Abstract
Growth inhibition by the pantothenate analog N-pentylpantothenamide (N5-Pan) has been attributed to the accumulation of acyl carrier protein carrying a prosthetic group modified by incorporation of N5-Pan. This was attributed to an inability of the AcpH acyl carrier protein phosphodiesterase to cleave the N5-Pan-modified prosthetic group from the protein moiety. We report that AcpH readily removes the N5-Pan-modified prosthetic group both in vivo and in vitro and show that N5-Pan blocks coenzyme A synthesis.
The N-substituted pantothenamide antimetabolites are analogs of the coenzyme A (CoA) precursor, pantothenic acid. These compounds have been utilized as antimicrobial agents (2, 18, 23), as probes of the active site of pantothenate kinase (21), and to posttranslationally modify and visualize carrier proteins in vivo (14).
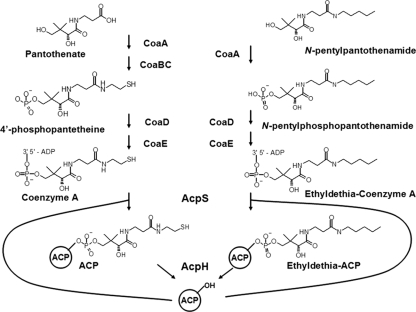
N-Pentylpantothenamide (N5-Pan), the most studied of these compounds, has been shown to participate in three of four CoA synthetic reactions (Fig. 1) leading to the formation of the inactive CoA analog ethyldethia-CoA (18). Acyl carrier protein (ACP), the carrier of acyl intermediates of fatty acid synthesis (1), requires the posttranslational attachment of a prosthetic group, 4′-phosphopantetheine, derived from CoA for metabolic function. Holo-ACP synthase (AcpS), the enzyme that catalyzes this reaction in Escherichia coli (7, 15), also uses ethyldethia-CoA as a substrate in vivo, leading to the accumulation of inactive ethyldethia-ACP and subsequent inhibition of fatty acid synthesis (23). Zhang and coworkers proposed that ACP phosphodiesterase (AcpH), the enzyme that hydrolyzes holo-ACP to apo-ACP and 4′-phosphopantetheine (19, 20), is unable to use ethyldethia-ACP as a substrate (23). This was postulated to lead to the observed accumulation of this ACP species and cause the antimicrobial activity of N5-Pan. However, the gene encoding AcpH was unknown at that time, precluding in vivo testing of this hypothesis. We report that AcpH readily hydrolyzes ethyldethia-ACP and that increasing or abolishing AcpH levels in vivo had only very modest effects on sensitivity to N5-Pan.
FIG. 1.
Incorporation of N-pentylpantothenamide into ethyldethia-CoA and ethyldethia-ACP. Pantothenate is converted into CoA in four synthetic steps. N5-Pan participates in three of these steps to form the CoA analog ethyldethia-CoA, which is utilized as a prosthetic group donor by holo-ACP synthase (AcpS) to form the inactive ethyldethia-ACP. Holo- and ethyldethia-ACP are hydrolyzed by ACP phosphodiesterase (AcpH).
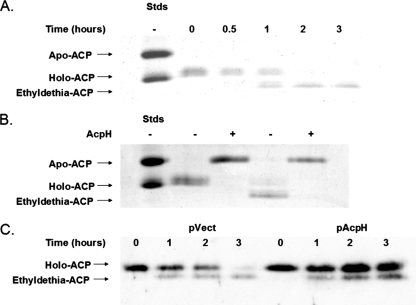
Strain JT6 was constructed by transduction of E. coli K-12 strain UB1005 to tetracycline resistance by using strain CAG12184 (tolC::Tn10) (17), which lacks the outer membrane exit duct of tripartite efflux pumps. An exponentially growing culture of strain JT6 in minimal M9 medium containing 0.4% glycerol and 0.1% vitamin-free Casamino Acids was supplemented with 200 μg/ml N5-Pan synthesized by the method of Strauss and Begley (18). Samples of the culture were periodically removed, cell extracts were prepared, and ACP pools were visualized by conformationally sensitive gel electrophoresis (5). As reported by other workers (23), a rapidly migrating ACP species corresponding to ethyldethia-ACP, which became the dominant ACP species, was observed (Fig. 2A).
FIG. 2.
Accumulation of ethyldethia-ACP in vivo and hydrolysis by AcpH in vitro. (A) Accumulation of ethyldethia-ACP upon supplementation of an exponentially growing culture of strain JT6 with 200 μg/ml N5-Pan, visualized by conformationally sensitive gel electrophoresis, followed by Coomassie blue staining. Stds is a mixture of purified apo-ACP and holo-ACP. (B) Ethyldethia-ACP is a substrate for ACP phosphodiesterase in vitro. Holo-ACP or ethyldethia-ACP (5 μM) was treated with 10 pmol of AcpH for 1 h. (C) The ACP pools of cultures of strain JT6 transformed with pBAD322 as a vector control (pVect) or pJT5 expressing AcpH from an arabinose-inducible promoter (pAcpH) after supplementation with 100 μg/ml N5-Pan were visualized by Western blotting with anti-ACP antibody (6).
