Abstract
Bacillus anthracis, the causative agent of anthrax, can produce fatal disease when it is inhaled or ingested by humans. Dalbavancin, a novel, semisynthetic lipoglycopeptide, has potent activity, greater than that of vancomycin, against Gram-positive bacteria and a half-life in humans that supports once-weekly dosing. Dalbavancin demonstrated potent in vitro activity against B. anthracis (MIC range, ≤0.03 to 0.5 mg/liter; MIC50 and MIC90, 0.06 and 0.25 mg/liter, respectively), which led us to test its efficacy in a murine inhalation anthrax model. The peak concentrations of dalbavancin in mouse plasma after the administration of single intraperitoneal doses of 5 and 20 mg/kg of body weight were 15 and 71 mg/kg, respectively. At 20 mg/kg, the dalbavancin activity was detectable for 6 days after administration (terminal half-life, 53 h), indicating that long intervals between doses were feasible. The mice were challenged with 50 to 100 times the median lethal dose of the Ames strain of B. anthracis, an inoculum that kills untreated animals within 4 days. The efficacy of dalbavancin was 80 to 100%, as determined by the rate of survival at 42 days, when treatment was initiated 24 h postchallenge with regimens of 15 to 120 mg/kg every 36 h (q36h) or 30 to 240 mg/kg every 72 h (q72h). A regimen of ciprofloxacin known to protect 100% of animals was tested in parallel. Delayed dalbavancin treatment (beginning 36 or 48 h postchallenge) with 60 mg/kg q36h or 120 mg/kg q72h still provided 70 to 100% survival. The low MICs and long duration of efficacy in vivo suggest that dalbavancin may have potential as an alternative treatment or for the prophylaxis of B. anthracis infections.
Bacillus anthracis, the causative agent of anthrax, is primarily a disease of animals. In humans, it can produce a fatal disease if spores are inhaled or ingested (18, 20). Of great concern is the potential use of this organism as a biological weapon, as demonstrated in a recent outbreak in the United States (8, 9, 41). Even though there are antibiotic treatments for B. anthracis infection, the possibility of emerging natural resistance, as well as engineered resistance, remains of great concern (26). Penicillin had long been the treatment of choice for anthrax, and ciprofloxacin (twice daily for 60 days) is the current standard for postexposure prophylaxis of inhalational anthrax. However, there have been reports of ß-lactamase-producing strains and treatment failures with penicillin (3, 15, 20, 35), as well as resistance to ciprofloxacin and other potential therapeutic antibiotics (4, 10, 42). With the added concern of possible engineered resistance in a biological threat agent setting, it becomes important to expand the spectrum of antibiotics available for the treatment of anthrax.
Dalbavancin is a novel, semisynthetic lipoglycopeptide antibiotic with potent in vitro and in vivo activity and bactericidal activity against Gram-positive bacteria (21, 27, 29, 30, 36, 37, 44). Its long half-life, which allows for once-weekly intravenous (i.v.) treatment of human infections (16, 28, 43), suggested that dalbavancin might provide a potentially convenient alternative for the treatment or prophylaxis of anthrax. We evaluated the in vitro activity of dalbavancin against a diverse set of B. anthracis strains and its efficacy in an established murine inhalational anthrax challenge model (24). Because repeated i.v. dosing is not feasible in murine models, we also compared the intraperitoneal (i.p.) and i.v. pharmacokinetic (PK) profiles of dalbavancin in mice.
MATERIALS AND METHODS
B. anthracis strains.
The 30 B. anthracis strains used in this study were from the United States Army Medical Research Institute of Infectious Diseases (USAMRIID) collection and included the Ames strain and strains isolated from human and animal infections worldwide. The eight genotype clades identified by Keim et al. (31) were represented.
Susceptibility to dalbavancin.
MICs were determined in triplicate by the broth microdilution method in cation-adjusted Mueller-Hinton broth (CAMHB), according to the methodology of the Clinical and Laboratory Standards Institute (CLSI) (11, 12). The final antibiotic concentrations were 0.03 to 64 μg/ml. After 18 to 24 h of incubation at 35°C, the MICs were determined both visually and spectrophotometrically (600 nm; SpectroMax M2 spectrometer; Molecular Devices, Columbia, MD). The quality control strain Staphylococcus aureus ATCC 29213 was tested in parallel (12).
Pharmacokinetics.
