs are depicted in Table 3. Neither artemisinin nor OZ277 was antagonized by either nonperoxidic analog; all four combinations were additive. Measured ΣFIC values for the K1 strain (data not shown) differed from those of the NF54 strain by less than 1.4-fold. We also tested the same combinations with the checkerboard method, and in accordance with the ΣFIC data shown in Table 3, we observed no increase in the IC50s of artemisinin and OZ277 (data not shown) with increasing concentrations of their nonperoxidic analogs.
TABLE 3.
In vitro interactions of either artemisinin or OZ277 and the nonperoxidic analogs deoxyartemisinin and carbaOZ277 against the NF54 strain of P. falciparuma
| Drug combination | Mean ΣFIC50 ± SD at a ratio ofb: |
Mean ΣFIC50 ± SD | Interaction | ||
|---|---|---|---|---|---|
| 1:3 | 1:1 | 3:1 | |||
| Artemisinin + deoxyartemisinin | 1.2 ± 0.0 | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 | Addition |
| OZ277 + carbaOZ277 | 1.4 ± 0.1 | 1.3 ± 0.1 | 1.2 ± 0.1 | 1.3 ± 0.1 | Addition |
| Artemisinin + carbaOZ277 | 1.1 ± 0.1 | 1.1 ± 0.0 | 1.1 ± 0.1 | 1.1 ± 0.0 | Addition |
| OZ277 + deoxyartemisinin | 1.2 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | Addition |
Values are the means and standard deviations from ≥3 experiments.
The 1:1, 1:3, and 3:1 ratios refer to fixed-concentration ratios of compound A to compound B.
DISCUSSION
The antagonism observed with combinations of either artemisinin or OZ277 with the TEMPO analogs supports the hypothesis that the formation of carbon-centered radicals is critical for the activities of these two antimalarial peroxides. At first glance, it may seem surprising that the TEMPO analogs showed a tendency toward greater antagonism with artemisinin than they did with OZ277. However, based on subtle differences in the reaction pathways of the carbon-centered radicals derived from artemisinin and OZ277, this outcome is not unexpected. First, both primary and secondary carbon-centered radicals formed from artemisinin self-quench (16, 23) in intramolecular reactions to a greater extent than did the secondary carbon-centered radical formed from OZ277 and its parent ozonide, OZ03 (28). Second, for data obtained under identical experimental conditions (5), significantly less heme alkylation was observed for artemisinin and its semisynthetic derivatives dihydroartemisinin, artemether, and sodium artesunate than for a series of equipotent ozonides, including OZ03 and OZ277.
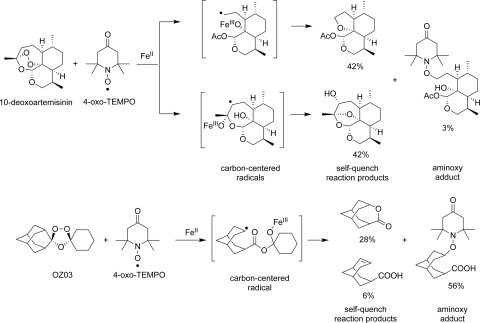
In Fig. 3, the very different reaction outcomes between iron(II) and the semisynthetic artemisinin, 10-deoxoartemisinin (14), and OZ03 (28), the core substructure of OZ277, are illustrated. The reactions were conducted under identical conditions in two different laboratories. In contrast to the efficient reaction of OZ03 with 4-oxo-TEMPO in the presence of ferrous acetate to form the radical-trapped aminoxy adduct in a yield of 56% accompanied by a 34% combined yield of the self-quench reaction products (28), the same reaction with 10-deoxoartemisinin formed the radical-trapped aminoxy adduct in only 3% yield accompanied by an 85% combined yield of the self-quench reaction products (14). This low reaction efficiency of 10-deoxoartemisinin with 4-oxo-TEMPO derives from the greater tendency of artemisinin-derived carbon-centered radicals to self-quench, resulting in a lower proportion of radicals available for subsequent chemical reactions. This explains why radical traps such as TEMPO and its analogs have a greater antagonistic effect on artemisinin than on OZ277.
