Abstract
Staphylococcal enterotoxins are potent activators for human T cells and cause lethal toxic shock. Rapamycin, an immunosuppressant, was tested for its ability to inhibit staphylococcal enterotoxin B (SEB)-induced activation of human peripheral blood mononuclear cells (PBMC) in vitro and toxin-mediated shock in mice. Stimulation of PMBC by SEB was effectively blocked by rapamycin as evidenced by the inhibition of tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), IL-6, IL-2, gamma interferon (IFN-γ), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), MIP-1β, and T-cell proliferation. In vivo, rapamycin protected 100% of mice from lethal shock, even when administered 24 h after intranasal SEB challenge. The serum levels of MCP-1 and IL-6, after intranasal exposure to SEB, were significantly reduced in mice given rapamycin versus controls. Additionally, rapamycin diminished the weight loss and temperature fluctuations elicited by SEB.
Staphylococcal exotoxins are among the most common etiological agents that cause toxic shock syndrome (28-30, 38, 44). The disease is characterized by fever, hypotension, desquamation of skin, and dysfunction of multiple organ systems (8, 38, 41). These toxins bind directly to the major histocompatibility complex (MHC) class II molecules on antigen-presenting cells and subsequently stimulate T cells expressing specific Vβ elements on T-cell receptors (9, 15, 24, 29, 35, 42). Staphylococcal enterotoxin B (SEB) and the distantly related toxic shock syndrome toxin 1 are also called superantigens because they induce massive proliferation of T cells (29). In vitro and in vivo studies show that these superantigens induce high levels of various proinflammatory cytokines, and these potent mediators cause lethal shock in animal models (1, 6, 22, 27, 37, 39, 45, 51, 55). SEB also causes food poisoning (4, 21, 52) and is a potential bioterrorism threat agent, as humans are extremely sensitive to this superantigen, especially by inhalation (28). There is currently no effective therapeutic treatment for SEB-induced shock except for the use of intravenous immunoglobulins (11). Various in vitro experiments identified inhibitors to counteract the biological effects of SEB, only some of which were successful in ameliorating SEB-induced shock in experimental models (1, 25-27, 51).
Rapamycin is a relatively new FDA-approved drug used to prevent graft rejection in renal transplantation, as it shows less nephrotoxicity than do calcineurin inhibitors (14, 40, 43, 48). Recent studies reveal other uses in animal models of cancer (23, 34), diabetic nephropathy (36), bleomycin-induced pulmonary fibrosis (31), liver fibrosis (5), and tuberous sclerosis (32). Rapamycin binds intracellularly to FK506-binding proteins, specifically FKBP12; the rapamycin-FKBP12 complex then binds to a distinct molecular target called mammalian target of rapamycin (mTOR) (reviewed in reference 48). Rapamycin inhibits mTOR activity, prevents cyclin-dependent kinase activation, and affects G1-to-S-phase transition (16, 48). Other studies identified mTOR as the conserved serine-threonine kinase for sensing cellular stress, and rapamycin promotes anabolic cellular processes in response to stress signals (20, 47, 50, 54). The mTOR pathway regulates myogenesis (13), cell cycle arrest (20), adipocyte differentiation (3), and insulin signaling (47, 50). The immunological effects of rapamycin include regulation of T-cell activation (48); differentiation, expansion, and preservation of regulatory T cells (2, 10, 19, 46); downregulation of dendritic cells (12, 53); and granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced neutrophil migration (17). Rapamycin impairs dendritic cell maturation and function by inhibiting the expression of adhesion molecule ICAM-1 (12, 53). Thus, rapamycin has a broad spectrum of effects and interferes with the activation of multiple cell types of the immune system.
Based on the potent immunosuppressive effects of rapamycin, we investigated the therapeutic impact of rapamycin on SEB-mediated toxic shock. The therapeutic efficacy of rapamycin in SEB-induced toxic shock was investigated by using a lethal murine model with intranasal delivery of SEB (22). This “double-hit” murine model relies on two low doses of SEB without the use of sensitizing agents such as lipopolysaccharide (LPS) or galactosamine to induce lethal shock (6, 27, 33, 37, 45). In this “SEB-only” toxic shock model, SEB was administered intranasally (i.n.) and another dose of SEB was strategically given intraperitoneally (i.p.) 2 h later to induce systemic cytokine release and pulmonary inflammation with lethality as an endpoint. We examined the effect of rapamycin on proinflammatory cytokines and chemokines induced by SEB in vitro using human peripheral blood mononuclear cells (PBMC) as a first step to test its immunological effects on SEB activation.
MATERIALS AND METHODS
Reagents.
Purified SEB was obtained from Toxin Technology (Sarasota, FL). The endotoxin content of these preparations was <1 ng of endotoxin/mg protein, as determined by the Limulus amoebocyte lysate assay (BioWhittaker, Walkersville, MD). Human cytokine enzyme-linked immunosorbent assay (ELISA) kits and assay reagents were purchased from R&D Systems (Minneapolis, MN). Mouse cytokine ELISA reagents were obtained from Pharmingen (San Diego, CA). Rapamycin and all other reagents were from Sigma (St. Louis, MO).
Cell cultures.
Human PBMC were isolated by Ficoll-Hypaque density gradient centrifugation of heparinized blood from healthy human donors. The PBMC (106 cells/ml) were cultured at 37°C in 24-well plates containing RPMI 1640 medium and 10% heat-inactivated fetal bovine serum. Cells were stimulated with SEB (200 ng/ml) for 16 h, and the supernatants were harvested and analyzed for tumor necrosis factor alpha (TNF-α), interleukin 1β (IL-1β), IL-6, IL-2, gamma interferon (IFN-γ), monocyte chemoattractant protein 1 (MCP-1), macrophage inflammatory protein 1α (MIP-1α), and MIP-1β by ELISA as described previously (25, 26). Rapamycin, when present, was added simultaneously with SEB.
Human T-cell proliferation assays.
