Abstract
The in vitro antibacterial activities of nemonoxacin (TG-873870), a novel nonfluorinated quinolone, against 770 clinical isolates were investigated. Nemonoxacin (tested as its malate salt, TG-875649) showed better in vitro activity than ciprofloxacin and levofloxacin against different species of staphylococci, streptococci, and enterococci, Neisseria gonorrhoeae, and Haemophilus influenzae. The in vitro activity of TG-875649 was also comparable to or better than that of moxifloxacin against these pathogens, which included ciprofloxacin-resistant, methicillin-resistant Staphylococcus aureus and levofloxacin-resistant Streptococcus pneumoniae.
Antimicrobial resistance is a global public health threat (6, 7). In Taiwan, multidrug and fluoroquinolone resistances are common in both Gram-negative and Gram-positive pathogens from inpatients as well as outpatients (3, 4, 8-10). For example, as many as 80% of nosocomial Staphylococcus aureus strains in Taiwan are methicillin-resistant S. aureus (MRSA) strains, of which 80% are fluoroquinolone resistant (9). Based on a national surveillance program of >1,200 pneumococcal isolates from recent years, the levofloxacin resistance level was 10% among non-penicillin-susceptible strains (isolates with penicillin MIC > 2 μg/ml) (3).
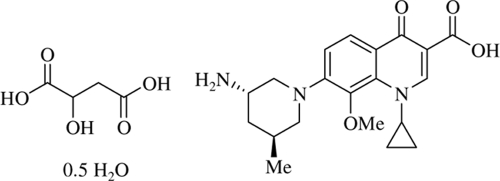
Development of new antibiotics is one of the means of combating multidrug-resistant bacteria (6). Nemonoxacin (TG-873870) is a novel nonfluorinated quinolone, and TG-875649 is a malate salt of nemonoxacin. The chemical structure of TG-875649 is shown in Fig. 1. The present study examined the in vitro antibacterial activity of TG-875649 against a spectrum of Gram-positive and Gram-negative clinical isolates in Taiwan.
FIG. 1.
Chemical structure of TG-875649, a malate salt of nemonoxacin (TG-873870). Me, methyl.
(This study was presented in part at the 47th Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 17 to 20 September 2007 [5].)
Nonduplicate bacterial strains were selected from a pool of clinical isolates previously studied under a national surveillance program in Taiwan (4, 9). Isolates were selected to include those with known resistance to different classes of antibiotics in addition to fluoroquinolones (levofloxacin and/or ciprofloxacin); thus, multidrug-resistant bacteria comprised a larger proportion than the wild type. A total of 770 isolates were tested, among which 688 (89.4%) were isolated in the year 2004, with the remainder isolated from 1998 to 2002. MICs were determined by the broth microdilution (BMD) method, using custom-made 96-well microtiter panels containing different antimicrobial agents at various concentrations (Trek Diagnostics, West Essex, England). The test procedure followed the instructions of the MIC panel manufacturer and guidelines of the Clinical and Laboratory Standards Institute (CLSI) (1, 2). For most species, 50 μl of the 0.5 McFarland standard organism suspension was transferred to 10 ml of cation-adjusted Mueller-Hinton broth (CAMHB) to obtain a final inoculum of 5 × 105 CFU/ml at 100 μl/well. For Proteus mirabilis, a 1 × 105-CFU/ml inoculum was used to avoid an inoculum effect. For fastidious organisms, 100 μl of the 0.5 McFarland standard suspension was transferred to 10 ml of MHB containing 3% lysed horse blood for Neisseria gonorrhoeae and streptococci and to 10 ml of Haemophilus Test Medium (HTM) broth for Haemophilus influenzae. All MIC plates were incubated in 35°C ambient air overnight except those for N. gonorrhoeae, which were incubated in 5% CO2. Appropriate quality control strains were included during each test run. The CAMHB and HTM were purchased from Trek Diagnostics. All other media were purchased from BBL (Becton Dickinson Microbiology System, Cockeysville, MD).