The procedures used with the mice were approved by the Animal Care Committee at Vicuron Pharmaceuticals, Inc. (Gerenzano, Italy). Female ICR mice weighing 23 to 27 g were utilized. Single dalbavancin doses of 5 and 20 mg/kg of body weight were administered i.p. or i.v. (via the tail vein) in 5% glucose solution. Blood samples were collected by cardiac puncture, while the mice were under halothane anesthesia, at 0.08, 0.25, 0.5, 1, 2, 4, 8, 12, 24, 48, 72, 96, and 144 h after dosing. Three animals per route, dose, and time point were utilized. The blood was collected in heparinized tubes, which were centrifuged to prepare the plasma. The plasma samples were stored at −20°C until analysis. Dalbavancin concentrations were determined by a microbiological agar diffusion assay that measures the total drug concentration, as described previously (6). The values of the PK parameters were determined by noncompartmental analysis (WinNonlin software, version 3.3; Pharsight).
Efficacy studies.
The research was conducted in compliance with the Animal Welfare Act and other U.S. statutes and regulations governing the use of animals and adhered to the principles stated in the Guide for the Care and Use of Laboratory Animals (39). The facility where this research was conducted is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International.
Female BALB/c mice (age, 6 to 8 weeks) were challenged by aerosol with between 50 and 100 times the established 50% lethal dose (3.4 × 104 CFU) of a spore preparation of the Ames strain of B. anthracis (24). In the postexposure prophylaxis model, antibiotic treatment (administered in 0.2 ml i.p.) was initiated 24 h after challenge. Treatment groups (10 mice per group) received dalbavancin once every 36 h (q36h) at doses ranging from 15 to 120 mg/kg or every 72 h (q72h) at doses ranging from 30 to 240 mg/kg for 14 days. A regimen of ciprofloxacin known to protect 100% of animals (30 mg/kg twice daily for 14 days) was tested in parallel. In the postexposure treatment experiments, the administration of dalbavancin at 60 mg/kg q36h or 120 mg/kg q72h was initiated at later times (36 or 48 h) after challenge, when symptoms of infection could have appeared (24). Control mice received phosphate-buffered saline (PBS). The mice were monitored for survival for 42 days, at which time the surviving animals were killed and their organs were harvested to determine the tissue bacterial burden. For animals that died or that were moribund at earlier times, their organs were harvested at those times. Lungs, spleens, and the mediastinum region (lymph nodes) were aseptically removed, weighed, and homogenized in 1 ml of sterile water. Homogenates were serially diluted 10-fold in water, and 100-μl aliquots were plated on sheep blood agar. To determine the numbers of CFU of the anthrax spores, homogenates were heat shocked for 15 min at 65°C to kill vegetative cells, serially diluted, and plated as described above.
RESULTS
Dalbavancin susceptibility of B. anthracis strains.
Dalbavancin demonstrated potent in vitro activity. The MICs for the 30 strains of B. anthracis ranged from ≤0.03 to 0.5 μg/ml (Fig. 1), with the MIC50 being 0.06 μg/ml and the MIC90 being 0.25 μg/ml. In comparison, the vancomycin MICs ranged from 1 to 4 μg/ml. For the three MIC determinations, the MICs for the individual strains never varied by more than a single dilution.
FIG. 1.
Distribution of susceptibilities to dalbavancin and vancomycin for 30 B. anthracis strains. Red bars, dalbavancin; black bars, vancomycin.
Dalbavancin PKs in mouse plasma.
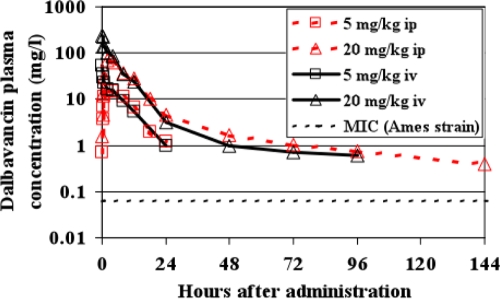
The concentrations of dalbavancin (bound and unbound) in plasma at different sampling times after i.p. administration are shown in Fig. 2, and the values of the PK and the pharmacodynamic (PD) parameters for the 5- and 20-mg/kg doses are presented in Table 1. The peak plasma concentrations (Cmax) of dalbavancin, attained 2 h after i.p. administration, were 15.2 and 71.3 μg/ml with the 5-mg/kg and 20-mg/kg doses, respectively. The terminal half-life achieved with the 20-mg/kg dose was 53 h. At the dose of 20 mg/kg i.p., dalbavancin was detectable (≥0.4 μg/ml) in plasma for 6 days after administration. From 2 h on, the levels in plasma closely followed the kinetics obtained with i.v. administration of the same doses (Fig. 2). However, the areas under the concentration-time curves (AUCs; calculated to infinity) were somewhat lower after i.p. administration (176 and 848 mg·h/liter with the 5- and 20-mg/kg doses, respectively) than after i.v. administration (200 and 1,071 mg·h/liter, respectively; data not shown). As observed in other studies with animals and humans (6, 16, 27, 33, 34), the PKs of dalbavancin in mice were dose proportional, on the basis of comparisons of the AUCs achieved with doses of 5 and 20 mg/kg.
