Abstract
Two neuraminidase (NA) inhibitors, zanamivir (Relenza) and oseltamivir phosphate (Tamiflu), have been licensed for use for the treatment and prophylaxis of influenza. We have reported on laninamivir (code name, R-125489), a novel neuraminidase inhibitor, and have discovered that the laninamivir prodrug CS-8958 worked as a long-acting neuraminidase inhibitor in a mouse influenza virus infection model when it is intranasally administered. In this study, CS-8958 was administered just once 7 days before infection and showed significant efficacy in vivo. The efficacy of a single administration of CS-8958 after viral infection was then compared with that of repeated administrations of oseltamivir phosphate or zanamivir in mice and ferrets. CS-8958 showed efficacy superior or similar to the efficacies of the two licensed NA inhibitors. CS-8958 also significantly reduced the titers of an oseltamivir-resistant H1N1 virus with a neuraminidase H274Y substitution in a mouse infection model. These results suggest that since CS-8958 is characteristically long lasting in the lungs, it may be ideal for the prophylaxis and treatment of influenza.
Influenza is a serious respiratory illness which can be debilitating and which causes complications that lead to hospitalization and death, especially in elderly individuals. This respiratory disease is caused by influenza A and B viruses, which are pathogens that are highly contagious for humans. Influenza A viruses are classified into subtypes on the basis of the antigenicities of hemagglutinin (HA) and neuraminidase (NA) molecules. To date, 16 HA subtypes (H1 to H16) and 9 NA subtypes (N1 to N9) have been reported. Seasonal influenza or influenza epidemics are caused by influenza A virus H1N1 and H3N2 and influenza B virus (22), and every year the global burden of influenza epidemics is believed to be 3.5 million cases of severe illness and 300,000 to 500,000 deaths (6), before the new pandemic in 2009.
In the last 100 years, humans have experienced three influenza pandemics: the first in 1918 (H1N1), the second in 1957 (H2N2), and the third in 1968 (H3N2) (22). In 2009, a new swine-origin influenza virus (H1N1) infected humans (20) and caused a pandemic. WHO has reported more than 400,000 confirmed cases worldwide as of 18 October 2009 (http://www.who.int/csr/don/2009_10_23/en/index.html). Another possible concern is a pandemic caused by highly pathogenic avian influenza (HPAI) H5N1 viruses. Since 2003, the number of humans infected with the HPAI H5N1 virus has increased, and the fatality rate is high. More than 444 cases infected with the H5N1 virus and as many as 262 deaths were reported as of 27 November 2009 (http://www.who.int/csr/disease/avian_influenza/country/en/). Thus, there is considerable concern that such highly pathogenic viruses will cause sustained human-to-human transmission and the next global pandemic.
Two countermeasures, vaccinations and treatment with antivirals, are available to control human influenza. Although vaccinations play a critical role in influenza prophylaxis, they are an insufficient tool both for prophylaxis and against a pandemic virus. Therefore, antivirals are an important tool that may be used to mitigate influenza pandemics. Currently, two types of anti-influenza virus drugs are available: M2 ion channel blockers (adamantane) (5) and NA inhibitors. However, adamantane-resistant viruses readily emerge and are already prevalent worldwide among the seasonal influenza viruses (both the H1N1 and the H3N2 subtypes) (1, 3). The pandemic 2009 H1N1 viruses are also adamantane resistant (9). Moreover, the emergence of adamantane-resistant HPAI H5N1 viruses has prevented the use of adamantane for the treatment of infections caused by these viruses (4). The adamantane drugs have not been recommended for use for the treatment or chemoprophylaxis of influenza in the United States since the 2005 influenza season (1, 2). The second and most recently developed class of drugs with activities against influenza A and B viruses are the NA inhibitors, which bind to the NA surface glycoprotein of newly formed virus particles and prevent their efficient release from the host cell (8). Two NA inhibitors, zanamivir (inhaled drug, 10 mg/dose; Relenza) and oseltamivir (oral drug, 75 mg/dose; Tamiflu), are currently licensed for use. Both drugs require twice-daily administration for treatment. Oseltamivir is predominant and is used worldwide for the treatment of influenza, and the generation and circulation of oseltamivir-resistant seasonal influenza viruses have become major concerns (10, 11, 15, 17, 18). In particular, the worldwide prevalence of neuraminidase H274Y oseltamivir-resistant mutants of seasonal H1N1 virus have been reported, and 95% of H1N1 isolates tested from the fourth quarter of 2008 to January 2009 (WHO, http://www.who.int/csr/disease/influenza/H1N1webupdate20090318%20ed_ns.pdf) and almost all the H1N1 isolates tested since October 2008 in the United States (CDC, http://www.cdc.gov/flu/weekly/) were reported to be oseltamivir resistant. As well, a number of oseltamivir-resistant pandemic 2009 H1N1 viruses (7) and HPAI H5N1 viruses (18) have already appeared, although their appearance is still sporadic. These epidemics of oseltamivir-resistant influenza viruses therefore necessitate the development of alternative antiviral agents.
We found a new strong neuraminidase inhibitor, laninamivir (code name, R-125489), and reported that CS-8958 (laninamivir octanoate or the laninamivir prodrug) worked as a long-acting neuraminidase inhibitor (12, 16, 23). Laninamivir potently inhibited the neuraminidase activities of various influenza A and B viruses, including subtypes N1 to N9 and oseltamivir-resistant viruses (23), as well as pandemic 2009 H1N1 virus (14). Due to the long retention of R-125489 in mouse lungs after the intranasal administration of CS-8958 (16), the intranasal administration of a single dose of CS-8958 showed efficacy superior to the efficacies of zanamivir and oseltamivir in mouse models of infection with influenza A virus and seasonal and current pandemic strains (14, 23).
In this report, the in vivo efficacy of a single administration of CS-8958 was compared with the efficacies of repeated administrations of zanamivir (intranasal) and oseltamivir (oral) in mouse or ferret models of influenza A and B virus infection and the administration of oseltamivir in a mouse model of H274Y virus infection. We demonstrate the great potential of the single administration of CS-8958 as an alternative treatment against influenza viruses, including oseltamivir-resistant mutants.
MATERIALS AND METHODS
Compounds.
CS-8958 (molecular weight, 472.53) and zanamivir (molecular weight, 332.31) were synthesized, and oseltamivir phosphate was extracted and purified from commercially available oseltamivir by Daiichi Sankyo Co., Ltd. The chemical structure of CS-8958 is indicated elsewhere (23).