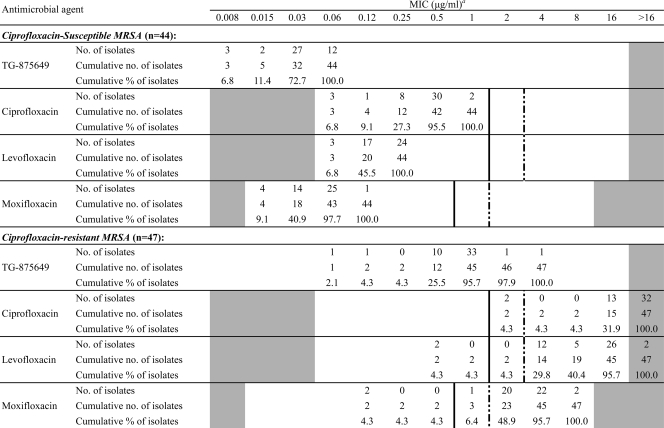
Table 1 lists the antibacterial activities (MIC range, MIC50, and MIC90) of TG-875649 against various species of Gram-positive and Gram-negative pathogens in comparison with those of 3 fluoroquinolones (FQs) (ciprofloxacin, levofloxacin, and moxifloxacin) and nonquinolone agents. TG-875649 was more active than ciprofloxacin and levofloxacin against all staphylococcal isolates, including 150 S. aureus, with 4- to >32-fold lower MIC90s. In addition, against ciprofloxacin-resistant (CIPr) MRSA, the MIC90 of TG-875649 (1 μg/ml) was 4-fold lower than that of moxifloxacin. Of the 47 CIPr MRSA isolates, only 2 (4.3%) and 3 (6.4%) isolates had levofloxacin and moxifloxacin MICs of ≤1 μg/ml, respectively (Table 2), while the majority (45 isolates; 95.7%) had TG-875649 MICs of ≤1 μg/ml (Table 2). Among the 50 enterococci tested, 17 (7 Enterococcus faecalis strains and 10 E. faecium strains) were vancomycin resistant. The MIC90s of TG-875649 were at least 2-fold lower than those of the 3 FQs for both E. faecalis and E. faecium.
TABLE 1.
Antibacterial activities of TG-875649, a malate salt of nemonoxacin (TG-873870), and reference compounds against Gram-positive and Gram-negative bacteria
| Organism (n) and compound | MIC (μg/ml) |
||
|---|---|---|---|
| Range | 50% | 90% | |
| Staphylococcus aureus | |||
| Methicillin susceptible (59) | |||
| TG-875649 | 0.015-1 | 0.03 | 0.12 |
| Ciprofloxacin | 0.12->16 | 0.5 | 2 |
| Levofloxacin | ≤0.06-16 | 0.25 | 1 |
| Moxifloxacin | ≤0.015-4 | 0.06 | 0.12 |
| Ceftriaxone | 2-8 | 4 | 4 |
| Oxacillin | ≤0.25-2 | 0.5 | 1 |
| Linezolid | 0.5-2 | 2 | 2 |
| Tigecycline | 0.06-0.25 | 0.12 | 0.25 |
| Vancomycin | 0.5-2 | 1 | 1 |
| Methicillin resistant, ciprofloxacin | |||
| susceptible (44) | |||
| TG-875649 | ≤0.008-0.06 | 0.03 | 0.06 |
| Ciprofloxacin | ≤0.06-1 | 0.5 | 0.5 |
| Levofloxacin | ≤0.06-0.25 | 0.25 | 0.25 |
| Moxifloxacin | ≤0.015-0.12 | 0.06 | 0.06 |
| Ceftriaxone | 8->8 | >8 | >8 |
| Oxacillin | 4->8 | >8 | >8 |
| Linezolid | 2 | 2 | 2 |
| Tigecycline | 0.06-0.5 | 0.25 | 0.25 |
| Vancomycin | 1-2 | 1 | 1 |
| Methicillin resistant, ciprofloxacin | |||
| resistant (47) | |||
| TG-875649 | 0.06-4 | 1 | 1 |