FIG. 3.
Reaction of 10-deoxoartemisinin and OZ03 with 1.5 equivalents of ferrous acetate in the presence of a 2-fold molar excess of 4-oxo-TEMPO.
Since artemisinin appears to react less efficiently with heme than OZ277, why do these two drugs have such similar potencies against P. falciparum (18)? To answer this question, one might consider that the physicochemical properties of the OZ277-heme and artemisinin-heme covalent adducts (Fig. 4) are quite different in that the OZ277-heme adduct (5) has an additional negative charge due to a carboxylate functional group, whereas the artemisinin-heme covalent adduct (24) retains the ionization characteristics of heme. The more lipophilic artemisinin-heme covalent adduct may mediate the antiplasmodial effects of artemisinin more efficiently than the more polar OZ277-heme covalent adduct mediates those of OZ277. For example, the more lipophilic artemisinin-heme covalent adduct would be expected to have a stronger interaction with relatively hydrophobic membrane lipids (6, 13) in the food vacuole.
FIG. 4.
Structures of artemisinin-heme and OZ277-heme covalent adducts.
The combination experiments with either artemisinin or OZ277 along with the nonperoxidic analogs deoxyartemisinin and carbaOZ277 revealed that the latter had no effect on the antimalarial activities of the former. This outcome is precisely what one would expect if the antimalarial properties of peroxides are derived solely from irreversible bond-forming interactions with parasite targets. If, on the other hand, this irreversible chemistry occurred only after a reversible interaction with one or more parasite targets, one would have expected to observe substantial antagonism by the nonperoxidic analogs, reminiscent of substrate protection against enzyme inactivation by suicide enzyme inhibitors (1). From these data, we can conclude that the antimalarial properties of peroxides such as artemisinin and OZ277 do not derive from reversible interactions with parasite targets. Furthermore, previous work (18) demonstrating the peroxide bond-dependent activity of artemisinin and OZ277 against hemoglobin-digesting protozoa (such as plasmodia) is consistent with the idea that the irreversible interactions of peroxides with parasite targets are iron dependent.
Acknowledgments
This investigation received financial support from the Medicines for Malaria Venture.
Footnotes
Published ahead of print on 22 December 2009.
REFERENCES
- 1.Ables, R. H., and A. L. Maycock. 1976. Suicide enzyme inactivators. Acc. Chem. Res. 9:313-319. [Google Scholar]
- 2.Asawamahasakda, W., I. Ittarat, Y. M. Pu, H. Ziffer, and S. R. Meshnick. 1994. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob. Agents Chemother. 38:1854-1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bhisutthibhan, J., X.-Q. Pan, P. A. Hossler, D. J. Walker, C. A. Yowell, J. Carlton, J. R. Dame, and S. R. Meshnick. 1998. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J. Biol. Chem. 273:16192-16198. [DOI] [PubMed] [Google Scholar]
- 4.Brossi, A., B. Venugopalan, L. Dominguez Gerpe, H. J. C. Yeh, J. L. Flippen-Anderson, P. Buchs, X. D. Luo, W. K. Milhous, and W. Peters. 1988. Arteether, a new antimalarial drug: synthesis and antimalarial properties. J. Med. Chem. 31:645-650. [DOI] [PubMed] [Google Scholar]