Cells.
Madin-Darby canine kidney (MDCK) cells were obtained from the American Type Culture Collection (ATCC CCL-34) and were purchased from DS Pharma Biomedical Co., Ltd. (Japan). The cells were maintained in minimum essential medium containing 10% fetal bovine serum, 50 units/ml penicillin, and 50 μg/ml streptomycin. The cells were cultured in 5% CO2 at 37°C.
Viruses.
The influenza viruses A/Puerto Rico/8/34 (A/PR/8/34, H1N1), B/Hong Kong/5/72, and B/Malaysia/2506/2004 were provided by the National Institute of Infectious Diseases, Japan. A/Yokohama/67/2006 clone 1 (wild type) and clone 11 (an H274Y oseltamivir-resistant mutant) were provided by the Yokohama City Institute of Health.
Animals.
Female BALB/c mice (age, 5 to 6 weeks; specific pathogen free; Japan SLC, Inc., or Charles River Laboratories Japan, Inc.) and ferrets (weight, 600 g to 800 g; Marshall BioResources) were kept in a controlled room throughout the experiments. The conditions in the room were as follows: the temperature was 23 ± 2°C, the relative humidity was 55% ± 10%, and a 12-h light, 12-h dark cycle was used. We observed the mice for survival daily from day 0 to day 20. All the experimental procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Daiichi Sankyo Co., Ltd.
Infection and administration.
The mice were anesthetized with ether-CHCl3 (1:1) or 2.5% isoflurane and were infected intranasally on day 0 with 50 μl of the number of PFU of the influenza virus per mouse indicated in the figure legends. The anesthetized mice were administered 50 μl (intranasally) or 200 μl (orally) of the compound dissolved in saline at the indicated doses and at the times mentioned in the appropriate figures or figure legends. For the life-prolonging-effect experiments, each administration group contained 8 to 12 mice, and the surviving mice were observed daily from day 0 to day 20. For the virus titration experiments, all the lungs from three mice were excised at the indicated time point mentioned in the appropriate figure legends. The lungs were then homogenized in 1 ml of phosphate-buffered saline (PBS) containing 0.4% bovine serum albumin (BSA), 50 units/ml penicillin, and 50 μg/ml streptomycin (PBS-BSA/PS) with an homogenizer. After centrifugation of the homogenates at 2,200 × g for 5 min at 4°C, the supernatants were collected for virus titration by a plaque assay.
The ferrets were intramuscularly anesthetized with 1 ml/kg of 40 mg/ml ketamine and 3 mg/ml xylazine and infected with 1,000 PFU/100 μl of B/Malaysia/2506/2004 at 0 h. At 4 h postinfection (hpi), saline, 0.05 μmol/kg (corresponding to 0.024 mg/kg of body weight) or 0.5 μmol/kg (corresponding to 0.24 mg/kg) of CS-8958, or 0.5 μmol/kg (corresponding to 0.17 mg/kg) of zanamivir was administered in 500 μl intranasally once and 25 mg/kg of oseltamivir phosphate was administered in 0.5 ml orally twice daily from 4 hpi. Nasal wash specimens were collected from the ferrets at 1, 2, and 3 days postinfection (dpi), as follows. The anesthetized ferrets were placed in a supine position with the head facing downward, and 2.5 ml of PBS-BSA/PS was poured onto the palate with a syringe. The solution draining from the nose was collected, and the volume of the solution was measured. After centrifugation at 2,200 × g for 5 min at 4°C, the supernatants were collected for virus titration by a plaque assay. The lack of antibodies to the influenza virus in the blood of the ferrets was confirmed by a hemagglutination inhibition assay.
Plaque assay.
The samples were serially diluted 10-fold with minimum essential medium containing 0.2% BSA, 50 units/ml penicillin, and 50 μg/ml streptomycin. MDCK cells were grown to confluence in six-well plates and washed with PBS, and 200 μl of each diluted sample was added to the plates in duplicate. After the cells were incubated at 37°C under 5% CO2 in an incubator for 1 h, they were washed with PBS. Then, 2.5 ml of modified Eagle medium containing 0.2% BSA, 25 mM HEPES buffer, 0.01% DEAE-dextran, 1 μg/ml of trypsin, 0.001% phenol red, and 0.6% agar was added to the wells. The plates were placed in the CO2 incubator for 2 days. After the agar medium was removed, 0.1% crystal violet in 19% methanol was added to the wells to fix and stain the cells. The number of plaques on each well was counted.
Statistical analysis.
For the life-prolonging-effect experiments, the survival proportions (in percent) and the median survival times (MSTs; the number of days with a survival rate equal to 50%) were calculated by the Kaplan-Meier method, and a log-rank test based on a joint ranking method was carried out to compare the results for the groups treated with compound with those for the group treated with saline. The statistical adjustment for multiple comparisons was done by the Bonferroni method, if necessary. For the virus titration experiments, based on the logarithms (log10 PFU/lungs) of the virus titers, a two-way analysis of variance (ANOVA) was carried out for all the titers on the titration days, and a Dunnett test was carried out for the titers for each day. An analysis of covariance (ANCOVA; a parallel-line assay) in which the logarithm of the dose was used as a covariate was carried out to estimate the relative potency between CS-8958 and zanamivir, determined as the area under the curve (AUC; in percent) based on the means of the virus titers. In addition, the 95% confidence intervals for the relative potency were estimated by use of the Fieller theorem, in which the AUC was calculated by use of the trapezoidal rule on the basis of the means of the virus titers (PFU/lungs) on days 1, 2, 3, and 4 for each dose. The analyses were performed with SAS system release 6.12 or 8.2 for Windows (SAS Institute, Inc.). P values of less than 0.05 were considered statistically significant. P values are provided throughout the text and in the figure legends, as appropriate.
RESULTS
As CS-8958 is a prodrug (16) and the active metabolite, laninamivir (R-125489), works as a neuraminidase inhibitor in animals, the doses used in this study are stated on a mole basis, and for the experiments comparing the activities of CS-8958 and zanamivir, the doses are stated on a weight basis as well.
Efficacy of prophylactic administration of CS-8958.