| Ciprofloxacin | 2->16 | >16 | >16 |
| Levofloxacin | 0.5->16 | 16 | 16 |
| Moxifloxacin | 0.12-8 | 4 | 4 |
| Ceftriaxone | 8->8 | >8 | >8 |
| Oxacillin | >8 | >8 | >8 |
| Linezolid | 1-2 | 2 | 2 |
| Tigecycline | 0.12-0.5 | 0.25 | 0.5 |
| Vancomycin | 1-2 | 2 | 2 |
| Coagulase negative staphylococci | |||
| Methicillin resistant (68)a | |||
| TG-875649 | 0.03-8 | 0.12 | 0.5 |
| Ciprofloxacin | 0.12->16 | 0.5 | >16 |
| Levofloxacin | 0.12->16 | 0.5 | 8 |
| Moxifloxacin | 0.03->8 | 0.12 | 2 |
| Ceftriaxone | 4->8 | >8 | >8 |
| Oxacillin | 0.5->8 | >8 | >8 |
| Linezolid | ≤0.25-4 | 1 | 2 |
| Tigecycline | 0.06-0.5 | 0.25 | 0.5 |
| Vancomycin | 0.25-4 | 2 | 2 |
| Enterococcus faecalis (31)b | |||
| TG-875649 | 0.12-4 | 0.25 | 4 |
| Ciprofloxacin | 1->16 | 2 | >16 |
| Levofloxacin | 1->16 | 2 | >16 |
| Moxifloxacin | 0.25->8 | 0.5 | >8 |
| Linezolid | 1-2 | 1 | 2 |
| Tigecycline | 0.06-0.25 | 0.25 | 0.25 |
| Vancomycin | 1->16 | 2 | >16 |
| Enterococcus faecium (19)b | |||
| TG-875649 | 0.5-8 | 4 | 8 |
| Ciprofloxacin | 2->16 | >16 | >16 |
| Levofloxacin | 2->16 | >16 | >16 |
| Moxifloxacin | 1->8 | >8 | >8 |
| Linezolid | 1-2 | 2 | 2 |
| Tigecycline | 0.03-0.25 | 0.06 | 0.12 |
| Vancomycin | 0.5-16 | 8 | >16 |
| Escherichia coli | |||
| Ciprofloxacin resistant (43) | |||
| TG-875649 | 2->16 | >16 | >16 |
| Ciprofloxacin | 2->16 | >16 | >16 |
| Levofloxacin | 1->16 | >16 | >16 |
| Moxifloxacin | 1->8 | >8 | >8 |
| Ceftazidime | ≤1->128 | 16 | 64 |
| Ceftriaxone | 0.06->8 | >8 | >8 |
| Cefepime | ≤0.06->8 | 0.5 | >8 |
| Imipenem | ≤0.12-2 | 0.5 | 0.5 |
| Piperacillin | 2->128 | >128 | >128 |
| Tigecycline | 0.12-0.5 | 0.25 | 0.5 |
| Ciprofloxacin susceptible (37) | |||
| TG-875649 | 0.015-4 | 1 | 2 |
| Ciprofloxacin | ≤0.06-1 | 0.25 | 1 |
| Levofloxacin | ≤0.06-2 | 0.5 | 1 |
| Moxifloxacin | ≤0.015-2 | 0.5 | 1 |
| Ceftazidime | ≤1-32 | 1 | 16 |
| Ceftriaxone | ≤0.03->8 | 0.06 | >8 |
| Cefepime | ≤0.06->8 | ≤0.06 | 8 |
| Imipenem | ≤0.12-1 | 0.25 | 0.5 |
| Piperacillin | 2->128 | >128 | >128 |
| Tigecycline | 0.12-0.5 | 0.25 | 0.5 |
| Klebsiella pneumoniae (30) | |||
| TG-875649 | 0.25->16 | 4 | >16 |
| Ciprofloxacin | ≤0.06->16 | 4 | >16 |