PBMC (105 cells/well) were plated in triplicate with SEB (200 ng/ml), with or without various concentrations of rapamycin, for 48 h at 37°C in 96-well microtiter plates. Cells were pulsed with 1 μCi/well of [3H]thymidine (New England Nuclear, Boston, MA) during the last 5 h of culture, as previously described (25). Cells were harvested onto glass fiber filters, and incorporated [3H]thymidine was measured by liquid scintillation. In experiments investigating the effectiveness of rapamycin after a short pulse, PBMC (7 × 105 cells/tube) were cultured with SEB (200 ng/ml), with or without rapamycin, for 3 h at 37°C in 4-ml polypropylene round-bottomed tubes. Cells were centrifuged at 1,200 × g for 10 min, and supernatants were removed at the end of 3 h. Cells (105 cells/well) were recultured with fresh medium without rapamycin and SEB and replated in triplicates for 45 h at 37°C in 96-well microtiter plates. Cells were pulsed with [3H]thymidine and harvested as described above.
Murine model of SEB-induced toxic shock.
Male C3H/HeJ mice (National Cancer Institute, Frederick, MD), weighing ∼20 g each (7 to 10 weeks old), were housed in conventional microisolator cages. Sterile temperature/identification transponders (IPTT-300; Biomedic Data Systems, Maywood, NJ) were implanted subcutaneously into each animal 5 to 10 days before SEB exposure, and temperatures were monitored twice daily. Initial weight of animals was recorded 3 to 7 days before SEB exposure, and weight changes were recorded once daily after SEB challenge. SEB was administered i.n. (50 μl) with a micropipette and i.p. (200 μl) with a tuberculin syringe (26-gauge, 3/8-inch needle). All intranasal doses were administered to mice previously anesthetized with an intramuscularly (i.m.) injected mixture of ketamine (2.4 mg/kg of body weight), acepromazine (0.024 mg/kg), and xylazine (0.27 mg/kg). There were 2 h of elapsed time between the first intranasal dose of 5 μg SEB/mouse and the second i.p. dose of 2 μg SEB/mouse, as this was the optimal time and dose previously determined to cause toxic shock without the use of synergistic agents (22). Mice exposed to both doses of SEB succumbed to death between 96 and 120 h, and lethal endpoints were recorded up to 168 h after the first toxin dose. Controls consisted of C3H/HeJ mice given two doses of either bovine serum albumin (BSA; Sigma Chemical Corp.) or saline 2 h apart, similar to those of SEB-exposed mice (i.e., by i.n. and i.p. routes). For therapeutic investigations, mice (n = 10) were given rapamycin i.n. at specific times after intranasal SEB exposure as described for each series of experiments. Intranasal rapamycin (0.7 mg/kg of body weight) in sterile saline (Sigma Chemical Corp., St. Louis, MO) was administered with a micropipette at the designated time points. This was followed by rapamycin (1.6 mg/kg) in sterile saline administered i.p. with a tuberculin syringe at 24, 48, 72, and 96 h after intranasal SEB administration. A group of mice (n = 10) received delayed treatment with i.n. rapamycin (0.7 mg/kg) at 24 h and subsequent i.p. doses of rapamycin (1.6 mg/kg) at 30, 48, 72, and 96 h. For comparison of effectiveness of therapeutics against SEB, mice (n = 10) were treated with another potent immunosuppressant, the corticosteroid dexamethasone. Intranasal dexamethasone (1.2 mg/kg) in sterile saline was administered at 2 and 5 h followed by i.p. doses of dexamethasone (5 mg/kg) in sterile saline at 24, 48, 72, and 96 h after intranasal SEB administration. Another group of mice (n = 10) received delayed treatment of i.n. dexamethasone at 5 h followed by i.p. doses of dexamethasone at 24, 48, 72, and 96 h after SEB exposure. Animals were monitored twice daily for illness and death for 96 h and as needed. Temperature and weight changes were measured daily up to 96 to 120 h. Temperature data were calculated as the mean temperature reading ± standard deviation (SD) of each group (n = 10 mice per group). The total number of mice dead versus alive was recorded at 168 h. Mice were followed for survival for 2 to 8 weeks.
Animal research was conducted in compliance with the Animal Welfare Act as well as other federal statutes and regulations. The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International. All efforts adhered to principles stated in the Guide for Care and Use of Laboratory Animals (37a).
Previous results indicated the optimal timing for serum cytokine collection after mice were given a dual dose of SEB (22). Sera were collected from anesthetized mice by cardiac puncture at 5 and 24 h after SEB exposure. Rapamycin (0.7 mg/kg) was administered at 2 and 5 h after intranasal SEB administration. Serum cytokine/chemokine levels from individual mice (n = 5 mice per time point), were analyzed for cytokines/chemokines using specific ELISA as described previously (22, 27, 39). Background levels of each cytokine/chemokine, all found to be negligible, were derived from a prebleed of the same mice performed 2 to 4 days before each experiment. For mice receiving delayed treatment with rapamycin at 24 h, whole lungs (n = 5) were excised from euthanized animals at 51 h post-toxin challenge, with and without treatment with rapamycin; weighed; and frozen at −70°C. Immediately before cytokine assays, lungs were thawed and homogenized for 30 min at 4°C in lysis buffer (Cell Signaling Technology, Danvers, MA) to a 7-mg/ml concentration with complete protease inhibitor (Roche Applied Science, Indianapolis, IN). Homogenates were centrifuged at 500 × g for 10 min, and supernatants were harvested and then filtered through a 0.45-μm membrane (Millipore, Bedford, MA). Supernatants from lung homogenates were assayed for MCP-1, IL-2, and IL-6 by ELISA as described above.
Cytokine detection.
The levels of human cytokines (TNF-α, IL-1, IL-6, IL-2, and IFN-γ) and chemokines (MCP-1, MIP-1α, and MIP-1β) in culture supernatants from PBMC or murine mediators (TNF-α, IL-1, IL-6, IL-2, IFN-γ, and MCP-1) in serum were measured via a sandwich ELISA by using cytokine-specific antibodies according to the manufacturer's instructions (25-27, 39). Recombinant cytokines (20 to 1,000 pg/ml) represented the standards for calibration, and the detection limit of all assays was 20 pg/ml.