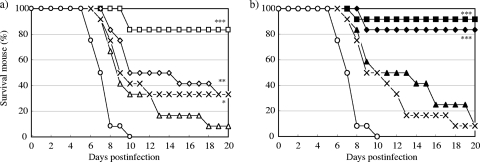
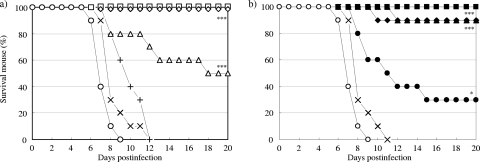
The survival curves for mice treated by a single intranasal administration of zanamivir or CS-8958 at various doses 7 days before infection with A/PR/8/34 (500 PFU) are shown in Fig. 1. All the control mice had died by 10 dpi. All groups of mice treated with both compounds showed prolonged survival. The doses of 5.2 μmol/kg (1.7 mg/kg) of zanamivir and 0.78 μmol/kg (0.37 mg/kg) of CS-8958 significantly prolonged survival (P = 0.0102 and P < 0.0001, respectively). At these doses, treatment with zanamivir and CS-8958 resulted in the survival of one-third of the mice (number of surviving mice/number of mice used, 4/12) and a survival rate of about 83% (10/12), respectively, at day 20.
FIG. 1.
In vivo efficacy of prophylactic intranasal administration of zanamivir or CS-8958 in mouse/influenza A virus infection model. Mice were infected with influenza A virus (A/PR/8/34, 500 PFU/mouse) on day 0 (n = 12). Zanamivir was intranasally administered at 0 μmol/kg (open circles; saline), 5.2 μmol/kg (multiplication signs), 10 μmol/kg (open triangles), 21 μmol/kg (open diamonds), or 42 μmol/kg (open squares) (a) and CS-8958 was intranasally administered at 0 μmol/kg (open circles; saline), 0.20 μmol/kg (multiplication signs), 0.39 μmol/kg (closed triangles), 0.78 μmol/kg (closed diamonds), or 1.5 μmol/kg (closed squares) (b) 7 days before infection. Zanamivir doses of 5.2, 10, 21, and 42 μmol/kg correspond to 1.7, 3.3, 7.0, and 14 mg/kg, respectively; and CS-8958 doses of 0.20, 0.39, 0.78, and 1.5 μmol/kg correspond to 0.095, 0.18, 0.37, and 0.71 mg/kg, respectively. The number of surviving mice was monitored until 20 dpi. The differences were not significant for the group treated with zanamivir at 10 μmol/kg and were significant for the groups treated with zanamivir at 5.2, 21, and 42 μmol/kg (P = 0.0102, P = 0.0029, and P < 0.0001, respectively) compared with the results for the saline group. The differences in the results were not significant for the groups treated with CS-8958 at 0.20 and 0.39 μmol/kg and were significant for the groups treated with CS-8958 at 0.78 and 1.5 μmol/kg (P < 0.0001 for both) compared with the results for the saline-treated group.
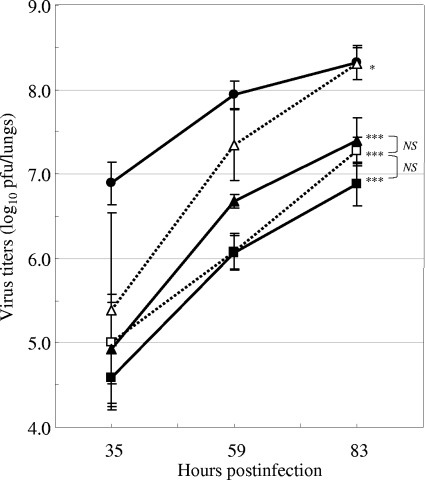
Under almost the same experimental conditions used in the experiment whose results are reported in Fig. 1, the virus titers in the mouse lungs were measured on days 1, 2, 3, and 4 after virus infection (Fig. 2). The maximum virus titers in the control mice were about 108 PFU/lungs at day 2. In all the mice treated with zanamivir or CS-8958, the virus titers were significantly reduced compared to those in the control group (P < 0.0001). The quantitative difference between the two compounds was determined on the basis of the AUC of the virus titers. The ratios of the AUCs of groups treated with one of the compounds to the AUC for the group treated with saline were calculated (Fig. 2, inset), and the relative potency between the compounds analyzed by the parallel-line assay (ANCOVA) was estimated to be 32.8, with the 95% confidence interval ranging from 18.1 to 62.3. This indicates that the decrease in the virus titers resulting from treatment with CS-8958 and zanamivir was significantly different (P = 0.0028).
FIG. 2.
Viral titers in the lungs of infected mice treated with zanamivir or CS-8958 7 days before influenza virus infection. Mice were intranasally administered zanamivir at 4.8 μmol/kg (closed squares), 14 μmol/kg (closed circles), or 43 μmol/kg (closed triangles) (a); CS-8958 at 0.18 μmol/kg (closed squares), 0.53 μmol/kg (closed circles), or 1.6 μmol/kg (closed triangles) (b); or saline (open circles; a and b) 7 days before infection with influenza A virus (A/PR/8/34, 500 PFU). The viral titers (log10 PFU/lungs) in the mouse lungs on days 1, 2, 3, and 4 are shown. Zanamivir doses of 4.8, 14, and 43 μmol/kg correspond to 1.6, 4.7, and 14 mg/kg, respectively; and CS-8958 doses of 0.18, 0.53, and 1.6 μmol/kg correspond to 0.085, 0.25, and 0.76 mg/kg, respectively. Each plot represents the mean ± standard deviation (n = 3). (Inset) AUCs (in percent) for zanamivir (closed circles) and CS-8958 (open circles) are plotted against the dose. The quantitative differences between the compounds were estimated to be 32.8 (P = 0.0028) by the parallel-line assay (ANCOVA).
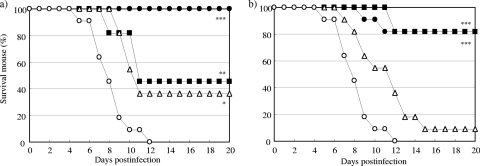
The improved efficacy achieved by prophylactic treatment with CS-8958 was again observed in an influenza B virus infection model. The survival curves for mice treated by the use of a single intranasal administration of zanamivir or CS-8958 at various doses 7 days before infection with B/Hong Kong/5/72 (1,500 PFU) are shown in Fig. 3. All the control mice had died by 12 dpi. All groups of mice treated with both compounds showed prolonged survival. Among the doses used, treatment with more than 3.3 μmol/kg (1.1 mg/kg) of zanamivir or 0.49 μmol/kg (0.23 mg/kg) of CS-8958 resulted in significantly prolonged survival compared with the saline-treated group (P = 0.0223 for 3.3 μmol/kg of zanamivir and P < 0.0001 for 0.49 μmol/kg of CS-8958). At these doses, treatment with zanamivir and CS-8958 resulted in survival rates of about 36% (number of surviving mice/number of mice used, 4/11) and about 82% (9/11), respectively, at day 20.