FIG. 2.
Pharmacokinetics of dalbavancin in mouse plasma after the administration of two separate single doses (5 or 20 mg/kg) by either the i.v. or the i.p. route. Each point is the mean for three mice.
TABLE 1.
Values of dalbavancin plasma PK-PD parameters for a single 5- or 20-mg/kg i.p. dose in mice
| Parametera | 5 mg/kg |
20 mg/kg |
||||
|---|---|---|---|---|---|---|
| Total dalbavancin | Free dalbavancinb | Total and free dalbavancin | Total dalbavancin | Free dalbavancin | Total and free dalbavancin | |
| Cmax (mg/liter) | 15.2 | 0.24 | 71.3 | 1.14 | ||
| Tmax (h) | 2 | 2 | ||||
| AUC (mg·h/liter)c | 176 | 2.8 | 848 | 13.6 | ||
| t1/2 (h) | 53.4 | 53.4 | ||||
| T > MICd (h) | 17.8 | 48 | ||||
| Cmax/MIC | 4 | 19 | ||||
| AUC/MIC | 46.7 | 227 | ||||
t1/2, half-life; T > MIC, time that the concentration in plasma is greater than the MIC. The other abbreviations are defined in the text.
Calculated with the assumption that 1.6% of dalbavancin is not protein bound in mouse plasma (2).
Estimated AUC from time zero to infinity. Bioavailability was 79% compared with that achieved by i.v. administration.
The MIC for the Ames strain is 0.06 μg/ml.
Efficacy of dalbavancin in the mouse inhalation anthrax model. (i) Postexposure prophylaxis model.
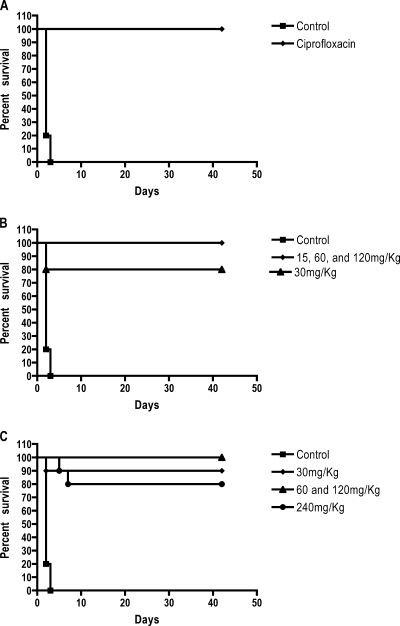
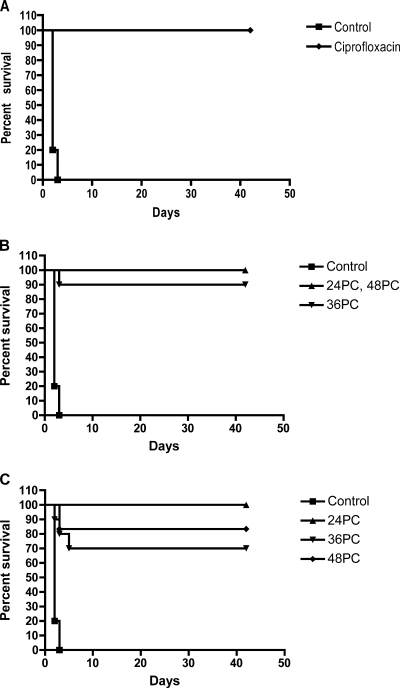
Dalbavancin, administered i.p. q36h (at ≥15 mg/kg) or q72h (at ≥30 mg/kg) for 14 days starting at 24 h postchallenge, provided significant protection (P < 0.001). Whereas all control mice died within 4 days postchallenge, 80 to 100% of the mice treated with dalbavancin survived with all dose regimens (Fig. 3). There was no indication of a dose-response relationship with the regimens of dalbavancin utilized. In comparison, as observed previously, 100% survival was obtained with a regimen of 30 mg/kg of ciprofloxacin twice daily for 14 days (24).
FIG. 3.
