Abstract
Antibiotic-induced bacteriolysis exacerbates inflammation and brain damage in bacterial meningitis. Here the quality and temporal kinetics of cerebrospinal fluid (CSF) inflammation were assessed in an infant rat pneumococcal meningitis model for the nonbacteriolytic antibiotic daptomycin versus ceftriaxone. Daptomycin led to lower CSF concentrations of interleukin 1β (IL-1β), IL-10, IL-18, monocyte chemoattractant protein 1 (MCP-1), and macrophage inflammatory protein 1 alpha (MIP-1α) (P < 0.05). In experimental pneumococcal meningitis, daptomycin treatment resulted in more rapid bacterial killing, lower CSF inflammation, and less brain damage than ceftriaxone treatment.
Up to half of the survivors of pneumococcal meningitis are left with neurological sequelae, the rate of which remained unchanged over the last few decades despite continuous improvements in therapy (16). In patients and in corresponding experimental models, brain injury caused by bacterial meningitis has been shown to prominently affect three brain structures, the cortex, the hippocampus, and the inner ear (2, 6). The different forms of tissue damage represent the morphological correlate of the functional deficits observed in survivors, including cerebral palsy, deficits in learning and memory, and hearing loss (9, 12).
Inflammation has been shown to play a key role in the pathophysiology leading to the development of brain damage consecutive to bacterial meningitis (11). Anti-inflammatory corticosteroids have been used as adjunctive therapy for bacterial meningitis, but conclusive evidence for a beneficial effect on brain damage, specifically in pediatric pneumococcal meningitis, is lacking (20). Prevention of the inflammatory reaction leads to less brain damage in experimental bacterial meningitis (14). Avoidance of the release of proinflammatory bacterial components upon use of nonbacteriolytic antibiotics is a promising strategic alternative to the use of corticosteroids (7, 14, 17). The nonbacteriolytic lipopeptide daptomycin was at least as efficient as ceftriaxone at eliminating bacteria from the cerebrospinal fluid (CSF) in experimental pneumococcal meningitis. Furthermore, daptomycin significantly lowered the CSF concentration of matrix-metalloproteinase 9 (MMP-9), an enzyme critically involved in the pathophysiology of brain damage, and in consequence caused less brain damage than ceftriaxone (7). Here we extended these observations by investigating the quality and temporal kinetics of CSF inflammation in infant rats with pneumococcal meningitis after treatment with daptomycin versus that with ceftriaxone.
All animal studies were approved by the Animal Care and Experimentation Committee of the Canton of Bern, Switzerland, and followed the Swiss national guidelines for performance of animal experiments. Eleven-day-old Wistar rats (n = 28; Charles River, Germany) were injected intracisternally (i.c.) with 10 μl of saline containing 1.5 × 104 CFU of Streptococcus pneumoniae (clinical isolate of a serotype 3 strain) as previously described (7, 10). Eighteen hours later, animals were randomly chosen to receive daptomycin (n = 14) (50 mg/kg body weight, administered subcutaneously [s.c.]; Cubicin, kindly provided by Cubist Pharmaceuticals, Lexington, MA) or ceftriaxone (n = 14) (100 mg/kg body weight given s.c.; Rocephine; Roche Pharma, Basel, Switzerland). The dosages of daptomycin and ceftriaxone used in this study are equal to those used in previously published work (7). Available data on pharmacokinetics/pharmacodynamics (PK/PD) of daptomycin in the CSF during experimental pneumococcal meningitis are derived from the rabbit model (3). For daptomycin, a comparable dosage in adult rats (40 mg/kg, s.c.) resulted in a maximum concentration of drug (Cmax) and an area under the concentration-time curve from 0 to 24 h (AUC0-24) in serum comparable to what is seen in humans with a 6- to 8-mg/kg dose given intravenously (i.v.) (15). More recently, a similar Cmax was also obtained in adult mice after a dosage of 25 mg/kg given i.p. (13). Based on a comparable body weight of infant rats and adult mice of approximately 25 to 30 g, a 50-mg/kg dosage, adjusted for an increased metabolism in younger animals, is expected to lead to comparable serum levels of daptomycin. CSF samples were obtained by puncture of the cisterna at defined time points after infection, i.e., 18, 20, and 24 h (n = 7 for each treatment group) and 40 h (n = 7 for daptomycin and n = 8 for ceftriaxone) after infection. A control experiment with untreated animals was not performed, because excessive mortality is observed at these time points without antibiotic treatment. Bacterial killing was significantly more rapid by therapy with daptomycin than by that with ceftriaxone 2 h after the initiation of therapy. Four hours of daptomycin therapy decreased CSF bacterial titers below the detection limit (<103 CFU/ml), leading to a more rapid sterilization of the CSF (see Fig. 2A).