FIG. 3.
In vivo efficacy of prophylactic intranasal administration of zanamivir or CS-8958 in a mouse/influenza B virus infection model. Mice were infected with influenza B virus (B/Hong Kong/5/72, 1,500 PFU/mouse) on day 0 (n = 11). Zanamivir was intranasally administered at 0 μmol/kg (open circles; saline), 3.3 μmol/kg (open triangles), 9.8 μmol/kg (closed squares), or 29 μmol/kg (closed circles) (a) and CS-8958 was intranasally administered at 0 μmol/kg (open circles; saline), 0.16 μmol/kg (open triangles), 0.49 μmol/kg (closed squares), or 1.5 μmol/kg (closed circles) (b) 7 days before infection. Zanamivir doses of 3.3. 9.8, and 29 μmol/kg correspond to 1.1, 3.3, and 9.6 mg/kg, respectively; and CS-8958 doses of 0.16, 0.49, and 1.5 μmol/kg of correspond to 0.076, 0.23, and 0.71 mg/kg, respectively. The number of surviving mice was monitored until 20 dpi. Differences were significant for the groups treated with zanamivir at 3.3, 9.8, and 29 μmol/kg (P = 0.0223, P = 0.0046, and P < 0.0001, respectively) compared with the results for the saline-treated group. The differences were not significant for the group treated with CS-8958 at 0.16 μmol/kg and were significant for the groups treated with CS-8958 at 0.49 and 1.5 μmol/kg (P < 0.0001 for both) compared with the results for the saline-treated group.
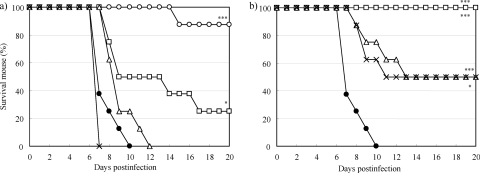
Next, 0.17 mg/kg of CS-8958 or 110 mg/kg of oseltamivir phosphate was administered to mice once intranasally and orally, respectively, at 7 days, 4 days, 1 day, and 12 h before infection with A/PR/8/34 (100 PFU); and the surviving mice were monitored (Fig. 4). The survival effects resulting from treatment with both compounds were observed to be administration time dependent. Significant survival benefits were achieved for the groups that were given oseltamivir phosphate only at 1 day and 12 h before infection (P = 0.0304 and P < 0.0001, respectively). Significantly prolonged survival effects, however, were achieved for all groups receiving CS-8958 (P = 0.0106 and 0.0011 for the mice dosed at 7 days and 4 days before infection, respectively, and P < 0.0001 for the other two groups). No significant survival effects were achieved for the groups of mice receiving 1.1 and 11 mg/kg of oseltamivir phosphate at the same dosing schedules indicated above (data not shown).
FIG. 4.
In vivo efficacy of prophylactic oral administration of oseltamivir phosphate and intranasal administration of CS-8958 in a mouse/influenza A virus infection model. Mice were infected with influenza A virus (A/PR/8/34, 100 PFU/mouse) on day 0 (n = 8). Oseltamivir phosphate at 110 mg/kg (a) and CS-8958 at 0.17 mg/kg (0.37 μmol/kg) (b) were administered at 12 h (open circles), 1 day (open squares), 4 days (open triangles), or 7 days (multiplication signs) before infection. Saline (closed circles) was administered intranasally at 7 days before infection. The number of surviving mice was monitored until 20 dpi. The prolonged survival effects for all groups administered CS-8958 were significant (P < 0.0001 for administration 12 h, 1 day, and 4 days before infection and P = 0.0106 for administration 7 days before infection). The survival effects for groups that received oseltamivir phosphate at 12 h and 1 day before infection were significant (P < 0.0001 and P = 0.0304, respectively).
Comparison of efficacies of repeated administration of zanamivir and a single administration of CS-8958.
Zanamivir is clinically administered by twice-daily inhalation of 10 mg/dose for 5 days. We next evaluated the efficacies of the single and repeated intranasal administrations of zanamivir and CS-8958 at a dose of 0.5 μmol/kg (0.17 mg/kg and 0.24 mg/kg, respectively). For the repeated administration, the administration started 11 h after infection with A/PR/8/34 (30 PFU) and continued twice daily. For the single administration, the administration was performed 11 hpi. The virus titers in the lungs were measured at 35, 59, and 83 hpi and are shown in Fig. 5. The virus titers of all four treated groups increased in a time-dependent manner, and the reductions in titers compared to those for the control group were significant (P = 0.0151 for a single administration of zanamivir and P < 0.0001 for the other three groups). The virus titers in the group that received a single administration of zanamivir were reduced at 35 and 59 hpi and then caught up to those of the control group at 83 hpi. The virus titers in the groups receiving single and repeated doses of CS-8958 were not significantly different (P = 0.0877). Finally, the reduction in virus titers achieved by a single administration of CS-8958 was compared to that achieved by repeated zanamivir administrations, but the difference was not statistically significant (P = 0.2184).
FIG. 5.
Virus titers in the lungs of infected mice treated with a single administration or repeated intranasal administrations of zanamivir or CS-8958. The virus titers (log10 PFU/lungs) in the mouse lungs after saline administration (closed circles), a single administration (open triangles) or repeated administrations (closed triangles) of zanamivir, and a single administration (open squares) or repeated administrations (closed squares) of CS-8958 are shown. The dose of both compounds used was 0.5 μmol/kg (0.17 mg/kg and 0.24 mg/kg for zanamivir and CS-8958, respectively). The virus titers were measured at 35, 59, and 83 h after infection with A/PR/8/34 (30 PFU) at 0 h. The repeated administrations started at 11 hpi and were then given twice daily. For the single administration, the compound was dosed at 11 hpi, and saline was then administered twice daily. Each plot represents the mean ± standard deviation (n = 3). No statistically significant differences were observed between a single administration of CS-8958 and repeated administrations of CS-8958 or between a single administration of CS-8958 and repeated administrations of zanamivir.
Comparison of efficacies of the repeated administration of oseltamivir phosphate and a single administration of CS-8958.
