Abstract
Thirty-nine new thiazolide/thiadiazolide compounds were compared with the nitrothiazole nitazoxanide for activity against Cryptosporidium parvum development in HCT-8 cells. Twenty-seven agents exerted ≥90% inhibition. Agents with a lower 50% inhibitory concentration (IC50) than nitazoxanide were either NO2 or halogen 5 substituted on the thiazole moiety. Other 5 substitutions such as methyl, C3H7, C6H11, H, SO2CH3, and SCH3 negatively impacted activity. Five-substituted deacetylated analogues exhibited higher IC50s than their acetylated counterparts. Halogeno-thiazolide/thiadiazolides may provide valuable nitro-free alternatives to nitazoxanide.
Human cryptosporidiosis is recognized as a threat to immunocompromised individuals and is responsible for diarrhea in immunocompetent children and adults (2). Few agents exhibited a suppressive effect on in vitro Cryptosporidium parvum growth, and none was effective in all situations in vivo (1, 19). The nitrothiazole nitazoxanide [2-acetyloxy-N-(5-nitro 2-thiazolyl)benzamide] (NTZ) has been approved for the treatment of diarrheas caused by C. parvum and Giardia duodenalis in immunocompetent adults and children from 12 months of age (7, 12, 18). Recently, NTZ-related thiazolide/thiadiazolide derivatives were reported to be active in vitro against anaerobic bacteria, influenza A virus, an extracellular protozoan, G. duodenalis, and two closely related apicomplexan protozoa, Neospora caninum and Sarcocystis neurona (6, 11, 14, 17, 20). In the present study, new thiazolide/thiadiazolide compounds were compared with NTZ for activity against C. parvum in vitro development.
Agents (Table 1 and Fig. 1) were synthesized at the Romark Center for Drug Discovery at the University of Liverpool, Liverpool, United Kingdom, and supplied by Romark Laboratories, L.C., Tampa, FL. Powder forms were solubilized in dimethyl sulfoxide (5 g/liter) and stored at −20°C until used. Final concentrations in cultures ranged from 0.1 to 10 mg/liter, corresponding to a maximum dimethyl sulfoxide concentration of 0.2% (vol/vol). HCT-8 cells (ATCC CRL 244; American Type Culture Collection, Manassas, VA) were cultured as previously described (8). Agent-induced effects on the viability of confluent HCT-8 cells were monitored by light microscopic inspection and a tetrazolium assay (CellTiter 96 AQueous nonradioactive cell proliferation assay; Promega, Madison, WI). Absent, mild, moderate, and severe effects were rated as described previously (8, 22). C. parvum oocysts of the Nouzilly isolate (a kind gift from M. Naciri, INRA, Nouzilly, France) were purified from calf feces and permitted to excystate as previously described (10). After being sieved through a 5-μm Nuclepore filter, 2.5 × 105 to 5 × 105 sporozoites were added to each confluent HCT-8 monolayer well. Two hours later, supernatants were removed and replaced with agent-containing or agent-free medium. Forty-six hours later, all parasite stages of methanol-fixed cultures were counted in 20 microscopic fields (×1,250) using indirect immunofluorescence as described previously (9, 10). Each set of experiments was done at least twice. Inhibitory activity (percent) was calculated as follows: [(mean number of parasite forms in treated cultures − mean number of parasite forms in untreated cultures)/mean number of parasite forms in untreated wells] × 100. Results are expressed as 50% inhibitory concentrations (IC50s); the IC50 was defined as the concentration (wt/vol) of an agent which resulted in a mean 50% inhibitory activity. For agents exhibiting ≥90% inhibitory activity, IC90s are similarly given. The significance of differences between the endpoint values of experimental and control cultures was determined by using Student's t test, thus assuming normal-like distributions of values. P values of <0.05 were considered statistically significant.
TABLE 1.