Determination of rapamycin in murine whole blood.
Blood was collected from rapamycin-treated mice (n = 5) by retro-orbital bleeding into EDTA tubes 1 h after intranasal administration of rapamycin. Blood was centrifuged at 12,000 × g for 3 min to remove cells. Supernatants were removed and stored at −70°C until analysis. Analysis of rapamycin was performed as previously described (18) using an Agilent Technologies series 1100 capillary high-pressure liquid chromatography (HPLC) instrument and an ion-trap mass spectrometer.
Statistical analysis.
The cytokine data were expressed as the mean ± SD and analyzed for significant differences by the Student t test with Stata (Stata Corp., College Station, TX). Statistical comparisons of survival data were performed by Fisher's exact test with Stata software (Stata Corp.). Differences were considered significant if P was <0.05.
RESULTS
Rapamycin inhibits SEB-induced cytokines from human PBMC.
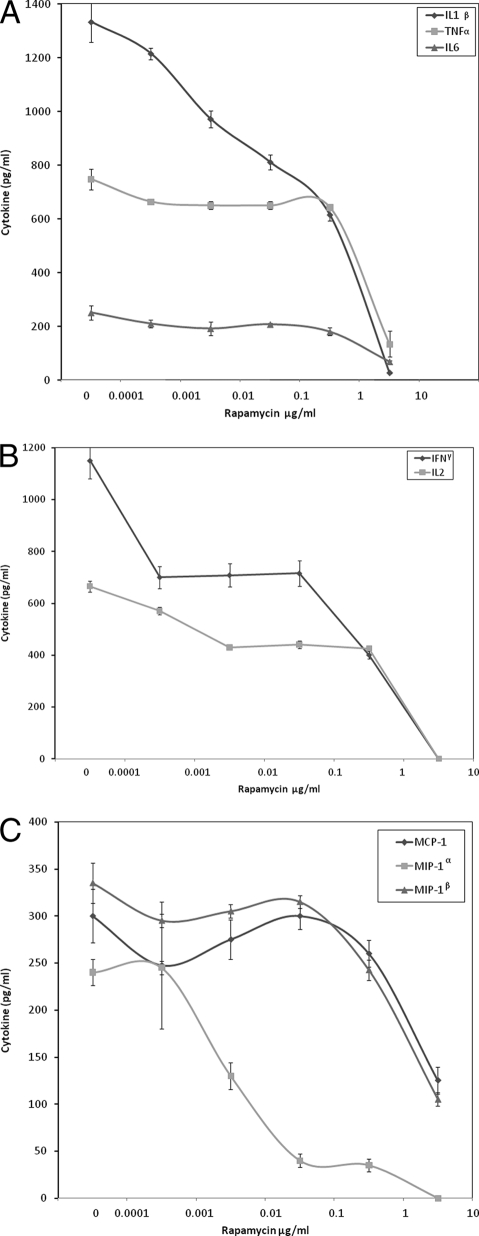
Because proinflammatory cytokines mediate the lethal effects of SEB, the effect of rapamycin on the production of these mediators was examined on human PBMC incubated with SEB. Figure 1 shows that rapamycin effectively blocked in a dose-dependent manner the production of TNF-α, IL-1β, IL-6, IL-2, and IFN-γ from PBMC incubated with SEB, achieving inhibition levels of 77%, 97%, 67%, 100%, and 100%, respectively, at 6.9 μg/ml of rapamycin compared to controls incubated with toxin alone (P < 0.05). The effect of rapamycin was more pronounced on T-cell cytokines, IL-2 and IFN-γ. At 6.9 ng/ml rapamycin, IL-2 and IFN-γ were suppressed by 34% and 36%, respectively, compared to cells stimulated with SEB alone (P < 0.05). Rapamycin also dose dependently reduced the production of chemokines MCP-1, MIP-1α, and MIP-1β from SEB-stimulated PBMC. This indicates that rapamycin inhibited both cytokines and chemokines produced in vitro by both T cells and monocytes in response to SEB.
FIG. 1.
Inhibition of TNF-α, IL-1β, and IL-6 (A); IL-2 and IFN-γ (B); and MCP-1, MIP-1α, and MIP-1β (C) production by human PBMC stimulated with SEB alone or in the presence of various concentrations of rapamycin. Values represent the means ± SDs of duplicate samples from three experiments.
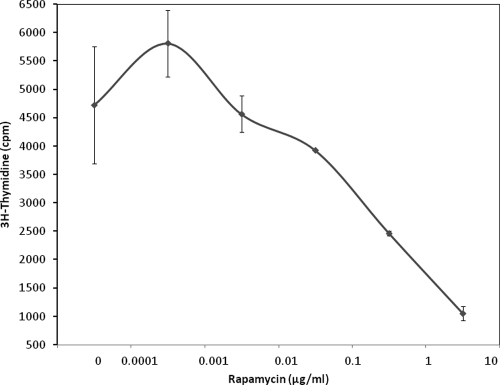
Rapamycin inhibits SEB-induced human T-cell proliferation.
In addition to increasing cytokine levels, SEB also stimulates T-cell proliferation. Therefore, the effect of rapamycin on SEB-induced proliferation of T cells was examined next. The results showed that rapamycin significantly decreased SEB-induced proliferation of T cells in a dose-response manner, with maximal inhibition (94%) achieved at the same concentration (6.9 μg/ml) that was most effective at blocking cytokine and chemokine release (Fig. 2). At 0.69 μg/ml rapamycin, T-cell proliferation was reduced by 56%. The lack of proliferation or cytokine release from PBMC was not due to the cytotoxic effect of rapamycin, as determined by trypan blue exclusion test (data not shown).
FIG. 2.
Inhibition of T-cell proliferation in PBMC stimulated with SEB alone or in the presence of various concentrations of rapamycin. Values are the means ± SDs of triplicate cultures and represent three experiments.