Oseltamivir phosphate is clinically administered by the twice-daily oral administration of 75 mg/dose as oseltamivir for 5 days. We evaluated the efficacy of repeated oral administrations of oseltamivir phosphate and a single intranasal administration of CS-8958 at various doses according to the life-prolonging effects achieved, and the results are shown in Fig. 6. For the repeated administration of oseltamivir phosphate, the administration started at 11 h after infection with A/PR/8/34 (100 PFU) and continued twice daily. The single intranasal administration of CS-8958 was performed only at 11 hpi. Treatment with more than 1.1 mg/kg of oseltamivir phosphate and 0.037 μmol/kg (0.017 mg/kg) of CS-8958 significantly prolonged survival (P = 0.0002 and P = 0.0123, respectively). At these doses, treatment with oseltamivir phosphate and CS-8958 resulted in the survival of about half of the mice (number of survival mice/number of mice used, 5/10) and 30% of the mice (3/10), respectively, at day 20.
FIG. 6.
In vivo efficacy of repeated administration of oseltamivir phosphate and a single administration of CS-8958 in a mouse influenza A virus infection model. Mice were infected with influenza A virus (A/PR/8/34, 100 PFU) on day 0 (0 h) (n = 10). For the group treated with oseltamivir phosphate, the oral administrations started at 11 hpi and were then continued twice daily until 120 hpi (twice daily for 5 days), and saline was administered intranasally at 11 hpi (a). For the group treated with CS-8958, the intranasal administration of CS-8958 was performed at 11 hpi, followed by the oral administration of saline twice daily for 5 days on the same schedule as the oseltamivir phosphate-treated group (b). For the control group, the intranasal administration of saline was performed at 11 hpi, followed by the oral administration of saline twice daily for 5 days on the same schedule as the oseltamivir phosphate-treated group. The doses of oseltamivir phosphate were 0.12 mg/kg (multiplication signs), 0.37 mg/kg (plus signs), 1.1 mg/kg (open triangles), 3.3 mg/kg (open diamonds), and 10 mg/kg (open squares); and the doses of CS-8958 were 0.012 μmol/kg (multiplication signs), 0.037 μmol/kg (closed circles), 0.11 μmol/kg (closed triangles), 0.33 μmol/kg (closed diamonds), and 1.0 μmol/kg (closed squares). Open circles (a and b), controls. CS-8958 doses of 0.012, 0.037, 0.11, 0.33, and 1.0 μmol/kg correspond to 0.0057, 0.017, 0.052, 0.16, and 0.47 mg/kg, respectively. The number of surviving mice was monitored until 20 dpi.
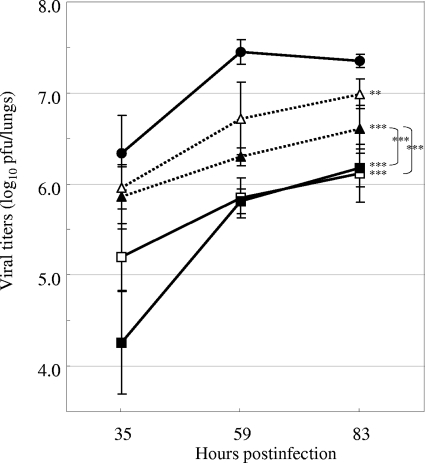
We next evaluated the efficacy of the repeated oral administration of oseltamivir phosphate or a single intranasal administration of CS-8958 at various doses according to the virus titers in the lungs of infected mice. The repeated administration of oseltamivir phosphate at doses of 1 and 10 mg/kg/dose started 11 hpi and continued twice daily. The single administration of CS-8958 at doses of 0.057 and 0.17 μmol/kg (0.027 or 0.080 mg/kg, respectively) was performed only at 11 hpi. The virus titers in the lungs were measured at 35, 59, and 83 hpi and are shown in Fig. 7. The virus titers in all four treated groups were significantly reduced in a time-dependent manner compared to those in the control group (P = 0.0021 for 1 mg/kg oseltamivir phosphate group and P < 0.0001 for the other three groups). The reductions in virus titer achieved with both doses of a single intranasal administration of CS-8958 were significant compared to those achieved with the repeated administrations of oseltamivir phosphate at the 10-mg/kg/dose (P = 0.0037 for the group treated with CS-8958 at 0.027 mg/kg and P < 0.0001 for the group treated with CS-8958 at 0.080 mg/kg).
FIG. 7.
Virus titers in the lungs of the infected mice treated with the repeated oral administration of oseltamivir phosphate or a single intranasal administration of CS-8958. Virus titers (log10 PFU/lungs) in the mouse lungs after saline administration (closed circles), the repeated administration of oseltamivir phosphate at 1 mg/kg (open triangles) or 10 mg/kg (closed triangles), and a single administration of CS-8958 at 0.057 μmol/kg (open squares) or 0.17 μmol/kg (closed squares) are shown. The virus titers were measured at 35, 59, and 83 h after infection with A/PR/8/34 (30 PFU) at 0 h. For the repeated administrations, oral administration of oseltamivir phosphate and then the intranasal administration of saline were performed at 11 hpi, followed by the oral administration of saline twice daily. For the single administration, CS-8958 was intranasally administered at 11 hpi and saline was orally administered on the same schedule indicated above. For the controls, saline was administered by both routes at 11 hpi and was orally administered at the rest of the time points. The CS-8958 dose of 0.17 μmol/kg corresponds to 0.080 mg/kg. Each plot represents the mean ± standard deviation (n = 3).
Efficacy of single administration of CS-8958 in a ferret infection model.
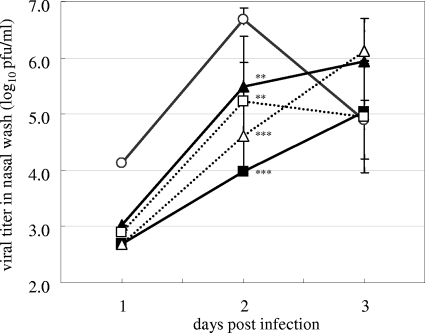
The efficacy of CS-8958 was investigated in ferrets, which are susceptible to influenza virus and which develop an upper respiratory tract infection as a result of influenza virus infection. At 4 hpi, ferrets infected with B/Malaysia/2506/2004 received intranasally one dose of saline, one dose of 0.05 or 0.5 μmol/kg (0.024 or 0.24 mg/kg, respectively) of CS-8958, or one dose of 0.5 μmol/kg (0.17 mg/kg) of zanamivir or they received 25 mg/kg of oseltamivir phosphate orally twice daily from 4 hpi. The virus titers in the nasal washes at 1, 2, and 3 dpi are shown in Fig. 8. As the numbers of cells in the noses of the ferrets susceptible to virus infection may be limited, the virus titers in the nasal washes from the untreated ferrets declined after 2 dpi. Therefore, the statistical analysis was performed on the basis of the AUC of the viral titers from 1 to 2 dpi. In this analysis, the nasal wash with a viral titer that was less than the lower limit of detection was defined as the lower limit of detection for the calculation of the AUC. The viral titers in the nasal washes of all the compound-treated ferrets were significantly reduced, and CS-8959 reduced the virus titers in a dose-dependent manner.