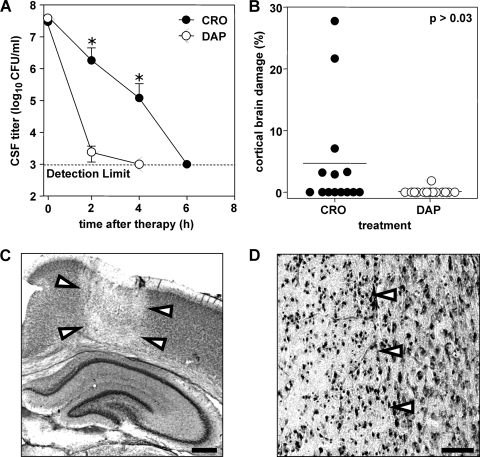
FIG. 2.
(A) CSF bacterial titers after antibiotic therapy. Sterilization of CSF was more rapid with therapy with daptomycin (DAP) than with therapy with ceftriaxone (CRO). Six hours after therapy, CSF was sterilized with daptomycin. At 2 and 4 h after therapy, bacterial titers differed significantly (P < 0.05, Mann-Whitney) between treatment groups. (B) Brain damage in experimental pneumococcal meningitis. The extent of cortical damage is significantly reduced (P = 0.02 Mann-Whitney) by daptomycin treatment versus that with ceftriaxone treatment. (C) Histopathology (overview). Cortical injury assessed by Nissl staining is characterized by wedge-shaped areas of decreased neuronal density (arrowheads), suggestive of ischemic necrosis (cresyl violet; original magnification, ×5; scale bar = 1 mm). (D) Histopathology. Focus of cortical neuronal loss (left side; arrowheads) containing neurons with morphological features of necrosis, including pyknotic nuclei, cell swelling, and fading of cytoarchitecture, is sharply demarcated from preserved brain tissue (right side; original magnification, ×200; scale bar, 50 μm; cresyl violet).
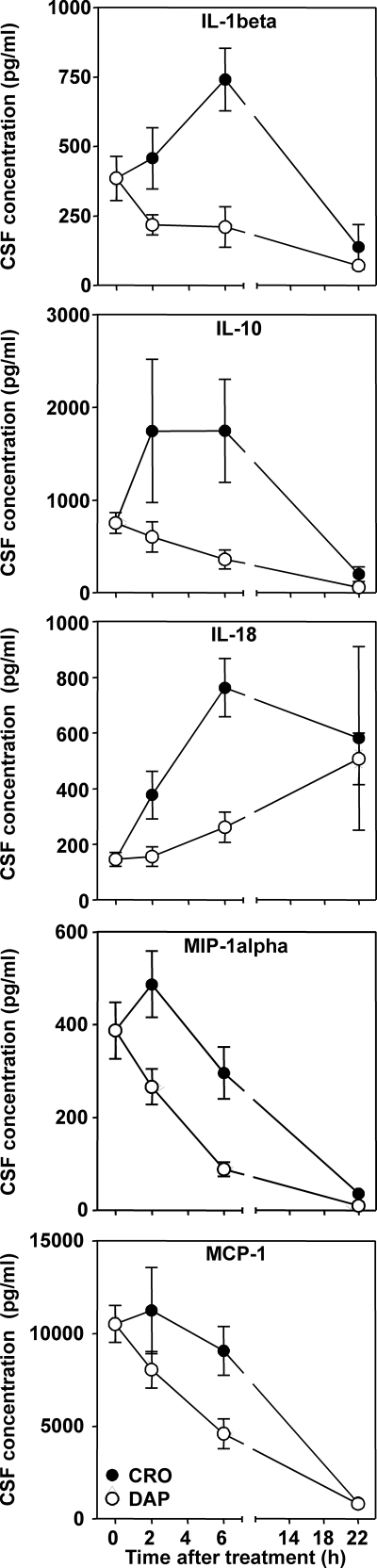
The CSF concentrations of defined inflammatory mediators (interleukin 1β [IL-1β], IL-2, IL-6, IL-10, IL-18, tumor necrosis factor alpha [TNF-α], gamma interferon [IFN-γ], granulocyte-macrophage colony-stimulating factor [GM-CSF], chemokine [C-X-C motif] ligand 1 [CXCL1], macrophage inflammatory protein 1 alpha [MIP-1α], and monocyte chemoattractant protein 1 [MCP-1]) were assessed, using a microsphere-based multiplex assay (Lincoplex; Millipore Corporation) as described previously (5). The addition of 1, 10, or 100 μg/ml of daptomycin or ceftriaxone to a mixture of cyto- and chemokines at known concentrations had no effect on the performance and the results of immunoassay (data not shown). Statistically significant (P < 0.05) differences in the profiles of IL-1β, IL-10, IL-18, MCP-1, and MIP-1α protein expression were found between the two therapeutic modalities, as determined by two-way analysis of variance ANOVA (Fig. 1). Ceftriaxone led to a marked increase in the CSF concentration of the above-detailed cyto- and chemokines at 2 to 6 h after the initiation of therapy, while the reaction to daptomycin treatment was limited to a moderate increase in IL-18 only (Fig. 1). It has been shown that daptomycin does not exhibit an immunomodulatory effect in an experimental endotoxin model of human whole blood (19). It is therefore unlikely that the lower CSF levels of cyto- and chemokines with treatment with daptomycin is due to an anti-inflammatory activity of daptomycin by itself.