Chemical structures and in vitro inhibitory activities on C. parvum development of NTZ and 39 new thiazolide/thiadiazolide derivatives
| Agent | Mol wt | Substitution |
IC50(mg/liter)a | IC90(mg/liter)a | |||||
|---|---|---|---|---|---|---|---|---|---|
| Thiazole ring |
Benzene ring |
||||||||
| X | Y | R1 | R2 | R3 | R4 | ||||
| NTZ | 307.3 | NO2 | H | OAcb | H | H | H | 1.2 | 10 |
| RM4801 | 279.3 | NO2 | H | OH | CH3 | H | H | 0.7 | 5.8e |
| RM4802 | 321.3 | NO2 | H | OAc | CH3 | H | H | 0.5 | 1e |
| RM4805 | 295.3 | NO2 | H | OH | OCH3 | H | H | 0.4 | 1e |
| RM4807 | 279.3 | NO2 | H | OH | H | H | CH3 | 0.5 | 0.9e |
| RM4809 | 279.3 | NO2 | H | OH | H | CH3 | H | 1.4 | 5e |
| RM4814 | 279.3 | NO2 | H | OMec | H | H | H | 5.5 | NAd |
| RM4815 | 279.3 | NO2 | CH3 | OH | H | H | H | 0.6 | 1e |
| RM4804 | 310.8 | Cl | H | OAc | CH3 | H | H | 0..4 | 1e |
| RM4848 | 254.7 | Cl | H | OH | H | H | H | 2.6 | 6e |
| RM4850 | 268.7 | Cl | H | OH | H | H | CH3 | 0.6 | 6.7e |
| RM4851 | 268.7 | Cl | H | OH | CH3 | H | H | 2..3 | 4.6e |
| RM4852 | 268.7 | Cl | H | OH | H | CH3 | H | 0.8 | 5.5e |
| RM4865 | 310.8 | Cl | H | OAc | H | H | CH3 | 0.1 | 3.8e |
| RM4803 | 355.2 | Br | H | OAc | CH3 | H | H | 0.6 | 5.6 |
| RM4806 | 371.2 | Br | H | OAc | OCH3 | H | H | 4.6 | NA |
| RM4819 | 313.2 | Br | H | OH | CH3 | H | H | 1.4 | 4.8e |
| RM4820 | 341.2 | Br | H | OAc | H | H | H | 0.6 | 4e |
| RM4821 | 355.2 | Br | H | OAc | H | H | CH3 | 1.7 | 4.4e |
| RM4822 | 355.2 | Br | H | OAc | H | CH3 | H | 0.7 | 3.7e |
| RM4823 | 355.2 | Br | CH3 | OAc | H | H | H | 2.1 | 4.8e |
| RM4832 | 299.1 | Br | H | OH | H | H | H | 2.5 | 8 |
| RM4847 | 313.2 | Br | H | OH | H | CH3 | H | 5.2 | 9.3 |
| RM4858 | 313.2 | Br | CH3 | OH | H | H | H | 2.9 | 9.1 |
| RM4859 | 327.2 | Br | CH3 | OH | CH3 | H | H | 0.1 | 1e |
| RM4860 | 389.3 | Br | C6H5 | OH | CH3 | H | H | 0.1 | 1e |
| RM4861 | 375.2 | Br | C6H5 | OH | H | H | H | 0.4 | 1 |
| RM4816f | 331.3 | CF3 | H | OAc | H | H | H | 0.7 | 6.6 |
| RM4854 | 238.2 | F | H | OH | H | H | H | 1.8 | 8.6 |
| RM4862 | 330.8 | C6H4Cl | H | OH | H | H | H | 6.1 | NA |
| RM4856 | 302.4 | C6H11 | H | OH | H | H | H | >10 | NA |
| RM4855 | 262.3 | C3H7 | H | OH | H | H | H | 6.7 | NA |
| RM4853 | 234.3 | CH3 | H | OH | H | H | H | >10 | NA |
| RM4857 | 220.3 | H | H | OH | H | H | H | >10 | NA |
| RM4813f | 263.3 | H | H | OAc | H | H | H | 5.0 | NA |
| RM4817f | 309.4 | SCH3 | H | OAc | H | H | H | 6.4 | 9.2 |
| RM4863 | 298.3 | SO2CH3 | H | OH | H | H | H | 6.9 | NA |
| RM4864 | 340.4 | SO2CH3 | H | OAc | H | H | H | 5.0 | NA |
| RM4818g | 510.5 | H | CH2COOCH2CH3 | OAc | H | H | H | >10 | NA |
| RM4824 | 348.4 | H | CH2COOCH2CH3 | OAc | H | H | H | 8.4 | NA |
Compared with a control treated with the same volume of dimethyl sulfoxide at 48 h postinfection.
Ac, acetyl.
Me, methyl.
NA, not applicable.
Maximal inhibitory activity of >99% (complete growth inhibition).
Thiadiazolide agent.
Benzamide N substitution with COC6H4OCOCH3.
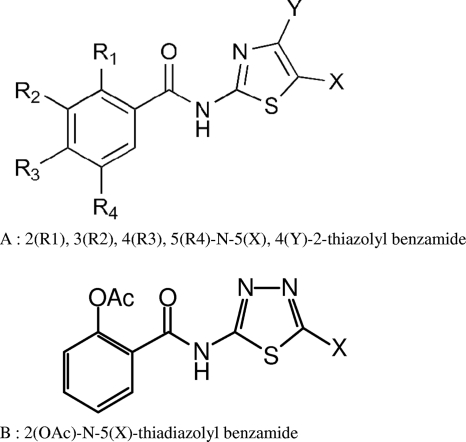
FIG. 1.