We also investigated whether a short pulse of rapamycin was sufficient to block SEB-induced T-cell proliferation. When rapamycin was removed after 3 h of incubation, T-cell proliferation was decreased by 40% with 6.9 μg/ml of rapamycin. Thus, partial inhibition of proliferation was obtained when rapamycin was present for a short period of 3 h.
Rapamycin protects mice from SEB-mediated shock.
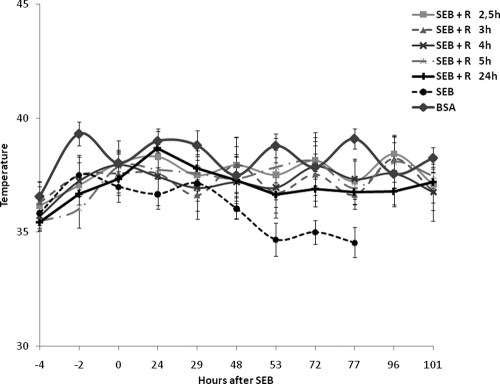
Since rapamycin was effective in attenuating the biological effects of SEB in vitro, we examined its efficacy in vivo. Table 1 shows that rapamycin significantly increased the survival rate among mice given two doses of SEB. We progressively increased the time between the exposure of mice to SEB and rapamycin treatment and found that mice were protected 100%, even when rapamycin was given 5 h after the SEB exposure (Table 1). Clinical signs of intoxication such as ruffled fur and lethargy observed with SEB-treated mice starting at 72 h were completely absent from the SEB-plus-rapamycin group. Additional data were collected regarding temperature fluctuations in mice treated with SEB and those treated with SEB plus rapamycin given at various times after SEB exposure. Mice given SEB experienced hypothermia starting at 48 h (Fig. 3). This hypothermic response, indicating systemic shock that mimicked those found in other murine models, was completely absent in rapamycin-treated, SEB-exposed mice. Rapamycin protected mice from systemic shock even when it was administered 5 h after SEB exposure, as seen from the normal temperature of rapamycin-treated animals. When rapamycin treatment was delayed further, all mice (n = 10) survived if rapamycin was administered i.n. at 24 h, followed by i.p. doses at 30, 48, 72, and 96 h. These mice also maintained normal body temperature. However, delaying treatment with rapamycin to 32 h resulted in only 20% protection.
TABLE 1.
Protective effects of rapamycin in vivo
| Toxin | % Alivea |
||||
|---|---|---|---|---|---|
| No drug | Drug given at time: |
||||
| 2 h and 5 h | 3 h | 4 h | 5 h | ||
| SEB | 0 | ||||
| SEB + rapamycin | 100 | 100 | 100 | 100 | |
| SEB + dexamethasone | 100 | ND | ND | 10 | |
The percentage of mice alive 168 h after intranasal SEB exposure. Rapamycin (0.7 mg/kg) was given i.n. at the designated time points after SEB exposure. All rapamycin treatment groups (n = 10) also received rapamycin (1.6 mg/kg) i.p. 24, 48, 72, and 96 h after SEB exposure. Dexamethasone (1.2 mg/kg) was given i.n. at the designated time points after SEB exposure. All dexamethasone-treated groups (n = 10) also received dexamethasone (5 mg/kg) i.p. 24, 48, 72, and 96 h after SEB exposure. There were no lethal effects among mice given PBS or BSA. ND, not determined.
FIG. 3.
Rapamycin attenuated the hypothermic response of C3H/HeJ mice treated with SEB. Body temperatures of mice exposed to BSA, SEB, and SEB plus rapamycin (0.7 mg/kg i.n.) at different time points after SEB exposure. Rapamycin (1.6 mg/kg) was administered i.p. to all mice at 24, 48, 72, and 96 h. Mice receiving rapamycin treatment delayed until 24 h also received i.p. doses of rapamycin (1.6 mg/kg) at 30, 48, 72, and 96 h following i.n. rapamycin (0.7 mg/kg) at 24 h. Points represent the means ± SDs for each group (n = 10).
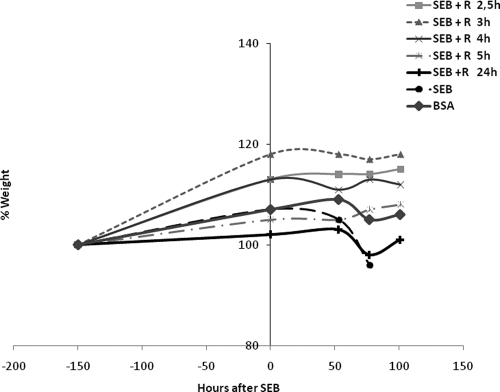
Weight loss is another prominent indicator of SEB-induced shock in other animal models of superantigen-induced disease (6, 33). We also examined the effect of rapamycin on the weight of animals after intranasal delivery of SEB. SEB-exposed mice experienced weight loss of 5% at 77 h whereas rapamycin-treated, SEB-exposed mice had weight gains of 8 to 17% over several days (Fig. 4). Rapamycin treatment ameliorated SEB-induced weight loss even when given 24 h after SEB exposure. Protection against temperature and weight fluctuations essentially paralleled the lethality results.
FIG. 4.
Rapamycin prevented weight loss in a murine SEB-mediated shock model. Weights of mice exposed to SEB or SEB plus rapamycin (0.7 mg/kg i.n.) at different time points after SEB exposure. Rapamycin (1.6 mg/kg) was administered i.p. to all mice at 24, 48, 72, and 96 h. Mice receiving rapamycin treatment delayed until 24 h also received i.p. doses of rapamycin (1.6 mg/kg) at 30, 48, 72, and 96 h following i.n. rapamycin (0.7 mg/kg) at 24 h. Points represent the means ± SDs for each group (n = 10).