FIG. 1.
Profile of cyto-/chemokine concentration in the CSF for treatment with daptomycin versus that with ceftriaxone at different time points (2, 6, and 40 h) after initiation of therapy. The concentrations of IL-1β, IL-10, IL-18, MCP-1, and MIP-1α were significantly (P < 0.05, two-way ANOVA) lower in daptomycin-treated animals.
For histopathological examination of brain damage, animals were sacrificed at 40 h after infection. Twelve coronal brain sections per animal were evaluated for neuronal injury of the cortex (Fig. 2C and D) and hippocampus, as described previously (5). The area of cortical necrosis was expressed as the percentage of the total area of cortex in each section, and the mean value per animal was calculated. Treatment with daptomycin versus that with ceftriaxone significantly reduced the occurrence (1/14 versus 6/14; P < 0.08, Fischer's exact test) and severity of cortical damage (0.13% ± 0.5% versus 4.7% ± 8.8% of total cortical volume; n = 14 for each group; P = 0.03, Mann Whitney) (Fig. 2B). Apoptosis in the dentate gyrus was not significantly different between the two treatment groups (data not shown).
Daptomycin disrupts membrane functions of Gram-positive bacteria. It has also recently been shown to bind to YycG, interfering with the function of this key sensor kinase, leading to cell death without lysis (1). Accordingly, the release of [3H]choline from the cell wall of labeled bacteria was diminished in daptomycin-treated rabbits in comparison to results with ceftriaxone during experimental pneumococcal meningitis (18). In the present experimental model, treatment with daptomycin compared to that with ceftriaxone led to a more rapid decrease in CSF bacterial titers and a reduction in the occurrence of cortical neuronal injury (7). A decrease in the inflammatory reaction, as suggested by a significant difference in metalloprotease-9 activity 22 h after treatment, was proposed as a factor contributing to the improved outcome with daptomycin (7). In the present study, we extended these observations by focusing on the quality and temporal kinetics of the inflammatory reaction over 22 h after antibiotic therapy, a critical time with respect to the pathophysiological mechanisms leading to neuronal injury. From the 11 cyto- and chemokines measured, significantly lower concentrations of IL-1β, IL-10, IL-18, MCP-1, and MIP-1α were documented in the CSF of daptomycin-treated animals than in that of ceftriaxone-treated animals. Although not significant, CSF levels of IL-6, CXCL1, and TNF-α were also lower in daptomycin-treated animals.