FIG. 8.
Virus titers in the nasal washes of infected ferrets treated by the repeated administration of oseltamivir phosphate and a single intranasal administration of zanamivir or CS-8958. The virus titers (log10 PFU/ml) in the nasal washes of ferrets treated by the twice-daily oral administration from 4 hpi of 25 mg/kg of oseltamivir phosphate (dotted lines, squares) or by the single intranasal administration at 4 hpi of 0.5 μmol/kg (0.17 mg/kg) of zanamivir (dotted lines, triangles), 0.05 μmol/kg (0.024 mg/kg; solid lines, triangles) or 0.5 μmol/kg (0.24 mg/kg; solid lines, squares) of CS-8958, or saline (solid lines, open circles) are shown. The virus titers (log10 PFU/ml of nasal wash) were measured at 1, 2, and 3 dpi. Each plot represents the mean ± standard deviation (n = 5). No viruses were detected in the nasal washes of one ferret in the saline-treated group, three of the ferrets in the oseltamivir-treated group, and four of ferrets in the zanamivir-treated group and both of the CS-8958 groups at 1 dpi and in the nasal washes of one of the ferrets in the high-dose CS-8958 group at 2 dpi. In those cases, the mean viral titers were calculated by using their lower limits of detection, and the standard deviation values were not calculated. By using the mean viral titers, oseltamivir phosphate, zanamivir, a low dose of CS-8958, and a high dose of CS-8958 showed statistically significant reductions in viral titers from 1 to 2 dpi (P = 0.0013, <0.0001, 0.0081, and < 0.0001, respectively.)
Efficacy of single administration of CS-8958 to H274Y mutant of H1N1 virus isolated from a patient.
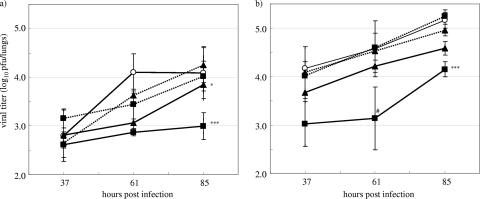
The efficacy of CS-8958 in a mouse model of infection with an oseltamivir-resistant H274Y virus was investigated. Mice infected with oseltamivir-sensitive A/Yokohama/67/2006 clone 1 or H274Y oseltamivir-resistant A/Yokohama/67/2006 clone 11 received 1.0 or 10 mg/kg of oseltamivir phosphate orally twice daily from 13 hpi or 0.057 or 0.17 μmol/kg (0.027 or 0.080 mg/kg, respectively) of CS-8958 intranasally once at 13 hpi. The virus titers in the lungs were measured at 37, 61, and 85 hpi; and the results are shown in Fig. 9. At the doses described above, oseltamivir phosphate reduced the virus titers in the lungs of the mice infected with an oseltamivir-sensitive virus, but essentially no reduction in the viral titer was detected in those infected with the resistant H274Y mutant. On the other hand, CS-8958 at 0.17 μmol/kg (0.080 mg/kg) strongly reduced the virus titers in the lungs of the mice infected with either virus (P = 0.0001 for sensitive virus, P < 0.0001 for resistant virus).
FIG. 9.
Virus titers in the lungs of oseltamivir-resistant or -sensitive virus-infected mice treated by the repeated oral administration of oseltamivir or a single intranasal administration of CS-8958. Mice infected with oseltamivir-sensitive A/Yokohama/67/2006 clone 1 (a) or H274Y oseltamivir-resistant A/Yokohama/67/2006 clone 11 (b) were orally administered oseltamivir phosphate twice daily from 13 hpi with 1.0 mg/kg (dotted lines, triangles) or 10 mg/kg (dotted lines, squares), were intranasally administered CS-8958 once at 13 hpi at 0.057 μmol/kg (0.027 mg/kg; solid lines, triangles) or 0.17 μmol/kg (0.080 mg/kg; solid lines, squares), or were administered saline (solid lines, open circles). The viral titers (log10 PFU/lungs) in the lungs were measured at 37, 61, and 85 hpi, and the lower limit of detection was 2.4 (log10 PFU/lungs). Each plot represents the mean ± standard deviation (n = 3). For the plot indicated by “#,” the viral titer was calculated to be 2.4 (log10 PFU/lungs) for 1 out of 3 mice because no viruses were recovered from the mouse lungs.
DISCUSSION
After the intranasal administration of CS-8958 to mice, CS-8958 was quickly converted to the active metabolite, laninamivir, and the laninamivir generated was retained in the lungs with a long half-life of 41.4 h (16). Therefore, we evaluated the efficacy of a single intranasal administration of CS-8958 in mouse and ferret influenza virus infection models.
The efficacy of a single administration of CS-8958 at various doses 7 days before infection was investigated in influenza A and B virus infection models. Figures 1 to 3 show that treatment with CS-8958 and zanamivir resulted in a dose-dependent survival rate and reduction of the virus titers. The effects of CS-8958 were superior to those of zanamivir, and the differences in the doses were about one to one and half orders of magnitude. Although oseltamivir phosphate administered orally at 110 mg/kg within 1 day before infection provided a significant survival effect, 0.17 mg/kg (0.37 μmol/kg) of CS-8958 administered intranasally even 7 days before infection provided a significant survival effect (Fig. 4). The half-life of zanamivir in the respiratory tract after inhalation or intranasal administration was estimated to be 2.8 h in humans (21), and it seemed to be less than 1 h in mice (16). The half-life of oseltamivir carboxylate in blood after the oral administration of oseltamivir phosphate was 7.0 h in rats (19). Therefore, the weak efficacy of the prophylactic administration of zanamivir and oseltamivir phosphate may be due to their rapid clearance from the lungs, the target organ of influenza virus infection in mice. The quantitative difference between CS-8958 and zanamivir was estimated to be 32.8, which was calculated on the basis of the AUCs of the virus titers (Fig. 2). Similarly, under the experimental conditions used in the present study, to achieve significant survival, CS-8958 doses of 0.37 to 0.78 μmol/kg (0.17 to 0.37 mg/kg; Fig. 1 and 4) were required for H1N1 influenza A virus and 0.49 μmol/kg (0.23 mg/kg) was required for influenza B virus. These doses correspond to 20 to 40 mg for humans, which are the doses used for the clinical studies (see below), and are realistic doses for once-weekly administration for prophylaxis.