The blood concentration of rapamycin in mice after oral dosing was determined by mass spectrophotometry to establish the in vivo concentration for effective treatment as previously described (18). The blood level of rapamycin 1 h after an i.n. dose of 0.7 mg/kg was 492 ± 204 ng/ml. At 97 h after multiple dosing (i.n. doses of 0.7 mg/kg at 2 and 5 h followed by i.p. doses of 1.6 mg/kg at 30, 48, 72, and 96 h), rapamycin reached 622 ± 282 ng/ml. The latter concentration likely represented the highest concentration of rapamycin achieved in this dosing regimen, as the concentration of rapamycin reportedly peaked 1 h after dosing (49). In contrast, the blood concentration of rapamycin at 97 h dropped to 38 ± 15 ng/ml after the use of lower doses of rapamycin (0.03 mg/kg i.n. at 2 and 5 h and 0.08 mg/kg i.p. at 24, 48, 72, and 96 h) which were totally unprotective against SEB-mediated shock.
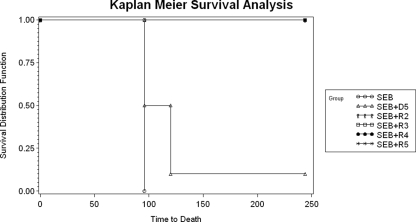
We also compared the efficacy of rapamycin against that of a potent immunosuppressant, the corticosteroid dexamethasone. Dexamethasone was 100% effective in preventing lethality when mice (n = 10) were treated with i.n. dexamethasone at 2 and 5 h, followed by daily i.p. doses at 24, 48, 72, and 96 h (Table 1). However, delaying treatment with dexamethasone until 5 h after SEB exposure resulted in 90% lethality. Thus, rapamycin is a more effective therapeutic post-SEB exposure than dexamethasone in vivo (Fig. 5).
FIG. 5.
Survival analysis of mice treated with SEB (i), SEB plus dexamethasone starting at 5 h (D5) post-SEB exposure (ii), SEB plus rapamycin starting at 2 h (R2) post-SEB exposure (iii), SEB plus rapamycin starting at 3 h (R3) post-SEB exposure (iv), SEB plus rapamycin starting at 4 h (R4) post-SEB exposure (v), and SEB plus rapamycin starting at 5 h (R5) post-SEB exposure (vii). Time to death is in hours after SEB exposure.
Rapamycin attenuates serum levels of IL-6 and MCP-1 in vivo.
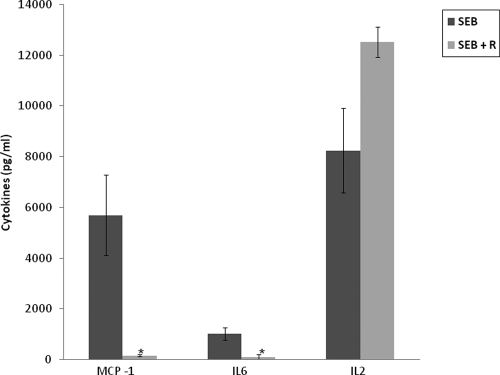
Based upon the strong inhibitory effects of rapamycin on SEB-mediated T-cell proliferation and cytokine production in vitro, the potential therapeutic role of rapamycin in vivo was further investigated in mice. Previous studies showed that elevated serum levels of MCP-1, IL-6, and IL-2 are a prominent feature of SEB-mediated toxic shock with intranasal delivery of SEB. Therefore, the in vivo effect of rapamycin on serum cytokine concentrations was examined in mice after SEB administration. Peak levels of MCP-1 and IL-6 were reduced by 90% and 80%, respectively, in SEB-plus-rapamycin-treated mice versus SEB controls without rapamycin (Fig. 6). Serum concentrations of IL-2 remained unexplainably high in contrast to the in vitro inhibitory effect of rapamycin on IL-2 in SEB-stimulated human PBMC. We further examined serum IL-2 concentrations at later time points. There was no IL-2 present at 24, 51, and 75 h in both SEB- and SEB-plus-rapamycin-treated mice (data not shown). Thus, the elevated serum IL-2 at 5 h was transient with rapamycin treatment and did not affect lethality or the overall well-being of animals based on temperature and weight changes.
FIG. 6.
Peak serum levels of MCP-1, IL-6, and IL-2 in mice (n = 5) treated with SEB alone or SEB plus rapamycin (0.7 mg/kg i.n. at 2 h). Values represent the means ± SDs of duplicate samples. The asterisk indicates P < 0.05 compared with mice treated with SEB.
Since rapamycin was also effective when administered i.n. at 24 h after SEB exposure, lungs were excised at 51 h post-SEB challenge for evaluation of the effect of rapamycin on lung cytokines. Lung MCP-1, IL-2, and IL-6 were attenuated by 56%, 80%, and 65%, respectively, in mice treated with rapamycin compared to the SEB-exposed group not treated with rapamycin.
DISCUSSION
The present study demonstrates for the first time that rapamycin effectively inhibited SEB-mediated production of TNF-α, IL-1β, IL-6, IL-2, IFN-γ, MCP-1, MIP-1α, and MIP-1β by human PBMC in vitro. Besides decreasing the levels of proinflammatory cytokines in vitro, rapamycin also completely blocked SEB-induced proliferation of T cells. T-cell cytokines, IL-2 and IFN-γ, were more sensitive to low concentrations (ng/ml) of rapamycin. As excessive release of cytokines mediates the pathogenic effects of SEB in vivo, the use of rapamycin, an immunosuppressant, was a seemingly logical choice for our studies in vivo. Our study is the first to demonstrate that rapamycin was 100% effective in protecting mice from SEB-mediated shock. Previous studies of drug treatment for SEB-mediated shock models indicate a very narrow therapeutic window of treatment with different types of inhibitors to reduce the biological effects of SEB (1, 6, 27, 51). As demonstrated in this study, the potent immunosuppressant dexamethasone was not protective when administered 5 h after SEB exposure but was protective when given at 2 h post-exposure to SEB. In a lipopolysaccharide (LPS)-synergized murine model of SEB-induced shock, “protective” peptides derived from conservative regions of SEB, even when given 1 h before SEB exposure, resulted in 83% protection (51). Our in vivo studies here indicate a wider therapeutic window for rapamycin and show that rapamycin, even when given 24 h after SEB exposure, still afforded 100% protection against mortality and temperature and weight fluctuations. Serum levels of MCP-1 and IL-6 were also markedly reduced in mice treated with rapamycin compared to control SEB-exposed mice. These results demonstrated the potency of rapamycin even when given post-SEB exposure. Clearly, the serum cytokine, temperature, and weight data revealed the protective effects of rapamycin after SEB exposure. Although we could not explain the high levels of IL-2 in vivo with rapamycin treatment, we found that this effect was transient and did not affect survival. Rapamycin given as late as 24 h also reduced cytokines and chemokines in lung tissues. Other effects of rapamycin on cells of the immune system and mTOR pathway, not explored fully in this study, likely contributed to its potency.