Postexposure survival by use of prophylaxis against anthrax. Dalbavancin treatment was initiated 24 h after challenge. The mice were monitored for survival for 42 days, and there were10 mice per treatment group. (A) Ciprofloxacin at 30 mg/kg q12 (control). (B) Dose range given q36h. Dalbavancin doses versus control, P < 0.001; dalbavancin doses versus ciprofloxacin, P > 0.05. (C) Dose range given q72h. Dalbavancin doses versus control, P < 0.001; dalbavancin doses versus ciprofloxacin, P > 0.05.
(ii) Postexposure treatment model.
By 36 to 42 h postchallenge, clinical signs and deaths due to inhalational anthrax become evident in the mouse model (24, 32, 38). The efficacies of therapeutic agents, including ciprofloxacin and doxycycline, are significantly reduced when they are administered after that time (24). Delayed treatment with dalbavancin provided 70 to 100% protection with i.p. administration of 60 mg/kg q36h or 120 mg/kg q72h starting at 36 or 48 h postchallenge (Fig. 4). In comparison, ciprofloxacin, administered at 30 mg/kg i.p. twice daily for 14 days beginning at 48 h postchallenge, protected 100% of the mice. Thus, intermittent dalbavancin treatment provided significant protection (P < 0.001 compared to the results for the controls), even when therapy was delayed until 36 or 48 h postchallenge, and its efficacy was at least comparable to that of ciprofloxacin administered twice daily (P > 0.05). The differences in survival obtained with ciprofloxacin treatment in this study and those obtained in previous studies (24) are most likely due to variations in the final spore challenge dose within and between experiments that are inherent to the aerosol system. Due to this variation, the number of deaths prior to the initiation of the treatment at 48 h give different numbers of animals between groups at the initiation of treatment.
FIG. 4.
Efficacy of delayed treatment with dalbavancin against anthrax. Delayed treatment with dalbavancin was compared to treatment with ciprofloxacin at 30 mg/kg q12 initiated at 48 h postchallenge. The mice were monitored for survival for 42 days. 24 PC, 36 PC, and 48 PC, initiation of treatment at 24, 36, and 48 h postchallenge (PC). (A) Ciprofloxacin treatment (n = 4). (B) Treatment with dalbavancin at 60 mg/kg q36. Each cohort had 10 mice at the time of initiation of treatment at 36 h and 48 h postchallenge. (C) Treatment with dalbavancin at 120 mg/kg q72. The numbers of mice in each cohort at the time of initiation of treatment were seven for 36 h postchallenge and six for 48 h postchallenge. Dalbavancin treatments versus controls, P < 0.001; dalbavancin treatments versus ciprofloxacin, P > 0.05.
Bacterial culture results 42 days postexposure showed small numbers of residual spores in the lungs, and these numbers were equivalent to the numbers observed in previous studies with ciprofloxacin-treated and cured animals (24). The other tissues were negative for the growth of any spores or vegetative forms of B. anthracis.
DISCUSSION
Dalbavancin is a lipoglycopeptide antibiotic that is being developed for the treatment of complicated skin and skin structure infections (cSSSIs), predominantly those caused by S. aureus and beta-hemolytic streptococci, organisms against which it has shown greater potency than existing glycopeptide therapeutic agents in vitro and in animal models (13, 21, 27, 29, 30, 33, 36, 44). Limited data suggest that the potent activity of dalbavancin extends to most other Gram-positive species (22, 23, 29, 30, 44); however, our study provides the first evidence of such activity against B. anthracis (dalbavancin MIC90, 0.25 μg/ml; vancomycin MIC, 2 μg/ml). Because of its long half-life, dalbavancin has shown efficacy as a regimen of two once-weekly doses against human cSSSIs and catheter-related cases of bacteremia (28, 43) and has been efficacious at a single dose in some animal infection models (2, 13, 27, 33). However, inhalational anthrax is a severe infection requiring extended treatment courses in humans. In the murine model, the standard treatment against which new agents are measured is 30 mg/kg ciprofloxacin twice daily for 14 days.
Dalbavancin is administered i.v. to humans, and this route has been utilized in studies with rat and rabbit models of infection (13, 27, 33). Because multiple i.v. administrations are not feasible in a murine model, we compared the PK profiles of dalbavancin after the administration of single i.p. and i.v. doses. At the doses utilized in the mouse PK studies, the PKs of dalbavancin appeared to be linear with the dose, as in other mammalian species, including humans (5, 6, 16, 17, 27, 33, 34, 40). It therefore seemed reasonable to extrapolate the values of the PK parameters obtained with the lower doses to the higher i.p. doses used in the infection studies. The distribution-elimination portion of the concentration-versus-time curves was almost superimposable for the two routes of administration, although the AUC was approximately 25% higher when the drug was given by the i.v. route.