Zanamivir and oseltamivir phosphate are licensed drugs that require twice-daily administration of a dose of 10 mg and 75 mg (as a free form), respectively, for 5 days. We next compared the efficacy of a single intranasal administration of CS-8958 with the efficacies achieved with repeated administrations of zanamivir (administered intranasally) and oseltamivir phosphate (administered orally) after the viral infection. No significant differences in the AUCs of the virus titers were observed between the single administration of a CS-8958 and the repeated administration of zanamivir (P = 0.2184) (Fig. 5). Interestingly, no significant difference between the results obtained by single and repeated administrations of CS-8958 was observed (P = 0.0877). This suggests that a single intranasal administration of CS-8958 may show efficacy similar to that achieved by the repeated intranasal administration of zanamivir and that multiple doses of CS-8958 will not be required to achieve a level of efficacy similar to that achieved with zanamivir.
The survival effects and the reduction in viral titers achieved with a single intranasal administration of CS-8958 and repeated oral administrations of oseltamivir phosphate are compared in Fig. 6 and 7, respectively. The efficacy of a single intranasal administration of CS-8958 was far superior to that of the repeated oral administration of oseltamivir phosphate according to both indexes of in vivo efficacy. The doses of CS-8958 in the experiments showing significant efficacy were 0.017 to 0.027 mg/kg, which is a realistic dose for use for the treatment of humans.
The superior efficacy was also confirmed in a ferret infection model (Fig. 8). Under our experimental conditions with B/Malaysia/2506/2004 virus infection, the virus titers reached a level as high as 106.68 PFU/ml in 2 ml of nasal wash at 2 dpi. In addition, no virus was detected in the blood of ferrets infected with B/Malaysia/2506/2004 before 5 to 6 dpi, as determined by the hemagglutination inhibition assay (data not shown). Therefore, we believe that the decline in the virus titers in the nasal washes of the untreated ferrets after 2 dpi was due to the limited number of nose cells which are susceptible to virus infection in ferrets. The ratio of AUCs of the virus titers of the compound-treated group to those of the control group at 1 and 2 dpi were 0.21% and 0.88% for the intranasal administration of 0.5 μmol/kg of CS-8958 (a single administration) and zanamivir (repeated administrations), respectively, and 3.0% for the repeated oral administration of oseltamivir phosphate.
Finally, the efficacy of CS-8958 against an oseltamivir-resistant influenza virus with the H274Y mutation was investigated in the mouse infection model. It was confirmed that a single intranasal administration of CS-8958 reduced the titers of oseltamivir-sensitive and -resistant viruses in mouse lungs in a dose-dependent manner.
In contrast to the currently available drugs, it is expected that a single inhalation of CS-8958 might be sufficient to treat influenza and that once-weekly inhalation might be sufficient for prevention. This CS-8958 dosing regimen will allow better compliance by patients with influenza and is a desirable characteristic for prophylaxis, especially when it is used as part of the measures against pandemic influenza that are required. Phase 1 clinical trials have been completed, and it was confirmed that the laninamivir in the plasma of healthy human volunteers who inhaled CS-8958 had a half-life of about 3 days (13), suggesting that a single inhalation of CS-8958 acts as a long-acting NA inhibitor in humans as well. Phase 3 and 2/3 clinical trials for confirmation of the efficacy of a single inhalation of 20 mg and 40 mg of CS-8958 in adults (A. Watanabe et al., personal communication) and children (N. Sugaya and Y. Ohashi, personal communication) have been completed. In addition, CS-8958 is expected to show activity against oseltamivir-resistant viruses, as well as against the current and next pandemic influenza viruses.
Acknowledgments
We thank T. Odagiri and M. Obuchi (National Institute of Infectious Diseases, Japan) for providing the influenza viruses and C. Kawakami (Yokohama City Institute of Health) for providing the oseltamivir-sensitive and -resistant mutants.
All work reported here was financially supported by Daiichi Sankyo Co., Ltd.
We are all employees of Daiichi Sankyo Co., Ltd. and do not have any financial conflicts of interest.
Footnotes
Published ahead of print on 4 January 2010.