The rapamycin doses used in vivo in this study were in the same range as those used previously to reduce murine adjuvant arthritis and carcinogen-induced lung tumors (7, 18). Peak blood concentrations of rapamycin also agreed with those used in murine models (18). In healthy subjects, blood concentrations of rapamycin reached 78 ± 18 ng/ml 1 h after administration of 15 mg of rapamycin (49). Rapamycin is used for extended periods of years to prevent graft rejection in renal transplantation. In these patients, long-term accumulative toxic effects must be taken into consideration. The doses of rapamycin in the present study were similar to those used in animal models of disease (7, 18), and the short therapeutic course of treatment described in this study suggests that rapamycin can be considered a good candidate for transition to clinical trials to treat SEB-induced shock. Obviously, different doses have to be tried and adjusted to limit toxicity in humans. Given the small dose discrepancy of rapamycin in previous mouse models of disease and similar disease in humans, lower doses of drug would likely be effective in treating SEB-induced shock in humans.
The pathophysiology and treatment of shock with Staphylococcus aureus-secreting superantigens such as SEB and toxic shock syndrome toxin 1 are much more complex. Further studies are needed for animal model development to establish in vivo models that can separate effects of bacterial infection and toxic effects of many bacterial virulence factors, including superantigens. With the continuing rise of vancomycin- and methicillin-resistant S. aureus strains, there is an urgent need for new antibacterials against drug-resistant microorganisms. A logical extension of this study is to apply rapamycin in an S. aureus infection model with bacteria producing SEB and other superantigens.
In conclusion, our in vivo studies showed that the beneficial effects of rapamycin in SEB-induced shock included increased survival, minimal temperature and weight change, and a dramatic reduction in the serum levels of MCP-1 and IL-6. These findings are significant because rapamycin can be given as late as 24 h after intranasal SEB exposure. Previous studies indicate a good correlation between superantigen toxicity in mice and elevated levels of proinflammatory cytokines (6, 22, 33, 39, 45). The promising findings of this study make rapamycin a potential agent to mitigate SEB-induced shock in humans hours after SEB exposure via the respiratory route. Because rapamycin has been safely administered to critically ill transplant patients without significant toxicity even after 2 years of use, it might easily be transitioned for use against SEB-induced shock in humans.
Acknowledgments
The views expressed in this publication are those of the author and do not reflect the official policy or position of the Department of the Army, the Department of Defense, or the U.S. Government.
The source of support was the Defense Threat Reduction Agency.
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Arad, G., R. Levy, D. Hillman, and R. Kaempfer. 2000. Superantigen antagonist protects against lethal shock and defines a new domain for T-cell activation. Nat. Med. 6:414-421. [DOI] [PubMed] [Google Scholar]
- 2.Battaglia, M., A. Stabilini, and M. G. Roncarolo. 2005. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood 105:4743-4748. [DOI] [PubMed] [Google Scholar]
- 3.Bell, A., L. Grunder, and A. Sorisky. 2000. Rapamycin inhibits human adipocyte differentiation in primary culture. Obes. Res. 8:249-254. [DOI] [PubMed] [Google Scholar]
- 4.Bergdoll, M. S. 1988. Monkey feeding test for staphylococcal enterotoxin. Methods Enzymol. 165:324-333. [DOI] [PubMed] [Google Scholar]
- 5.Biecker, E., A. DeGottardi, M. Neef, M. Unternahrer, V. Schneider, M. Ledermann, H. Sagesser, S. Shaw, and R. Reichen. 2005. Long-term treatment of bile duct-ligated rats with rapamycin (sirolimus) significantly attenuates liver fibrosis: analysis of the underlying mechanisms. J. Pharmacol. Exp. Ther. 313:952-961. [DOI] [PubMed] [Google Scholar]
- 6.Blank, C., A. Luz, S. Bendigs, A. Erdmann, H. Wagner, and K. Heeg. 1997. Superantigen and endotoxin synergize in the induction of lethal shock. Eur. J. Immunol. 27:825-833. [DOI] [PubMed] [Google Scholar]
- 7.Blazar, B., R. P. A. Taylor, S. N. Sehgal, and D. A. Vallera. 1994. Rapamycin, a potent inhibitor of T cell function, prevents graft rejection in murine recipients of allogeneic T-cell depended donor marrow. Blood 83:600-609. [PubMed] [Google Scholar]
- 8.Chesney, P. J., J. P. Davis, W. K. Purday, P. J. Wand, and R. W. Chesney. 1981. Clinical manifestations of toxic shock syndrome. JAMA 246:741-748. [PubMed] [Google Scholar]
- 9.Choi, Y., B. Kotzin, L. Hernon, J. Callahan, P. Marrack, and J. Kappler. 1989. Interaction of Staphyloccocus aureus toxin “superantigens” with human T cells. Proc. Natl. Acad. Sci. U. S. A. 86:8941-8945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coenen, J. J., H. J. Koenen, E. van Rijssen, L. B. Hilbrands, and I. Joosten. 2006. Rapamycin, and not cyclosporin A, preserves the highly suppressive CD27+ subset of human CD4+CD25+ regulatory T cells. Blood 107:1018-1023. [DOI] [PubMed] [Google Scholar]