- 5.Creek, D. J., W. N. Charman, F. C. K. Chiu, R. J. Prankerd, Y. Dong, J. L. Vennerstrom, and S. A. Charman. 2008. Relationship between antimalarial activity and haem alkylation for spiro- and dispiro-1,2,4-trioxolane antimalarials. Antimicrob. Agents Chemother. 52:1291-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crespo, M. P., T. D. Avery, E. Hanssen, E. Fox, T. V. Robinson, P. Valente, D. K. Taylor, and L. Tilley. 2008. Artemisinin and a series of novel endoperoxide antimalarials exert early effects on digestive vacuole morphology. Antimicrob. Agents Chemother. 52:98-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Desjardins, R. E., C. J. Canfield, J. D. Haynes, and J. D. Chulay. 1979. Quantitative assessment of antimalarial activity in vitro by a semiatuomated microdilution technique. Antimicrob. Agents Chemother. 16:710-718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckstein-Ludwig, U., R. J. Webb, I. D. A. van Goethem, J. M. East, A. G. Lee, M. Kimura, P. M. O'Neill, P. G. Bray, S. A. Ward, and S. Krishna. 2003. Artemisinins target the SERCA of Plasmodium falciparum. Nature 424:957-961. [DOI] [PubMed] [Google Scholar]
- 9.Elliott, D. A., M. T. McIntosh, H. D. Hosgood III, S. Chen, G. Zhang, P. Baevova, and K. A. Joiner. 2008. Four distinct pathways of hemoglobin uptake in the malaria parasite Plasmodium falciparum. Proc. Natl. Acad. Sci. U. S. A. 105:2463-2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fivelman, Q. L., I. S. Adagu, and D. C. Warhurst. 2004. Modified fixed-ratio isobologram method for studying in vitro interactions between atovaquone and proguanil or dihydroartemisinin against drug-resistant strains of Plasmodium falciparum. Antimicrob. Agents Chemother. 48:4097-4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Francis, S. E., D. J. Sullivan, Jr., and D. E. Goldberg. 1997. Hemoglobin metabolism in the malarial parasite Plasmodium falciparum. Annu. Rev. Microbiol. 51:97-123. [DOI] [PubMed] [Google Scholar]
- 12.Garah, F. B.-E., J.-L. Stigliani, F. Coslédan, B. Meunier, and A. Robert. 2009. Docking studies of structurally diverse antimalarial drugs targeting PfATP6: no correlation between in silico binding affinity and in vitro antimalarial activity. ChemMedChem 4:1469-1479. [DOI] [PubMed] [Google Scholar]
- 13.Hartwig, C. L., A. S. Rosenthal, J. D'Angelo, C. E. Griffin, G. H. Posner, and R. A. Cooper. 2009. Accumulation of artemisinin trioxane derivatives within neutral lipids of Plasmodium falciparum malaria parasites is endoperoxide-dependent. Biochem. Pharmacol. 77:322-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes, R. K., W. C. Chan, C.-M. Lung, A.-C. Uhlemann, U. Eckstein, D. Taramelli, S. Parapini, D. Monti, and S. Krishna. 2007. The Fe2-mediated decomposition, PfATP6 binding, and antimalarial activities of artemisone and other artemisinins: the unlikelihood of C-centered radicals as bioactive intermediates. ChemMedChem 2:1480-1497. [DOI] [PubMed] [Google Scholar]
- 15.Huber, W., and J. Koella. 1993. A comparison of three methods of estimating EC50 in studies of drug resistance of malaria parasites. Acta Trop. 55:257-261. [DOI] [PubMed] [Google Scholar]
- 16.Jefford, C. W. 2001. Why artemisinin and certain synthetic peroxides are potent antimalarials. Implications for the mode of action. Curr. Med. Chem. 8:1803-1826. [DOI] [PubMed] [Google Scholar]
- 17.Jefford, C. W. 2007. New developments in synthetic peroxidic drugs as artemisinin mimics. Drug Discov. Today 12:487-495. [DOI] [PubMed] [Google Scholar]