The efficacy of dalbavancin in the B. anthracis spore challenge model in the mouse suggests that this new lipoglycopeptide may have potential as an alternative treatment for B. anthracis infections or as postexposure prophylaxis. The MIC of dalbavancin for the Ames strain was 0.06 μg/ml. If proportionality across the entire dose range is assumed, the doses used in this study yielded a theoretical total Cmax/MIC range of approximately 1,000 to 13,000 and an AUC/MIC range of about 10,000 to 150,000, on the basis of the extrapolation of the values of the PK parameters obtained with the 20-mg/kg single i.p. dose of dalbavancin (Table 1). In humans, a dalbavancin total plasma concentration of 20 μg/ml (corresponding to at least 1 μg/ml unbound drug) is maintained over the interval between weekly doses, and the free AUC over 7 days is about 756 mg·h/liter (5, 17, 40). On the basis of the findings of PD studies with S. aureus and Streptococcus pneumoniae in mice, these parameters predict 90% target attainment (the time that the concentration remains above the MIC or AUC/MIC) in humans for the infecting organisms, with the dalbavancin MICs being between 0.5 and >2 μg/ml (2, 17). This would provide 100% coverage of the B. anthracis strains, if it is assumed that the distribution in Fig. 1 is reasonably representative of that for the population.
In the mouse, only 1.6% of the dalbavancin in plasma has been estimated to be unbound to protein (2), whereas the value is 7% in humans (5). A greater extent of binding to mouse plasma proteins than to human plasma proteins has also been reported for oritavancin, another experimental lipoglycopeptide (25). If it is assumed that 1.6% of the dalbavancin is unbound and if dose proportionality is assumed, at the dosage regimens utilized in mice infected with the Ames strain of B. anthracis, the free Cmax/MIC and AUC/MIC ratios were estimated to be approximately 16 to 200 and 160 to 9,000, respectively. The actual ratios estimated for a single 20-mg/kg i.p. dose are shown in Table 1. In addition, although the half-life of dalbavancin is shorter in the mouse than in humans, the long half-life of dalbavancin can be estimated to provide a continuous free plasma concentration above the MIC90 (0.25 μg/ml) for B. anthracis for at least 24 h with the administration of a 120-mg/kg dose. Even at a dose of 20 mg/kg i.p., the MIC for the Ames strain was estimated to be exceeded for 24 to 48 h after the administration of a single dose. Note that the in vitro bactericidal activity of dalbavancin is time dependent, suggesting that the time that the concentration remains above the MIC may be an important PD parameter. This is of potential importance when it is considered that the germination of anthrax spores may occur between the time at which the Cmax is attained (Tmax) and the time of the next dose of dalbavancin. Although in the mouse neutropenic thigh infection model AUC/MIC was reported to be the critical PD parameter for the efficacy of dalbavancin against non-spore-forming Gram-positive bacteria (2), it is possible that the time that the concentration remains above the MIC may be more relevant for in vivo activity against B. anthracis.
Previous studies conducted with B. anthracis and ciprofloxacin in primates, which resulted in U.S. Food and Drug Administration (FDA) approval for use of the drug for the treatment of anthrax, were based on a successful empirical twice-daily regimen (19). Levofloxacin was granted FDA approval under subpart H, on the basis of the findings of studies conducted with mice and primates. The dosage regimens used in those studies with animals simulated the shape and magnitude (AUC from time zero to 24 h) of the levofloxacin concentration-time profile achieved in humans who received a 500-mg once-daily regimen (14). The regimens were effective in both species, and thus, the drug gained approval for use for the particular indication. In neither species was an exposure- or dose-response relationship identified. A more recent study with a related fluoroquinolone, gatifloxacin, showed that the PK-PD parameter predictive of a successful outcome was the AUC (1). While fluoroquinolones are different mechanistically and pharmacodynamically from dalbavancin, these approvals demonstrate the importance of understanding what the PD driver is when an indication for the use of a drug is sought by the use of data for animals alone.
Delayed-treatment experiments demonstrated that dalbavancin is effective in animals with active infections. Similar results were previously reported for ciprofloxacin (24). Currently, ciprofloxacin is the antibiotic recommended for use for both postexposure prophylaxis and the treatment of B. anthracis infections, with doxycycline or penicillin G being alternatives. One concern is that resistance to each of these antibiotics has been observed in B. anthracis, and clinical failures with β-lactam antibiotics have been documented (7). A second concern is the duration of treatment and the regimen required to ensure adequate protection from residual inhaled spores. Treatment for a minimum of 60 days is currently recommended. Its potent in vitro activity against B. anthracis, the potential for once-weekly dosing in humans, and its mechanism of action different from those of current antianthrax therapeutic agents suggest that dalbavancin could be an attractive alternative therapy for this disease.