- 11.Darenberg, J., B. Soderquist, B. H. Normark, and A. A. Norrby-Tegland. 2004. Differences in potency of intravenous polyspecific immunoglobulin G against streptococcal and staphylococcal superantigen: implications for therapy of toxic shock syndrome. Clin. Infect. Dis. 38:836-842. [DOI] [PubMed] [Google Scholar]
- 12.Das, S., A. Haddadi, S. Veniamin, and J. Samuel. 2008. Delivery of rapamycin-loaded nanoparticle down regulates ICAM-1 expression and maintains an immunosuppressive profile in human CD34+ progenitor-derived dendritic cells. J. Biomed. Mater. Res. A 85:983-992. [DOI] [PubMed] [Google Scholar]
- 13.Erbay, E., and J. Chen. 2001. The mammalian target of rapamycin regulates C2C12 myogenesis via a kinase-independent mechanism. J. Biol. Chem. 276:36079-36082. [DOI] [PubMed] [Google Scholar]
- 14.Flechner, S. M. 2009. Sirolimus in kidney transplantation indications and practical guidelines: de novo sirolimus-based therapy without calcineurin inhibitors. Transplantation 87:S1-S6. [DOI] [PubMed] [Google Scholar]
- 15.Fleischer, B., H. Schrezenmeier, and P. Conradt. 1989. T lymphocyte activation by staphylococcal enterotoxins: role of class II molecules and T cell surface structures. Cell. Immunol. 120:92-101. [DOI] [PubMed] [Google Scholar]
- 16.Frost, P., Y. Shi, B. Hoang, J. Gera, and A. Lichtenstein. 2009. Regulation of D-cyclin translation inhibition in myeloma cells treated with mammalian target of rapamycin inhibitors: rationale for combined treatment with extracellular signal-regulated kinase inhibitors and rapamycin. Mol. Cancer Ther. 8:83-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gomez-Cambronero, J. 2003. Rapamycin inhibits GM-CSF-induced neutrophil migration. FEBS Lett. 550:94-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Granville, C. A., N. Warfel, J. Tsurutani, and M. C. Hollander, et. al. 2007. Identification of a highly effective rapamycin schedule that markedly reduces the size, multiplicity, and phenotypic progression of tobacco carcinogen-induced murine lung tumors. Clin. Cancer Res. 13:2281-2289. [DOI] [PubMed] [Google Scholar]
- 19.Haxhinasto, S., D. Mathis, and C. Benoist. 2008. The AKT-mTOR axis regulates de novo differentiation of CD4+Foxp3+ cells. J. Exp. Med. 205:565-574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heitman, J., N. R. Movva, and M. N. Hall. 1991. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 253:905-909. [DOI] [PubMed] [Google Scholar]
- 21.Holmberg, S. D., and P. A. Blake. 1984. Staphylococcal food poisoning in the United States. New facts and old misconceptions. JAMA 251:487-489. [PubMed] [Google Scholar]
- 22.Huzella, L. M., M. J. Buckley, D. A. Alves, B. G. Stiles, and T. Krakauer. 2009. Central roles for IL-2 and MCP-1 following intranasal exposure to SEB: a new mouse model. Res. Vet. Sci. 86:241-247. [DOI] [PubMed] [Google Scholar]
- 23.Koehl, G. E., J. Andrassy, M. Guba, S. Richter, A. Kroemer, M. N. Scherer, M. Steinbauer, C. Graeb, H. J. Schlitt, K.-W. Jauch, and E. K. Geissler. 2004. Rapamycin protects allografts from rejection while simultaneously attacking tumors in immunosuppressed mice. Transplantation 77:1319-1326. [DOI] [PubMed] [Google Scholar]
- 24.Kotzin, B. L., D. Y. M. Leung, J. Kappler, and P. A. Marrack. 1993. Superantigens and their potential role in human disease. Adv. Immunol. 54:99-166. [DOI] [PubMed] [Google Scholar]
- 25.Krakauer, T. 1995. Inhibition of toxic shock syndrome toxin-induced cytokine production and T cell activation by interleukin 10, interleukin 4, and dexamethasone. J. Infect. Dis. 172:988-992. [DOI] [PubMed] [Google Scholar]
- 26.Krakauer, T. 1999. The induction of CC chemokines in human peripheral blood mononuclear cells by staphylococcal exotoxins and its prevention by pentoxifylline. J. Leukoc. Biol. 66:158-164. [DOI] [PubMed] [Google Scholar]
- 27.Krakauer, T., and M. Buckley. 2006. Dexamethasone attenuates staphylococcal enterotoxin B-induced hypothermic response and protects mice from superantigen-induced toxic shock. Antimicrob. Agents Chemother. 50:391-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madsen, J. M. 2001. Toxins as weapons of mass destruction. A comparison and contrast with biological-warfare and chemical-warfare agents. Clin. Lab. Med. 21:593-605. [PubMed] [Google Scholar]
- 29.Marrack, P., and J. Kappler. 1990. The staphylococcal enterotoxins and their relatives. Science 248:705-709. [DOI] [PubMed] [Google Scholar]
- 30.McCormick, J. K., J. M. Yarwood, and P. M. Schlievert. 2001. Toxic shock syndrome and bacterial superantigens: an update. Annu. Rev. Microbiol. 55:77-104. [DOI] [PubMed] [Google Scholar]
- 31.Mehrad, B., M. D. Burdick, and R. M. Strieter. 2009. Fibrocyte CXCR4 regulation as a therapeutic target in pulmonary fibrosis. Int. J. Biochem. Cell Biol. 41:1708-1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meikle, L., K. Pollizzi, A. Egnor, I. Kramvis, H. Lane, M. Sahin, and D. J. Kwiatkowski. 2008. Response of a neuronal model of tuberous sclerosis to mammalian target of rapamycin (mTOR) inhibitors: effects on mTORC1 and Akt signaling lead to improved survival and function. J. Neurosci. 28:5422-5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miethke, T., C. Wahl, K. Heeg, B. Echtenacher, P. H. Krammer, and H. Wagner. 1992. T cell-mediated lethal shock triggered in mice by the superantigen SEB: critical role of TNF. J. Exp. Med. 175:91-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mita, M. M., A. Mita, and E. K. Rowinsky. 2003. The molecular target of rapamycin (mTOR) as a therapeutic target against cancer. Cancer Biol. Ther. 2:S169-S177. [PubMed] [Google Scholar]