Chemical structures of the thiazolides (A) and thiadiazolides (B) used in this study.
High-yield complete (asexual and sexual stages) parasite development was obtained (8, 9). Assays were performed at host cell confluence to limit the apoptosis-inducing activity recently reported for NTZ and RM4819 in human enterocytic Caco-2 cells (16). At 1- and 5-mg/liter concentrations of all of the agents (i.e., 1.96 to 22.70 μM), no alteration of HCT-8 cells was observed after 48 h of contact. At a 10-mg/liter (25.69 to 37.22 μM) concentration, moderate alteration of cell viability was noted for RM4802 to -4805, RM4807, RM4815 to -4817, RM4819, RM4821, RM4848, RM4851, RM4852, RM4854, RM4858 to -4862, and RM4865. Twenty-seven of 39 thiazolides/thiadiazolides exerted ≥90% dose-dependent parasite inhibition, with IC90s ranging from 0.9 to 9.3 mg/liter (3.2 to 29.7 μM) (Table 1 and Fig. 2). For agents with both a lower IC50 than that of NTZ (P < 0.05) and alteration of cell viability (RM4802 to -4805, RM4807, RM4815, RM4816, RM4852, RM4859, RM4860, and RM4865), the ratio of the concentration affecting cell viability (31.12 to 37.22 μM) to the IC50 (0.32 to 2.98 μM) ranged from 13 to 100.
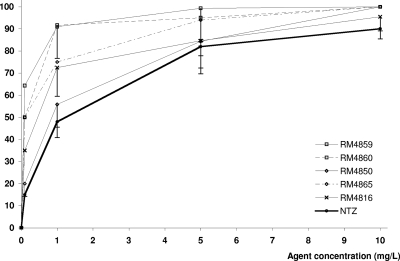
FIG. 2.
Inhibition curves of representative active compounds. RM4959 and RM4860 are 5-bromothiazolides; RM4850 (deacetylated metabolite of RM4865) and RM4865 are 5-chlorothiazolides; RM4816 is a thiadiazolide. Data, expressed as percent inhibition compared with untreated infected control cultures, represent the means of triplicate experiments. Error bars indicate standard deviations that were >5%.
Sixteen agents with IC50s lower than that of NTZ (P < 0.05) have either an NO2 or a halogen thiazole/thiadiazole X substitution (Table 1). Compared with NTZ, 5/7 NO2 X-substituted agents exhibited a lower IC50 (P < 0.05), 1/7 (RM4809) had an equivalent IC50 (P > 0.05), and 1/7 (RM4814, which contains a methoxy group at position R1 of the benzene ring) was considered inefficient.
All chlorothiazolides were strong inhibitors, regardless of the substitution on the benzene ring (IC50s ranging from 0.1 to 2.6 mg/liter, i.e., 0.3 to 10.2 μM), and four agents exhibited IC50s lower than that of NTZ (Table 1, P < 0.01). Of two F X-substituted agents, one was very active (CF3 substitution, RM4816), with an IC50 lower than that of NTZ (P < 0.05), and the other exerted limited activity (RM4854). CH3, C3H7, C6H11, H, SO2CH3, and SCH3 X substitutions negatively impacted the inhibitory activity, producing IC50s lower than that of NTZ (P < 0.05).
In the hydrophobic 5-bromothiazolide series, 12/13 agents exhibited ≥90% maximal inhibition, with IC50s ranging from 0.4 to 2.8 mg/liter (0.3 to 16.6 μM) (Table 1). Compared with NTZ, six agents (RM4803, RM4820, RM4822, RM4859, RM4860, and RM4861) exhibited lower IC50s (ranging from 0.1 to 0.7 mg/liter, i.e., 0.3 to 2 μM), one (RM4819) had a similar IC50 (P = 0.5), and six (RM4806, RM4821, RM4823, RM4832, RM4847, and RM4858) had higher IC50s (ranging from 1.7 to 5.2 mg/liter, i.e., 4.9 to 16.6 μM; P < 0.05).
Of three thiadiazolides, one (RM4816) was very active, with an IC50 lower than that of NTZ (P < 0.01), and two were less active than NTZ (P < 0.05).