Animal models alone will of necessity provide the fundamental efficacy data needed for the development of therapeutics active against biological threat agents, such as B. anthracis. The mouse anthrax inhalation model is the first step in meeting the FDA's “animal rule” (46). The results obtained with dalbavancin in this model warrant further study with mice and strongly indicate a need to progress to studies with the nonhuman primate model of B. anthracis infection.
Acknowledgments
The research described herein was sponsored by the Defense Threat Reduction Agency (project no. 02-4-2C-013). B.P.G. was an employee of Vicuron Inc., which was acquired by Pfizer Inc. after completion of this study. B.P.G. received an honorarium from Pfizer Inc. in connection with the development of the manuscript.
The opinions, interpretations, conclusions, and recommendations presented here are those of the authors and are not necessarily endorsed by the U.S. Army.
Footnotes
Published ahead of print on 4 January 2010.
REFERENCES
- 1.Ambrose, P. G., A. Forrest, W. A. Craig, C. M. Rubino, S. M. Bhavnani, G. L. Drusano, and H. S. Heine. 2007. Pharmacokinetics-pharmacodynamics of gatifloxacin in a lethal murine Bacillus anthracis inhalation infection model. Antimicrob. Agents Chemother. 51:4351-4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andes, D., and W. A. Craig. 2007. In vivo pharmacodynamic activity of the glycopeptide antibiotic dalbavancin. Antimicrob. Agents Chemother. 51:1633-1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bradaric, N., and V. Punda-Polic. 1992. Cutaneous anthrax due to penicillin-resistant Bacillus anthracis transmitted by an insect bite. Lancet 340:306-307. [DOI] [PubMed] [Google Scholar]
- 4.Brook, I., T. B. Elliott, H. I. Pryor II, T. E. Sautter, B. T. Gnade, J. H. Thakar, and G. B. Knudson. 2001. In vitro resistance of Bacillus anthracis Sterne to doxycycline, macrolides and quinolones. Int. J. Antimicrob. Agents 18:559-562. [DOI] [PubMed] [Google Scholar]
- 5.Buckwalter, M., and J. A. Dowell. 2005. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J. Clin. Pharmacol. 45:1279-1287. [DOI] [PubMed] [Google Scholar]
- 6.Cavaleri, M., S. Riva, A. Valagussa, M. Guanci, L. Colombo, J. Dowell, and M. Stogniew. 2005. Pharmacokinetics and excretion of dalbavancin in the rat. J. Antimicrob. Chemother. 55(Suppl. 2):ii31-ii35. [DOI] [PubMed] [Google Scholar]
- 7.Cavillo, J. D., F. R. Ramisse, M. Girardet, J. Vaissaire, M. Mock, and E. Hernandez. 2002. Antibiotic susceptibilities of 96 isolates of Bacillus anthracis isolated in France between 1994 and 2000. Antimicrob. Agents Chemother. 46:2307-2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.CDC. 2001. Update: investigation of anthrax associated with intentional exposure and interim public health guidelines. MMWR Morb. Mortal. Wkly. Rep. 50:889-897. [PubMed] [Google Scholar]
- 9.CDC. 2001. Investigation of bioterrorism-related anthrax and interim guidelines for exposure management and antimicrobial therapy. MMWR Morb. Mortal. Wkly. Rep. 50:909-919. [PubMed] [Google Scholar]
- 10.Choe, C., S. Bouhaouala, I. Brook, T. Elliott, and G. Knudson. 2000. In vitro development of resistance to ofloxacin and doxycycline in Bacillus anthracis Sterne. Antimicrob. Agents Chemother. 44:1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard, 7th ed. CLSI document M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 12.Clinical and Laboratory Standards Institute. 2008. Performance standards for antimicrobial susceptibility testing; 18th informational supplement. CLSI document M100-S18. Clinical and Laboratory Standards Institute, Wayne, PA.