| Levofloxacin | ≤0.06->16 | 2 | >16 |
| Moxifloxacin | 0.12->8 | 4 | >8 |
| Ceftazidime | ≤1->128 | 16 | >128 |
| Ceftriaxone | ≤0.03->8 | >8 | >8 |
| Cefepime | ≤0.06->8 | 2 | >8 |
| Imipenem | 0.25-32 | 0.5 | 4 |
| Piperacillin | 4->128 | >128 | >128 |
| Tigecycline | 0.25-2 | 0.5 | 1 |
| Enterobacter cloacae (30) | |||
| TG-875649 | 0.5->16 | 4 | >16 |
| Ciprofloxacin | 0.12->16 | 2 | >16 |
| Levofloxacin | 0.12->16 | 2 | 16 |
| Moxifloxacin | 0.25->8 | 2 | >8 |
| Ceftazidime | ≤1->128 | 128 | >128 |
| Ceftriaxone | 0.12->8 | >8 | >8 |
| Cefepime | ≤0.06-8 | 4 | >8 |
| Imipenem | 0.25-8 | 1 | 4 |
| Piperacillin | 4->128 | >128 | >128 |
| Tigecycline | 0.25-4 | 0.5 | 2 |
| Proteus mirabilis (30) | |||
| TG-875649 | 0.5->16 | 8 | >16 |
| Ciprofloxacin | ≤0.06->16 | 2 | >16 |
| Levofloxacin | ≤0.06->16 | 2 | >16 |
| Moxifloxacin | 0.25->8 | 8 | >8 |
| Ceftazidime | ≤1-8 | ≤1 | ≤1 |
| Ceftriaxone | ≤0.03->8 | ≤0.03 | >8 |
| Cefepime | ≤0.06->8 | ≤0.06 | 8 |
| Imipenem | 0.25->32 | 2 | 8 |
| Piperacillin | 1->128 | 32 | >128 |
| Tigecycline | 1-4 | 2 | 4 |
| Citrobacter freundii (30) | |||
| TG-875649 | 0.12->16 | 0.5 | 4 |
| Ciprofloxacin | ≤0.06->16 | ≤0.06 | 2 |
| Levofloxacin | ≤0.06->16 | 0.12 | 2 |
| Moxifloxacin | 0.12->8 | 0.5 | 8 |
| Ceftazidime | ≤1->128 | ≤1 | 64 |
| Ceftriaxone | ≤0.03->8 | 0.25 | >8 |
| Cefepime | ≤0.06->8 | ≤0.06 | >8 |
| Imipenem | 0.25-4 | 1 | 2 |
| Piperacillin | 2->128 | 16 | >128 |
| Tigecycline | 0.25-1 | 0.25 | 0.5 |
| Streptococcus pneumoniae | |||
| Levofloxacin susceptible (71) | |||
| TG-875649 | 0.06-0.25 | 0.12 | 0.12 |
| Ciprofloxacin | 1-16 | 2 | 4 |
| Levofloxacin | 0.5-2 | 1 | 2 |
| Moxifloxacin | 0.06-0.5 | 0.12 | 0.25 |
| Ceftriaxone | ≤0.06->8 | 1 | 2 |
| Linezolid | 0.5-2 | 1 | 1 |
| Tigecycline | ≤0.08-0.12 | 0.03 | 0.06 |
| Vancomycin | ≤0.12-1 | 0.5 | 0.5 |
| Levofloxacin-resistant (29) | |||
| TG-875649 | 0.5-8 | 1 | 2 |
| Ciprofloxacin | 8->16 | >16 | >16 |
| Levofloxacin | 8->16 | 16 | >16 |
| Moxifloxacin | 2->8 | 2 | 8 |
| Ceftriaxone | 0.12-8 | 1 | 2 |
| Linezolid | 0.5-2 | 1 | 1 |
| Tigecycline | 0.015-0.12 | 0.06 | 0.06 |
| Vancomycin | 0.25-0.5 | 0.5 | 0.5 |
| Streptococcus pyogenes (30) | |||
| TG-875649 | 0.06-0.12 | 0.12 | 0.12 |