The biological activity of NTZ was previously reported to depend on the nitro group, possibly via inhibition of an oxidoreductase (3, 13, 21). A 5-nitro group was found to be a prerequisite for the in vitro efficiency of RM4805 and RM4807 against G. duodenalis, although it was not sufficient since further modification of the benzene moiety could reduce or even abrogate their activities (14). In contrast, consistent with previous N. caninum, Besnoitia besnoiti, and S. neurona in vitro studies, the present data suggest that the nitro group on the thiazole ring is not required for the activity of thiazolides against intracellular protozoa, implying differences between the intracellular and extracellular protozoal targets (4, 6, 11, 12, 15).
As previously shown for NTZ and its biologically active deacetylated metabolite tizoxanide, both acetylated (at R1 on the benzene ring) thiazolide derivatives and their deacetylated counterparts, which represent their most probable primary metabolites, displayed excellent inhibitory effects, although they were lower for deacetylated agents (P = 0.03), consistent with previous observations for N. caninum with RM4822 and RM4847 (5, 10). Substitution of a bromo or chloro group for the nitro group at the 5 position did not significantly impact the activity of deacetylated compounds, provided that position R2 (or R3 or R4) was substituted with a methyl group in the 5-bromo or the 5-chloro series, respectively. A methyl group at position R3 in the 5-bromo series and a polar methoxy group in position R1 in the 5-nitro series notably reduced the efficacy of deacetylated compounds. These data suggest that both the thiazole and benzene residues serve as key pharmacophores, as reported for N. caninum and S. neurona (5, 6, 11).
The present report provides the first evidence of in vitro inhibitory activities of new NTZ-derived thiazolides/thiadiazolides against C. parvum. It is noteworthy that agents where the nitro group was eliminated, as well as nitrothiazolide, were active. Nitro-free agents and especially halogeno compounds may provide a valuable alternative to NTZ, depending on future studies of their in vivo activities.
Acknowledgments
We are grateful to R. Mancassola and M. Naciri, INRA, Nouzilly, France, for kindly providing C. parvum-infected calf feces. We thank C. Pidathala and M. Iqbal for their help in thiazolide synthesis.
This study was supported in part by a grant from the Romark Foundation and by grants from AFSSET (EST-2006/1/30) and Seine-Aval 2006.
Footnotes
Published ahead of print on 4 January 2010.
REFERENCES
- 1.Abubakar, I., S. H. Aliyu, C. Arumugam, N. K. Asman, and P. R. Hunter. 2007. Treatment of cryptosporidiosis in immunocompromised individuals: systematic review and meta-analysis. Br. J. Clin. Pharmacol. 63:387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen, X. M., J. S. Keithly, C. V. Paya, and N. F. La Russo. 2002. Cryptosporidiosis. N. Engl. J. Med. 346:1723-1731. [DOI] [PubMed] [Google Scholar]
- 3.Coombs, G. H., and S. Müller. 2002. Recent advances in the search for new anti-coccidial drugs. Int. J. Parasitol. 32:497-508. [DOI] [PubMed] [Google Scholar]
- 4.Cortes, H. C., N. Mueller, M. Esposito, M. Leitao, A. Naguleswaran, and A. Hemphill. 2007. In vitro efficacy of nitro- and bromo-thiazolyl-salicylamide compounds (thiazolides) against Besnoitia besnoiti infection in Vero cells. Parasitology 134:975-985. [DOI] [PubMed] [Google Scholar]
- 5.Esposito, M., N. Müller, and A. Hemphill. 2007. Structure-activity relationships from in vitro efficacies of the thiazolide series against the intracellular apicomplexan protozoan Neospora caninum. Int. J. Parasitol. 37:183-190. [DOI] [PubMed] [Google Scholar]
- 6.Esposito, M., R. Stettler, S. L. Moores, C. Pidathala, N. Müller, A. Stachulski, N. G. Berry, J. F. Rossignol, and A. Hemphill. 2005. In vitro efficacies of nitazoxanide and other thiazolides against Neospora caninum tachyzoites reveal antiparasitic activity independent of the nitro group. Antimicrob. Agents Chemother. 49:3715-3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fox, L. M., and L. D. Saravolatz. 2005. Nitazoxanide: a new thiazolide antiparasitic agent. Clin. Infect. Dis. 40:1173-1180. [DOI] [PubMed] [Google Scholar]