- 18.Kaiser, M., S. Wittlin, A. Nehrbass-Stuedli, Y. Dong, X. Wang, A. Hemphill, H. Matile, R. Brun, and J. L. Vennerstrom. 2007. Peroxide bond-dependent antiplasmodial specificity of artemisinin and OZ277 (RBx11160). Antimicrob. Agents Chemother. 51:2991-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meshnick, S. R. 2002. Artemisinin: mechanisms of action, resistance and toxicity. Int. J. Parasitol. 32:1655-1660. [DOI] [PubMed] [Google Scholar]
- 20.Meshnick, S. R., A. Thomas, A. Ranz, C.-M. Xu, and H.-Z. Pan. 1991. Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol. Biochem. Parasitol. 49:181-190. [DOI] [PubMed] [Google Scholar]
- 21.Novak, I., L. J. Harrison, B. Kovac, and L. M. Pratt. 2004. Electronic structure of persistent radicals: nitroxides. J. Org. Chem. 69:7628-7634. [DOI] [PubMed] [Google Scholar]
- 22.Olliaro, P., and T. N. C. Wells. 2009. The global portfolio of new antimalarial medicines under development. Clin. Pharmacol. Ther. 85:584-595. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill, P. M., and G. H. Posner. 2004. A medicinal chemistry perspective on artemisinin and related endoperoxides. J. Med. Chem. 47:2945-2964. [DOI] [PubMed] [Google Scholar]
- 24.Robert, A., Y. Coppel, and B. Meunier. 2002. Alkylation of heme by the antimalarial drug artemisinin. Chem. Commun. 2002:414-415. [DOI] [PubMed] [Google Scholar]
- 25.Robert, A., F. Benoit-Vical, C. Claparols, and B. Meunier. 2005. The antimalarial drug artemisinin alkylates heme in infected mice. Proc. Natl. Acad. Sci. U. S. A. 102:13676-13680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Snyder, C., J. Chollet, J. Santo-Tomas, C. Scheurer, and S. Wittlin. 2007. In vitro and in vivo interaction of synthetic peroxide RBx11160 (OZ277) with piperaquine in Plasmodium models. Exp. Parasitol. 115:296-300. [DOI] [PubMed] [Google Scholar]
- 27.Tang, Y., Y. Dong, and J. L. Vennerstrom. 2004. Synthetic peroxides as antimalarials. Med. Res. Rev. 24:425-448. [DOI] [PubMed] [Google Scholar]
- 28.Tang, Y., Y. Dong, X. Wang, K. Sriraghavan, J. K. Wood, and J. L. Vennerstrom. 2005. Dispiro-1,2,4-trioxane analogues of a prototype dispiro-1,2,4-trioxolane: mechanistic comparators for artemisinin in the context of reaction pathways with iron(II). J. Org. Chem. 70:5103-5110. [DOI] [PubMed] [Google Scholar]
- 29.Trager, W., and J. B. Jensen. 1976. Human malaria parasites in continuous cultures. Science 193:673-675. [DOI] [PubMed] [Google Scholar]
- 30.Uhlemann, A.-C., S. Wittlin, H. Matile, L. Y. Bustamante, and S. Krishna. 2007. Mechanism of antimalarial action of synthetic trioxolane RBX11160 (OZ277). Antimicrob. Agents Chemother. 51:667-672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vennerstrom, J. L., S. Arbe-Barnes, R. Brun, S. A. Charman, F. C. K. Chiu, J. Chollet, Y. Dong, A. Dorn, D. Hunziker, H. Matile, K. McIntosh, M. Padmanilayam, J. Santo Tomas, C. Scheurer, B. Scorneaux, Y. Tang, U. Urwyler, S. Wittlin, and W. N. Charman. 2004. Identification of an antimalarial synthetic trioxolane drug development candidate. Nature 430:900-904. [DOI] [PubMed] [Google Scholar]
- 32.White, N. J. 2008. Qinghoasu (artemisinin): the price of success. Science 320:330-334. [DOI] [PubMed] [Google Scholar]
- 33.Yang, Y.-Z., B. Little, and S. R. Meshnick. 1994. Alkylation of proteins by artemisinin. Effects of heme, pH, and drug structure. Biochem. Pharmacol. 48:569-573. [DOI] [PubMed] [Google Scholar]