| Ciprofloxacin | 0.25-4 | 1 | 4 |
| Levofloxacin | 0.25-2 | 1 | 2 |
| Moxifloxacin | 0.06-0.5 | 0.25 | 0.5 |
| Ceftriaxone | ≤0.06 | ≤0.06 | ≤0.06 |
| Linezolid | 0.5-1 | 1 | 1 |
| Tigecycline | 0.015-0.06 | 0.03 | 0.06 |
| Vancomycin | 0.25-0.5 | 0.5 | 0.5 |
| Streptococcus agalactiae (30) | |||
| TG-875649 | 0.12-2 | 0.12 | 0.25 |
| Ciprofloxacin | 1->16 | 1 | 2 |
| Levofloxacin | 0.5->16 | 1 | 2 |
| Moxifloxacin | 0.12->8 | 0.25 | 0.5 |
| Ceftriaxone | ≤0.06->8 | ≤0.06 | 0.12 |
| Linezolid | 1-2 | 1 | 1 |
| Tigecycline | 0.03-0.06 | 0.03 | 0.06 |
| Vancomycin | 0.5-1 | 0.5 | 1 |
| Viridans group streptococci (30) | |||
| TG-975649 | 0.06-0.25 | 0.12 | 0.25 |
| Ciprofloxacin | 0.25-4 | 1 | 4 |
| Levofloxacin | 0.25-2 | 1 | 2 |
| Moxifloxacin | 0.06-9,25 | 0.12 | 0.25 |
| Ceftriaxone | ≤0.06-1 | 0.25 | 0.5 |
| Linezolid | 0.5-2 | 1 | 2 |
| Tigecycline | 0.03-1 | 0.06 | 0.12 |
| Vancomycin | 0.5-1 | 0.5 | 1 |
| Acinetobacter baumannii (30) | |||
| TG-875649 | 0.06->16 | 4 | >16 |
| Ciprofloxacin | ≤0.06-16 | 4 | >16 |
| Levofloxacin | ≤0.06->16 | 2 | 16 |
| Moxifloxacin | 0.06->8 | 1 | >8 |
| Ceftazidime | 8->128 | 64 | >128 |
| Ceftriaxone | 8->8 | >8 | >8 |
| Cefepime | 4->8 | >8 | >8 |
| Imipenem | 0.5->32 | 2 | >32 |
| Piperacillin | 16->128 | >128 | >128 |
| Tigecycline | 0.12-2 | 1 | 2 |
| Pseudomonas aeruginosa (30) | |||
| TG-875649 | 1->16 | 16 | >16 |
| Ciprofloxacin | 0.12->16 | 4 | >16 |
| Levofloxacin | 0.25->16 | 8 | >16 |
| Moxifloxacin | 1->8 | >8 | >8 |
| Ceftazidime | 2->128 | 32 | >128 |
| Ceftriaxone | >8 | >8 | >8 |
| Cefepime | 1->8 | >8 | >8 |
| Imipenem | 1->32 | 8 | >32 |
| Piperacillin | 4->128 | 128 | >128 |
| Tigecycline | 2->8 | 8 | >8 |
| Haemophilus influenzae (30) | |||
| TG-875649 | ≤0.008-8 | 0.12 | 4 |
| Ciprofloxacin | ≥0.06-16 | 0.25 | 16 |
| Levofloxacin | ≥0.06-8 | 0.25 | 8 |
| Moxifloxacin | ≥0.015->8 | 0.25 | >8 |
| Ceftazidime | ≤1 | ≤1 | ≤1 |
| Ceftriaxone | ≤0.03-0.06 | ≤0.03 | ≤0.03 |
| Cefepime | ≤0.06-0.5 | 0.25 | 0.5 |
| Imipenem | ≤0.12-8 | 1 | 4 |
| Tigecycline | 0.12-1 | 0.5 | 1 |
Including 44 S. epidermidis coagulase-negative staphylococci and 24 non-S. epidermidis coagulase-negative staphylococci.