In a murine model, it has recently been shown that daptomycin and vancomycin, in combination with dexamethasone, were similarly active for the treatment of pneumococcal meningitis (13). The effect of dexamethasone was shown to only marginally affect the antibactericidal activity of daptomycin alone or in combination with ceftriaxone, although the penetration of daptomycin in the inflamed meninges was reduced (4).
Successful treatment of a patient with methicillin-resistant Staphylococcus aureus with daptomycin has recently been reported (8). But because the activity of daptomycin is limited against Gram-positive bacteria, clinical use as an empirical therapy of bacterial meningitis would require combination with a broad-spectrum antibiotic. Sequential therapy with a nonlytic antibiotic, i.e., rifampin with ceftriaxone, has been recently demonstrated to cause less brain injury (17). Important in the context of a prospective clinical application is the recent finding that the combination of daptomycin with ceftriaxone was shown to be more active than vancomycin plus ceftriaxone in experimental rabbit meningitis (4). The present evidence supports further investigations of the use of daptomycin in combination therapy for bacterial meningitis and how it influences the inflammatory reaction and the development of neurological damage.
Acknowledgments
This work was supported by the Swiss National Science Foundation (grant 310030-116257) and by unrestricted research grants from Cubist Pharmaceuticals, Lexington, MA, and Novartis Pharma Schweiz, AG, Bern, Switzerland.
Footnotes
Published ahead of print on 11 January 2010.
REFERENCES
- 1.Baltz, R. H. 2009. Daptomycin: mechanisms of action and resistance, and biosynthetic engineering. Curr. Opin. Chem. Biol. 13:144-151. [DOI] [PubMed] [Google Scholar]
- 2.Bifrare, Y. D., C. Gianinazzi, H. Imboden, S. L. Leib, and M. G. Täuber. 2003. Bacterial meningitis causes two distinct forms of cellular damage in the hippocampal dentate gyrus in infant rats. Hippocampus 13:481-488. [DOI] [PubMed] [Google Scholar]
- 3.Cottagnoud, P., M. Pfister, F. Acosta, M. Cottagnoud, L. Flatz, F. Kuhn, H. P. Muller, and A. Stucki. 2004. Daptomycin is highly efficacious against penicillin-resistant and penicillin- and quinolone-resistant pneumococci in experimental meningitis. Antimicrob. Agents Chemother. 48:3928-3933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Egermann, U., Z. Stanga, A. Ramin, F. Acosta, A. Stucki, P. Gerber, M. Cottagnoud, and P. Cottagnoud. 2009. The combination of daptomycin plus ceftriaxone was more active than vancomycin plus ceftriaxone in experimental meningitis after addition of dexamethasone. Antimicrob. Agents Chemother. 53:3030-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gehre, F., S. L. Leib, D. Grandgirard, J. Kummer, A. Bühlmann, F. Simon, R. Gäumann, A. S. Kharat, M. G. Täuber, and A. Tomasz. 2008. Essential role of choline for pneumococcal virulence in an experimental model of meningitis. J. Intern. Med. 264:143-154. [DOI] [PubMed] [Google Scholar]
- 6.Gerber, J., W. Bruck, C. Stadelmann, S. Bunkowski, H. Lassmann, and R. Nau. 2001. Expression of death-related proteins in dentate granule cells in human bacterial meningitis. Brain Pathol. 11:422-431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grandgirard, D., C. Schürch, P. Cottagnoud, and S. L. Leib. 2007. Prevention of brain injury by the nonbacteriolytic antibiotic daptomycin in experimental pneumococcal meningitis. Antimicrob. Agents Chemother. 51:2173-2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee, D. H., B. Palermo, and M. Chowdhury. 2008. Successful treatment of methicillin-resistant staphylococcus aureus meningitis with daptomycin. Clin. Infect. Dis. 47:588-590. [DOI] [PubMed] [Google Scholar]