- 13.Darouiche, R. O., and D. M. Mansouri. 2004. Dalbavancin compared with vancomycin for prevention of Staphylococcus aureus colonization of devices in an animal model. J. Infect. 50:206-209. [DOI] [PubMed] [Google Scholar]
- 14.Deziel, M. R., H. Heine, A. Louie, M. Kao, W. R. Byrne, J. Bassett, K. Bush, M. Kelly, and G. L. Drusano. 2005. Effective antimicrobial regimens for use in humans for therapy of Bacillus anthracis infections and postexposure prophylaxis. Antimicrob. Agents Chemother. 49:5099-5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doganay, M., and N. Aydin. 1991. Antimicrobial susceptibility of Bacillus anthracis. Scand. J. Infect. Dis. 23:333-335. [DOI] [PubMed] [Google Scholar]
- 16.Dorr, M. B., D. Jabes, M. Cavaleri, J. Dowell, G. Mosconi, A. Malabarba, R. J. White, and T. J. Henkel. 2005. Human pharmacokinetics and rationale for once-weekly dosing of dalbavancin, a semi-synthetic glycopeptide. J. Antimicrob. Chemother. 55(Suppl. 2):ii25-ii30. [DOI] [PubMed] [Google Scholar]
- 17.Dowell, J. A., B. P. Goldstein, M. Buckwalter, M. Stogniew, and B. Damle. 2008. Pharmacokinetic-pharmacodynamic modeling of dalbavancin, a novel glycopeptide antibiotic. J. Clin. Pharmacol. 48:1063-1068. [DOI] [PubMed] [Google Scholar]
- 18.Friedlander, A. M. 2000. Anthrax: clinical features, pathogenesis, and potential biological warfare threat. Curr. Clin. Top. Infect. Dis. 20:335-349. [PubMed] [Google Scholar]
- 19.Friedlander, A. M., S. L. Welkos, M. L. Pitt, J. W. Ezzell, P. L. Worsham, K. J. Rose, B. E. Ivins, J. R. Lowe, G. B. Howe, P. Mikesell, and W. B. Lawrence. 1993. Postexposure prophylaxis against experimental inhalation anthrax. J. Infect. Dis. 167:1239-1243. [DOI] [PubMed] [Google Scholar]
- 20.Gold, H. 1955. Anthrax: a report of one hundred seventeen cases. Arch. Intern. Med. 96:387-396. [DOI] [PubMed] [Google Scholar]
- 21.Goldstein, B. P., D. C. Draghi, D. J. Sheehan, P. Hogan, and D. F. Sahm. 2007. Bactericidal activity and resistance development profiling of dalbavancin. Antimicrob. Agents Chemother. 51:1150-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein, E. J., D. M. Citron, C. V. Merriam, Y. Warren, K. Tyrrell, and H. T. Fernandez. 2003. In vitro activities of dalbavancin and nine comparator agents against anaerobic gram-positive species and corynebacteria. Antimicrob. Agents Chemother. 47:1968-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goldstein, E. J., D. M. Citron, Y. A. Warren, K. L. Tyrrell, C. V. Merriam, and H. T. Fernandez. 2006. In vitro activities of dalbavancin and 12 other agents against 329 aerobic and anaerobic gram-positive isolates recovered from diabetic foot infections. Antimicrob. Agents Chemother. 50:2875-2879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heine, H. S., J. Bassett, L. Miller, J. M. Hartings, B. E. Ivins, M. L. Pitt, D. Fritz, S. L. Norris, and W. R. Byrne. 2007. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob. Agents Chemother. 51:1373-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heine, H. S., J. Basset, L. Miller, A. Basset, B. E. Ivins, D. Lehoux, F. F. Arhin, T. R. Parr, Jr., and G. Moeck. 2008. Efficacy of oritavancin in a murine model of Bacillus anthracis spore inhalation anthrax. Antimicrob. Agents Chemother. 52:3350-3357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inglesby, T. V., T. O'Toole, D. A. Henderson, J. G. Bartlett, M. S. Ascher, E. Eitzen, A. M. Friedlander, J. Gerberding, J. Hauer, J. Hughes, J. McDade, M. T. Osterholm, G. Parker, T. M. Perl, P. K. Russell, and K. Tonat. 2002. Anthrax as a biological weapon, 2002: updated recommendations for management. JAMA 287:2236-2252. [DOI] [PubMed] [Google Scholar]
- 27.Jabes, D., G. Candiani, G. Romano, C. Brunati, S. Riva, and M. Cavaleri. 2004. Efficacy of dalbavancin against methicillin-resistant Staphylococcus aureus in the rat granuloma pouch infection model. Antimicrob. Agents Chemother. 48:1118-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jauregui, L. E., S. Babazadeh, E. Seltzer, L. Goldberg, D. Krievins, M. Frederick, D. Krause, I. Satilovs, Z. Endzinas, J. Breaux, and W. O'Riordan. 2005. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin. Infect. Dis. 41:1407-1415. [DOI] [PubMed] [Google Scholar]
- 29.Jones, R. N., T. R. Fritsche, H. S. Sader, and B. P. Goldstein. 2005. Antimicrobial spectrum and potency of dalbavancin tested against clinical isolates from Europe and North America (2003): initial results from an international surveillance protocol. J. Chemother. 17:593-600. [DOI] [PubMed] [Google Scholar]
- 30.Jones, R. N., M. G. Stilwell, H. S. Sader, T. R. Fritsche, and B. P. Goldstein. 2006. Spectrum and potency of dalbavancin tested against 3322 gram-positive cocci isolated in the United States Surveillance Program (2004). Diagn. Microbiol. Infect. Dis. 54:149-153. [DOI] [PubMed] [Google Scholar]
- 31.Keim, P., L. B. Price, A. M. Klevytska, K. L. Smith, J. M. Schupp, R. Okinaka, P. J. Jackson, and M. E. Hugh-Jones. 2000. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J. Bacteriol. 182:2928-2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leffel, E. K., and L. M. Pitt. 2006. Anthrax, p. 77-94. In J. R. Swearengen (ed.), Biodefense. Research methodology and animal models. CRC Press, Boca Raton, FL.