Including 17 (7 E. faecalis strains and 10 E. faecium strains) vancomycin-resistant enterococci.
TABLE 2.
MIC distribution of TG-875649 and comparator fluoroquinolone agents against ciprofloxacin-susceptible and -resistant, methicillin-resistant S. aureus (MRSA)
MICs before vertical solid lines, between solid and dotted lines, and after dotted lines indicate susceptible, intermediate, and resistant breakpoints, respectively, based on CLSI interpretive criteria (2). The white fields denote range of dilutions tested for each agent. Values above the range denote MICs greater than the highest concentration tested. MICs equal to or lower than the lowest concentration tested are given as the lowest concentration.
Against levofloxacin-susceptible Streptococcus pneumoniae isolates, the MIC90 of TG-875649 (0.12 μg/ml) was severalfold lower than those of ciprofloxacin (4 μg/ml) and levofloxacin (2 μg/ml) and 2-fold lower than that of moxifloxacin (0.25 μg/ml). Against the 29 levofloxacin-resistant S. pneumoniae isolates studied, TG-875649 also had the lowest MIC90 (2 μg/ml), lower than those of the 3 FQs (8 to >16 μg/ml). The activity of TG-875649 was comparable to that of moxifloxacin against viridans group streptococci (MIC90 of 0.25 μg/ml for both drugs) and better than that of moxifloxacin against group A and group B streptococci (S. pyogenes and S. agalactiae, respectively), with MIC90s of 0.12 to 0.25 μg/ml for TG-875649 and 0.5 μg/ml for moxifloxacin. Three group B streptococcal isolates were FQ resistant, with ciprofloxacin and levofloxacin MICs of >16 μg/ml and a moxifloxacin MIC of 2 to >8 μg/ml, while the MICs of TG-875649 were lower, at 1 to 2 μg/ml (data not shown).
TG-875649 was less active against Gram-negative pathogens Enterobacteriaceae, Acinetobacter baumannii, and Pseudomonas aeruginosa. The MIC90s of TG-875649 and the 3 FQs against these species (except Citrobacter freundii and CIP-susceptible Escherichia coli) were nearly all equal to or greater than the highest concentrations tested. A look at the MIC50s revealed that the in vitro activities of TG-875649 were 2- to 4-fold less than the 3 FQs against most of these species. However, against Haemophilus influenzae, TG-875649 showed slightly better activity than the 3 FQs, with an MIC50 of 0.12 μg/ml (those of the 3 FQs were all 0.25 μg/ml), and its MIC90 was also ≥2-fold lower than those of the 3 FQs. It needs to be pointed out that the 30 H. influenzae isolates tested included 11 non-FQ-susceptible isolates. TG-875649 also showed slightly better activity against N. gonorrhoeae. Of the 10 N. gonorrhoeae isolates studied, only 2 were susceptible to ciprofloxacin. The ciprofloxacin, levofloxacin, and moxifloxacin MICs of the other 8 non-FQ-susceptible N. gonorrhoeae isolates ranged from 2 to 4, 1 to 4, and 0.5 to 2 μg/ml, respectively, but the MIC for TG-875649 was lower, at 0.25 to 1 μg/ml (data not shown).