- 35.Mollick, J. A., M. Chintagumpala, R. G. Cook, and R. R. Rich. 1991. Staphylococcal exotoxin activation of T cells. Role of exotoxin-MHC class II binding affinity and class II isotype. J. Immunol. 146:463-468. [PubMed] [Google Scholar]
- 36.Mori, H., K. Inoki, K. Masutani, Y. Wakabayashi, K. Komai, R. Nakagawa, K. L. Guan, and A. Yoshimura. 2009. The mTOR pathway is highly activated in diabetic nephropathy and rapamycin has a strong therapeutic potential. Biochem. Biophy. Res. Commun. 384:471-475. [DOI] [PubMed] [Google Scholar]
- 37.Nagaki, M., Y. Muto, H. Ohnishi, S. Yasuda, K. Sano, T. Naito, T. Maeda, T. Yamada, and H. Moriwaki. 1994. Hepatic injury and lethal shock in galactosamine-sensitized mice induced by the superantigen staphylococcal enterotoxin B. Gastroenterology 106:450-458. [DOI] [PubMed] [Google Scholar]
- 37a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 38.Proft, T., and J. D. Fraser. 2003. Bacterial superantigens. Clin. Exp. Immunol. 133:299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roy, C. J., K. L. Warfield, B. C. Welcher, R. F. Gonzales, T. Larsen, J. Hanson, C. S. David, T. Krakauer, and S. Bavari. 2005. Human leukocyte antigen-DQ8 transgenic mice: a model to examine the toxicity of aerosolized staphylococcal enterotoxin B. Infect. Immun. 73:2452-2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saunders, R. N., M. S. Metcalfe, and M. L. Nicholson. 2001. Rapamycin in transplantation: a review of the evidence. Kidney Int. 59:3-16. [DOI] [PubMed] [Google Scholar]
- 41.Schlievert, P. M. 1993. Role of superantigens in human disease. J. Infect. Dis. 167:997-1002. [DOI] [PubMed] [Google Scholar]
- 42.Scholl, P., A. Diez, W. Mourad, J. Parsonnet, R. S. Geha, and T. Chatila. 1989. Toxic shock syndrome toxin-1 binds to major histocompatibility complex class II molecules. Proc. Natl. Acad. Sci. U. S. A. 86:4210-4214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sehgal, S. N. 2003. Sirolimus: its discovery, biological properties, and mechanism of action. Transplant. Proc. 35:7S-14S. [DOI] [PubMed] [Google Scholar]
- 44.Stevens, D. L. 1996. The toxic shock syndromes. Infect. Dis. Clin. North Am. 10:727-746. [DOI] [PubMed] [Google Scholar]
- 45.Stiles, B. G., S. Bavari, T. Krakauer, and R. G. Ulrich. 1993. Toxicity of staphylococcal enterotoxins potentiated by lipopolysaccharide: major histocompatibility complex class II molecule dependency and cytokine release. Infect. Immun. 61:5333-5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Strauss, L., T. L. Whiteside, A. Knights, C. Bergmann, A. Knuth, and A. Zippelius. 2007. Selective survival of naturally occurring human CD4+CD25+Foxp3+ regulatory T cells cultured with rapamycin. J. Immunol. 178:320-329. [DOI] [PubMed] [Google Scholar]
- 47.Takano, A., I. Usui, T. Haruta, J. Kawahara, T. Uno, M. Iwata, and M. Kobayashi. 2001. Mammalian target of rapamycin pathway regulates insulin signaling via subcellular redistribution of insulin receptor substrate 1 and integrates nutritional signals and metabolic signals of insulin. Mol. Cell. Biol. 21:5050-5062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomson, A. W., H. R. Turnquist, and G. Raimondi. 2009. Immunoregulatory functions of mTOR inhibition. Nat. Rev. Immunol. 9:324-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thomson PDR. 2005. Physicians' desk reference, 59th ed., p. 3395-3402. Thomson PDR, Montvale, NJ.
- 50.Tremblay, F., A. Gagnon, A. Veilleux, A. Sorisky, and A. Marette. 2005. Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes. Endocrinology 146:1328-1337. [DOI] [PubMed] [Google Scholar]
- 51.Visvanathan, K., A. Charles, J. Bannan, P. Pugach, K. Kashfi, and J. B. Zabriskie. 2001. Inhibition of bacterial superantigens by peptides and antibodies. Infect. Immun. 69:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wieneke, A., D. Roberts, and R. J. Gilbert. 1993. Staphylococcal food poisoning in the United Kingdom, 1969-1990. Epidemiol. Infect. 110:519-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Woltman, A. M., S. W. van der Kooij, P. J. Coffer, R. Offringa, M. R. Daha, and C. van Kooten. 2003. Rapamycin specifically interferes with GM-CSF signaling in human dendritic cells, leading to apoptosis via increased p27KIP1 expression. Blood 101:1439-1445. [DOI] [PubMed] [Google Scholar]
- 54.Wullschleger, S., R. Loewith, and M. N. Hall. 2006. TOR signaling in growth and metabolism. Cell 124:471-484. [DOI] [PubMed] [Google Scholar]
- 55.Yeung, R. S., J. M. Penninger, T. Kundig, W. Khoo, P. S. Ohashi, G. Kroemer, and T. W. Mak. 1996. Human CD4 and human major histocompatibility complex class II (DQ6) transgenic mice: supersensitivity to superantigen-induced septic shock. Eur. J. Immunol. 26:1074-1082. [DOI] [PubMed] [Google Scholar]