- 33.Lefort, A., J. Pavie, L. Garry, F. Chau, and B. Fantin. 2004. Activities of dalbavancin in vitro and in a rabbit model of experimental endocarditis due to Staphylococcus aureus with or without reduced susceptibility to vancomycin and teicoplanin. Antimicrob. Agents Chemother. 48:1061-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leighton, A., A. B. Gottlieb, M. B. Dorr, D. Jabes, G. Mosconi, C. VanSaders, E. J. Mroszczak, K. C. Campbell, and E. Kelly. 2004. Tolerability, pharmacokinetics, and serum bactericidal activity of intravenous dalbavancin in healthy volunteers. Antimicrob. Agents Chemother. 48:940-945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lightfoot, N. F., R. J. D. Scott, and P. C. B. Turnbull. 1990. Antimicrobial susceptibility of Bacillus anthracis. Salisbury Med. Bull. 68(Suppl.):95-98. [Google Scholar]
- 36.Lin, G., K. Credito, L. M. Ednie, and P. C. Appelbaum. 2005. Antistaphylococcal activity of dalbavancin, an experimental glycopeptide. Antimicrob. Agents Chemother. 49:770-772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin, G., K. Smith, L. M. Ednie, and P. C. Appelbaum. 2005. Antipneumococcal activity of dalbavancin compared to other agents. Antimicrob. Agents Chemother. 49:5182-5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loving, C. L., M. Kennett, G. M. Lee, V. K. Grippe, and T. J. Merkel. 2007. Murine aerosol challenge model of anthrax. Infect. Immun. 75:2689-2698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, DC.
- 40.Nicolau, D. P., H. K. Sun, E. Seltzer, M. Buckwalter, and J. A. Dowell. 2007. Pharmacokinetics of dalbavancin in plasma and skin blister fluid. J. Antimicrob. Chemother. 60:681-684. [DOI] [PubMed] [Google Scholar]
- 41.Pile, J. C., J. D. Malone, E. M. Eitzen, and A. M. Friedlander. 1998. Anthrax as a potential biological warfare agent. Arch. Intern. Med. 158:429-434. [DOI] [PubMed] [Google Scholar]
- 42.Price, L. B., A. Volger, T. Pearson, J. D. Busch, J. M. Schupp, and P. Keim. 2003. In vitro selection and characterization of Bacillus anthracis mutants with high-level resistance to ciprofloxacin. Antimicrob. Agents Chemother. 47:2362-2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raad, I., R. Darouiche, J. Vazquez, A. Lentnek, R. Hachem, H. Hanna, B. Goldstein, T. Henkel, and E. Seltzer. 2005. Efficacy and safety of weekly dalbavancin therapy for catheter-related bloodstream infection caused by Gram-positive pathogens. Clin. Infect. Dis. 40:374-380. [DOI] [PubMed] [Google Scholar]
- 44.Streit, J. M., T. R. Fritsche, H. S. Sader, and R. N. Jones. 2004. Worldwide assessment of dalbavancin activity and spectrum against over 6,000 clinical isolates. Diagn. Microbiol. Infect. Dis. 48:137-143. [DOI] [PubMed] [Google Scholar]
- 45.U.S. Food and Drug Administration. 2002. New drug and biological drug products; evidence needed to demonstrate effectiveness of new drugs when human efficacy studies are not ethical or feasible. Final rule. Fed. Regist. 67:37988-37998. [PubMed] [Google Scholar]
- 46.U.S. Food and Drug Administration. 2002. Draft guidance for industry, inhalational anthrax (post-exposure)—development of antimicrobial drugs. CDER, U.S. Food and Drug Administration, Washington, DC.