In conclusion, nemonoxacin (tested as its malate salt, TG-875649) demonstrated better antibacterial activities than ciprofloxacin and levofloxacin against different species of Gram-positive bacteria, including staphylococci, streptococci, and enterococci, and against H. influenzae and N. gonorrhoeae. The in vitro activity of TG-875649 was also comparable to or better than that of moxifloxacin against these pathogens, including ciprofloxacin-resistant MRSA and levofloxacin-resistant S. pneumoniae. Based on these data, further studies of nemonoxacin pharmacology and development of bacterial resistance to nemonoxacin are warranted.
Acknowledgments
We thank Pei-Chen Chen, Hui-Yin Wang, and I-Wen Huang for microbiology laboratory assistance.
This study was supported in part by TaiGen Biotechnology Co., Taipei, Taiwan, and by an intramural grant from the National Health Research Institutes, Zhunan, Taiwan (96 A1-CLPP01-014).
Footnotes
Published ahead of print on 11 January 2010.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M07-A7, 8th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 2.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Lai, J. F., I. W. Huang, Y. R. Shiau, P. C. Chen, H. Y. Wang, and T. L. Lauderdale. 2009. Penicillin-nonsusceptibility and fluoroquinolone resistance in Streptococcus pneumoniae in Taiwan, 2002-2008, abstr. C2-1395. Abstr. 49th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 4.Lauderdale, T. L., L. C. McDonald, Y. R. Shiau, P. C. Chen, H. Y. Wang, J. F. Lai, and M. Ho. 2004. The status of antimicrobial resistance in Taiwan among gram-negative pathogens: the Taiwan surveillance of antimicrobial resistance (TSAR) program, 2000. Diagn. Microbiol. Infect. Dis. 48:211-219. [DOI] [PubMed] [Google Scholar]
- 5.Lauderdale, T. L., Y. R. Shiau, J. F. Lai, H. C. Chen, and C. H. R. King. 2007. In vitro antibacterial activity of nemonoxacin (TG-873870), a new non-fluorinated quinolone, against clinical isolates, abstr. E-1635. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 6.Levy, S. B., and B. Marshall. 2004. Antibacterial resistance worldwide: causes, challenges and responses. Nat. Med. 10:S122-S129. [DOI] [PubMed] [Google Scholar]
- 7.Livermore, D. M., and N. Woodford. 2006. The beta-lactamase threat in Enterobacteriaceae, Pseudomonas and Acinetobacter. Trends Microbiol. 14:413-420. [DOI] [PubMed] [Google Scholar]
- 8.Ma, L., C. J. Lin, J. H. Chen, C. P. Fung, F. Y. Chang, Y. K. Lai, J. C. Lin, L. K. Siu, and Taiwan Surveillance of Antimicrobial Resistance Project. 2009. Widespread dissemination of aminoglycoside resistance genes armA and rmtB in Klebsiella pneumoniae isolates in Taiwan producing CTX-M-type extended-spectrum beta-lactamases. Antimicrob. Agents Chemother. 53:104-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonald, L. C., T. L. Lauderdale, Y. R. Shiau, P. C. Chen, J. F. Lai, H. Y. Wang, and M. Ho. 2004. The status of antimicrobial resistance in Taiwan among Gram-positive pathogens: the Taiwan Surveillance of Antimicrobial Resistance (TSAR) programme, 2000. Int. J. Antimicrob. Agents 23:362-370. [DOI] [PubMed] [Google Scholar]
- 10.Yan, J. J., P. R. Hsueh, J. J. Lu, F. Y. Chang, J. M. Shyr, J. H. Wang, Y. C. Liu, Y. C. Chuang, Y. C. Yang, S. M. Tsao, H. H. Wu, L. S. Wang, T. P. Lin, H. L. M. Wu, H. M. Chen, and J. J. Wu. 2006. Extended-spectrum ß-lactamases and plasmid-mediated AmpC enzymes among clinical isolates of Escherichia coli and Klebsiella pneumoniae from seven medical centers in Taiwan. Antimicrob. Agents Chemother. 50:1861-1864. [DOI] [PMC free article] [PubMed] [Google Scholar]