REFERENCES
- 1.Bright, R. A., D. K. Shay, B. Shu, N. J. Cox, and A. I. Klimov. 2006. Adamantane resistance among influenza A viruses isolated early during the 2005-2006 influenza season in the United States. JAMA 295:891-894. [DOI] [PubMed] [Google Scholar]
- 2.Bright, R. A., D. Shay, J. Bresee, A. Klimov, N. Cox, and J. Ortiz. 2006. High levels of adamantane resistance among influenza A (H3N2) viruses and interim guidelines for use of antiviral agents—United States, 2005-06 influenza season. MMWR Morb. Mortal. Wkly. Rep. 55:44-46. [PubMed] [Google Scholar]
- 3.Bright, R. A., M. J. Medina, X. Xu, G. Perez-Oronoz, T. R. Wallis, et al. 2005. Incidence of adamantane resistance among influenza A (H3N2) viruses isolated worldwide from 1994 to 2005: a cause for concern. Lancet 366:1175-1181. [DOI] [PubMed] [Google Scholar]
- 4.Cheung, C. L., J. M. Rayner, G. J. Smith, P. Wang, T. S. Naipospos, et al. 2006. Distribution of amantadine-resistant H5N1 avian influenza variants in Asia. J. Infect. Dis. 193:1626-1629. [DOI] [PubMed] [Google Scholar]
- 5.Davies, W. L., R. R. Grunert, R. F. Haff, J. W. McGahen, E. M. Neumayer, et al. 1964. Antiviral activity of 1-adamantanamine (amantadine). Science 144:862-863. [DOI] [PubMed] [Google Scholar]
- 6.Fiore, A. E., D. K. Shay, P. Haber, J. K. Iskander, T. M. Uyeki, G. Mootrey, J. S. Bresee, and N. J. Cox. 2007. Prevention and control of influenza. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recommend. Rep. 56(RR-6):1-54. [PubMed] [Google Scholar]
- 7.Global Influenza Surveillance Network. 2009. Laboratory surveillance and response to pandemic H1N1 2009. Wkly. Epidemiol. Rec. 84:361-365. [PubMed] [Google Scholar]
- 8.Gubareva, L. V. 2004. Molecular mechanisms of influenza virus resistance to neuraminidase inhibitors. Virus Res. 103:199-203. [DOI] [PubMed] [Google Scholar]
- 9.Gubareva, L., M. Okomo-Adhiambo, V. Deyde, A. M. Fry, T. G. Sheu, R. Garten, C. Smith, J. Barnes, A. Myrick, M. Hillman, M. Shaw, C. Bridges, A. Klimov, and N. Cox. 2009. Update: drug susceptibility of swine-origin influenza A (H1N1) viruses, April 2009. MMWR Morb. Mortal. Wkly. Rep. 58:433-435. [PubMed] [Google Scholar]
- 10.Hatakeyama, S., N. Sugaya, M. Ito, M. Yamazaki, M. Ichikawa, K. Kimura, M. Kiso, H. Shimizu, C. Kawakami, K. Koike, K. Mitamura, and Y. Kawaoka. 2007. Emergence of influenza B viruses with reduced sensitivity to neuraminidase inhibitors. JAMA 297:1435-1442. [DOI] [PubMed] [Google Scholar]
- 11.Hayden, F. G. 2006. Antiviral resistance in influenza viruses—implications for management and pandemic response. N. Engl. J. Med. 354:785-788. [DOI] [PubMed] [Google Scholar]
- 12.Honda, T., S. Kubo, T. Masuda, M. Arai, Y. Kobayashi, and M. Yamashita. 2009. Synthesis and in vivo influenza virus-inhibitory effect of ester prodrug of 4-guanidino-7-O-methyl-Neu5Ac2en. Bioorg. Med. Chem. Lett. 19:2938-2940. [DOI] [PubMed] [Google Scholar]
- 13.Ishizuka, H., S. Yoshiba, H. Okabe, and K. Yoshihara. Clinical pharmacokinetics of laninamivir, a novel long-acting neuraminidase inhibitor, after single and multiple inhaled doses of its prodrug, CS-8958, in healthy male volunteers. J. Clin. Pharmacol., in press. [DOI] [PubMed]
- 14.Itoh, Y., K. Shinya, M. Kiso, T. Watanabe, Y. Sakoda, M. Hatta, Y. Muramoto, D. Tamura, Y. Sakai-Tagawa, T. Noda, S. Sakabe, M. Imai, Y. Hatta, S. Watanabe, C. Li, S. Yamada, K. Fujii, S. Murakami, H. Imai, S. Kakugawa, M. Ito, R. Takano, K. Iwatsuki-Horimoto, M. Shimojima, T. Horimoto, H. Goto, K. Takahashi, A. Makino, H. Ishigaki, M. Nakayama, M. Okamatsu, K. Takahashi, D. Warshauer, P. A. Shult, R. Saito, H. Suzuki, Y. Furuta, M. Yamashita, K. Mitamura, K. Nakano, M. Nakamura, R. Brockman-Schneider, H. Mitamura, M. Yamazaki, N. Sugaya, M. Suresh, M. Ozawa, G. Neumann, J. Gern, H. Kida, K. Ogasawara, and Y. Kawaoka. 2009. In vitro and in vivo characterization of new swine-origin H1N1 influenza viruses. Nature 460:1021-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiso, M., K. Mitamura, Y. Sakai-Tagawa, K. Shiraishi, C. Kawakami, K. Kimura, F. G. Hayden, N. Sugaya, and Y. Kawaoka. 2004. Resistant influenza A viruses in children treated with oseltamivir: descriptive study. Lancet 364:759-765. [DOI] [PubMed] [Google Scholar]
- 16.Koyama, K., M. Takahashi, M. Oitate, N. Nakai, H. Takakusa, S. Miura, and O. Okazaki. 2009. CS-8958, a prodrug of the novel neuraminidase inhibitor R-125489, demonstrates a favorable long retention profile in the mouse respiratory tract. Antimicrob. Agents Chemother. 53:4845-4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lackenby, A., O. Hungnes, S. G. Dudman, A. Meijer, W. J. Paget, A. J. Hay, and M. C. Zambon. 2008. Emergence of resistance to oseltamivir among influenza A (H1N1) viruses in Europe. Euro. Surveill. 13:1-2. [DOI] [PubMed] [Google Scholar]
- 18.Le, Q. M., M. Kiso, K. Someya, Y. T. Sakai, T. H. Nguyen, K. H. L. Nguyen, N. D. Pham, H. H. Ngyen, S. Yamada, Y. Muramoto, T. Horimoto, A. Takada, H. Goto, T. Suzuki, Y. Suzuki, and Y. Kawaoka. 2005. Avian flu: isolation of drug-resistant H5N1 virus. Nature 437:1108. [DOI] [PubMed] [Google Scholar]
- 19.Li, W., P. A. Escarpe, E. J. Eisenberg, K. C. Cundy, C. Sweet, K. J. Jakeman, J. Merson, W. Lew, M. Williams, L. Zhang, C. U. Kim, N. Bischofberger, M. S. Chen, and D. B. Mendel. 1998. Identification of GS 4104 as an orally bioavailable prodrug of the influenza virus neuraminidase inhibitor GS 4071. Antimicrob. Agents Chemother. 42:647-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Novel Swine-Origin Influenza A (H1N1) Virus Investigation Team. 2009. Emergence of a novel swine-origin influenza A (H1N1) virus in humans. N. Engl. J. Med. 360:2605-2615. [DOI] [PubMed] [Google Scholar]
- 21.Peng, A. W., S. Milleri, and D. S. Stein. 2000. Direct measurement of the anti-influenza agent zanamivir in the respiratory tract following inhalation. Antimicrob. Agents Chemother. 44:1974-1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wright, P. F., and R. G. Webster. 2001. Orthomyxoviruses, p. 1534-1579. In D. M. Knipe, P. M. Howley, D. E. Griffin, et al. (ed.), Fields virology, 4th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 23.Yamashita. M., T. Tomozawa, M. Kakuta, A. Tokumitsu, H. Nasu, and S. Kubo. 2009. CS-8958, a prodrug of the new neuraminidase inhibitor R-125489, shows long-acting anti-influenza virus activity. Antimicrob. Agents Chemother. 53:186-192. [DOI] [PMC free article] [PubMed] [Google Scholar]