- 8.Gargala, G., A. Baishanbo, L. Favennec, A. François, J. J. Ballet, and J. F. Rossignol. 2005. Inhibitory activities of epidermal growth factor receptor tyrosine kinase-targeted dihydroxyisoflavone and trihydroxydeoxybenzoin derivatives on Sarcocystis neurona, Neospora caninum, and Cryptosporidium parvum development. Antimicrob. Agents Chemother. 49:4628-4634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gargala, G., A. Delaunay, L. Favennec, P. Brasseur, and J. J. Ballet. 1999. Enzyme immunoassay detection of Cryptosporidium parvum inhibition by sinefungin in sporozoite infected HCT-8 enterocytic cells. Int. J. Parasitol. 29:703-709. [DOI] [PubMed] [Google Scholar]
- 10.Gargala, G., A. Delaunay, X. Li, P. Brasseur, L. Favennec, and J. J. Ballet. 2000. Efficacy of nitazoxanide, tizoxanide and tizoxanide glucuronide against Cryptosporidium parvum development in sporozoite-infected HCT-8 enterocytic cells. J. Antimicrob. Chemother. 46:57-60. [DOI] [PubMed] [Google Scholar]
- 11.Gargala, G., L. Le Goff, J. J. Ballet, L. Favennec, A. V. Stachulski, and J. F. Rossignol. 2009. In vitro efficacy of nitro- and halogeno-thiazolide/thiadiazolide derivatives against Sarcocystis neurona. Vet. Parasitol. 162:230-235. [DOI] [PubMed] [Google Scholar]
- 12.Hemphill, A., J. Müller, and M. Esposito. 2006. Nitazoxanide, a broad-spectrum thiazolide anti-infective agent for the treatment of gastrointestinal infections. Expert Opin. Pharmacother. 7:953-964. [DOI] [PubMed] [Google Scholar]
- 13.Hoffman, P. S., G. Sisson, M. A. Croxen, K. Welch, W. D. Harman, N. Cremades, and M. G. Morash. 2007. Antiparasitic drug nitazoxanide inhibits the pyruvate oxidoreductases of Helicobacter pylori, selected anaerobic bacteria and parasites, and Campylobacter jejuni. Antimicrob. Agents Chemother. 51:868-876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Müller, J., G. Rühle, N. Müller, J. F. Rossignol, and A. Hemphill. 2006. In vitro effects of thiazolides on Giardia lamblia WB clone C6 cultured axenically and in coculture with Caco2 cells. Antimicrob. Agents Chemother. 50:162-170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Müller, J., A. Naguleswaran, N. Müller, and A. Hemphill. 2008. Neospora caninum: functional inhibition of protein disulfide isomerase by the broad-spectrum anti-parasitic drug nitazoxanide and other thiazolides. Exp. Parasitol. 118:80-88. [DOI] [PubMed] [Google Scholar]
- 16.Müller, J., D. Sidler, U. Nachbur, J. Wastling, T. Brunner, and A. Hemphill. 2008. Thiazolides inhibit growth and induce glutathione-S-transferase Pi (GSTP1)-dependent cell death in human colon cancer cells. Int. J. Cancer 123:1797-1806. [DOI] [PubMed] [Google Scholar]
- 17.Pankuch, G. A., and P. C. Appelbaum. 2006. Activities of tizoxanide and nitazoxanide compared to those of five other thiazolides and three other agents against anaerobic species. Antimicrob. Agents Chemother. 50:1112-1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossignol, J. F., A. Ayoub, and M. S. Ayers. 2001. Treatment of diarrhea caused by Cryptosporidium parvum: a prospective randomized, double-blind, placebo-controlled study of nitazoxanide. J. Infect. Dis. 184:103-106. [DOI] [PubMed] [Google Scholar]
- 19.Rossignol, J. F. 2006. Nitazoxanide in the treatment of acquired immune deficiency syndrome-related cryptosporidiosis: results of the United States compassionate use program in 365 patients. Aliment. Pharmacol. Ther. 24:887-894. [DOI] [PubMed] [Google Scholar]
- 20.Rossignol, J. F., S. La Frazia, L. Chiappa, A. Ciucci, and M. G. Santoro. 2009. Thiazolides, a new class of anti-influenza molecules targeting viral hemagglutinin at post-translational level. J. Biol. Chem. 284:29798-29808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rotte, C., F. Stejskal, G. Zhu, J. S. Keithly, and W. Martin. 2001. Pyruvate: NADP+ oxidoreductase from the mitochondrion of Euglena gracilis and from the apicomplexan Cryptosporidium parvum: a biochemical relic linking pyruvate metabolism in mitochondriate and amitochondriate protists. Mol. Biol. Evol. 18:710-720. [DOI] [PubMed] [Google Scholar]
- 22.Theodos, C. M., J. K. Griffiths, J. D'Onfro, A. Fairfield, and S. Tzipori. 1998. Efficacy of nitazoxanide against Cryptosporidium parvum in cell culture and in animal models. Antimicrob. Agents Chemother. 42:1959-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]