- 9.Leib, S. L., C. Heimgartner, Y. D. Bifrare, J. M. Loeffler, and M. G. Täuber. 2003. Dexamethasone aggravates hippocampal apoptosis and learning deficiency in pneumococcal meningitis in infant rats. Pediatr. Res. 4:4. [DOI] [PubMed] [Google Scholar]
- 10.Loeffler, J. M., R. Ringer, M. Hablutzel, M. G. Täuber, and S. L. Leib. 2001. The free radical scavenger alpha-phenyl-tert-butyl nitrone aggravates hippocampal apoptosis and learning deficits in experimental pneumococcal meningitis. J. Infect. Dis. 183:247-252. [DOI] [PubMed] [Google Scholar]
- 11.Meli, D. N., S. Christen, S. L. Leib, and M. G. Täuber. 2002. Current concepts in the pathogenesis of meningitis caused by Streptococcus pneumoniae. Curr. Opin. Infect. Dis. 15:253-257. [DOI] [PubMed] [Google Scholar]
- 12.Meli, D. N., R. S. Coimbra, D. G. Erhart, G. Loquet, C. L. Bellac, M. G. Täuber, U. Neumann, and S. L. Leib. 2006. Doxycycline reduces mortality and injury to the brain and cochlea in experimental pneumococcal meningitis. Infect. Immun. 74:3890-3896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mook-Kanamori, B. B., M. S. Rouse, C. I. Kang, D. van de Beek, J. M. Steckelberg, and R. Patel. 2009. Daptomycin in experimental murine pneumococcal meningitis. BMC Infect. Dis. 9:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nau, R., and H. Eiffert. 2005. Minimizing the release of proinflammatory and toxic bacterial products within the host: a promising approach to improve outcome in life-threatening infections. FEMS Immunol. Med. Microbiol. 44:1-16. [DOI] [PubMed] [Google Scholar]
- 15.Sakoulas, G., G. M. Eliopoulos, J. Alder, and C. T. Eliopoulos. 2003. Efficacy of daptomycin in experimental endocarditis due to methicillin-resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 47:1714-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schuchat, A., K. Robinson, J. D. Wenger, L. H. Harrison, M. Farley, A. L. Reingold, L. Lefkowitz, and B. A. Perkins. 1997. Bacterial meningitis in the United States in 1995. Active Surveillance Team. N. Engl. J. Med. 337:970-976. [DOI] [PubMed] [Google Scholar]
- 17.Spreer, A., R. Lugert, V. Stoltefaut, A. Hoecht, H. Eiffert, and R. Nau. 2009. Short-term rifampicin pretreatment reduces inflammation and neuronal cell death in a rabbit model of bacterial meningitis. Crit. Care Med. 37:2253-2258. [DOI] [PubMed] [Google Scholar]
- 18.Stucki, A., M. Cottagnoud, V. Winkelmann, T. Schaffner, and P. Cottagnoud. 2007. Daptomycin produces an enhanced bactericidal activity compared to ceftriaxone, measured by [3H]choline release in the cerebrospinal fluid, in experimental meningitis due to a penicillin-resistant pneumococcal strain without lysing its cell wall. Antimicrob. Agents Chemother. 51:2249-2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thallinger, C., M. Rothenburger, C. Marsik, S. Wuenscher, M. Popovic, G. Endler, O. Wagner, and C. Joukhadar. 2008. Daptomycin does not exert immunomodulatory effects in an experimental endotoxin model of human whole blood. Pharmacology 81:57-62. [DOI] [PubMed] [Google Scholar]
- 20.van de Beek, D., J. de Gans, P. McIntyre, and K. Prasad. 2007. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst. Rev. 2007:CD004405. [DOI] [PubMed] [Google Scholar]