In the model previously proposed (23), a strain lacking AcpH would be resistant to N5-Pan. Moreover, due to the increased turnover of holo-ACP, a strain overproducing AcpH should be more sensitive to N5-Pan. In order to test these predictions, strain JT13 lacking acpH was constructed by transduction of strain JT6 to chloramphenicol resistance by using a P1 lysate made on the ΔacpH strain JT1 (19). Derivatives of strain JT6 transformed with plasmid pJT5, which expresses AcpH under arabinose control (19), or the empty vector pBAD322 (3) were also constructed. Contrary to our expectations, we observed that the acpH null mutant strain JT13 was about 2-fold more sensitive to N5-Pan than was the parent strain (Fig. 3A), whereas the strain overexpressing AcpH was approximately 2-fold more resistant to the analog (Fig. 3B).
FIG. 3.
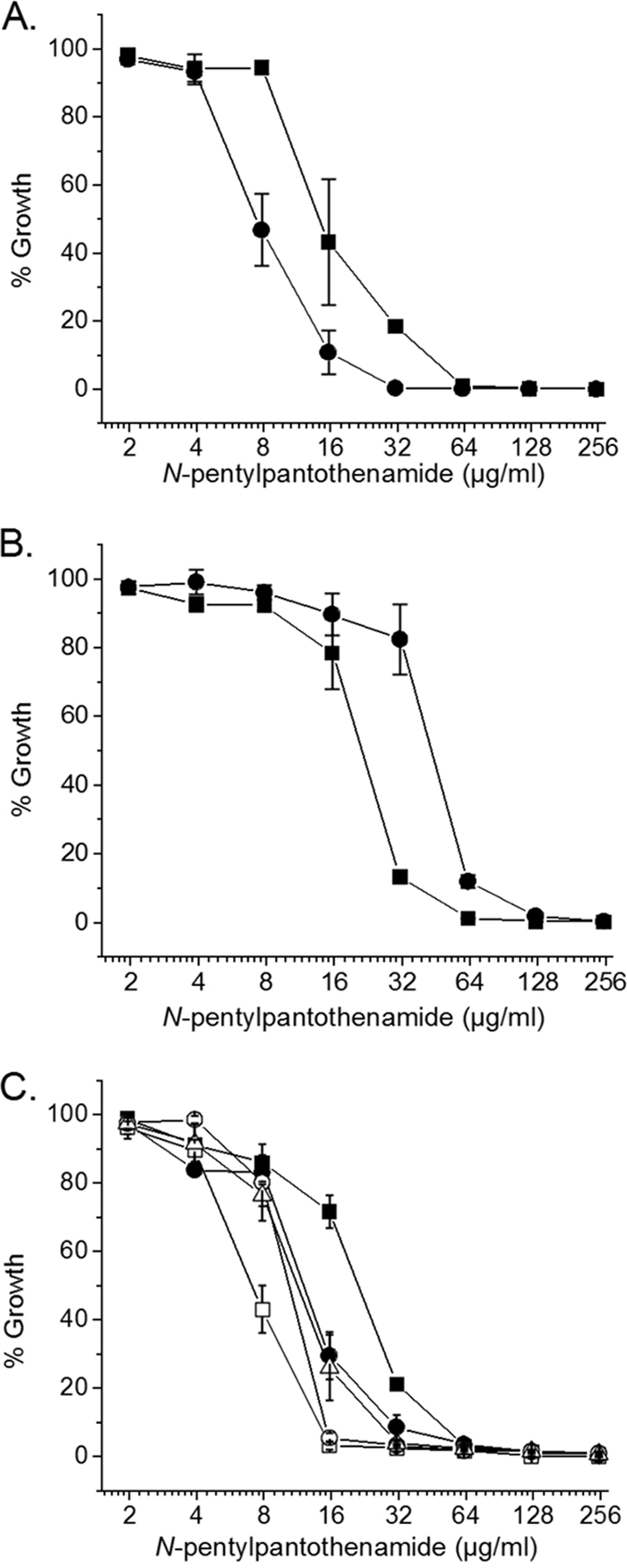
Growth inhibition by N5-Pan. (A) Strains JT6 (closed squares) and JT13 (ΔacpH) (closed circles). (B) JT6 transformed with pJT5 expressing AcpH under ParaBAD control (closed circles) or transformed with pBAD322 as an empty vector control (closed squares). (C) JT6 transformed with pBAD24 as an empty vector control (closed squares) or plasmids expressing CoaA (open squares), CoaD (closed circles), CoaE (open circles), or CoaBC (open triangles) under Para control. The error bars represent the standard deviations from the mean of the results for three independent experiments.
To examine the ACP pools of these strains, cultures were treated with 100 μg/ml N5-Pan, crude extracts of the cells were made, and the proteins were separated by conformationally sensitive gel electrophoresis, followed by electrophoretic transfer and Western blotting with a specific anti-ACP antibody (Fig. 2C). A significant pool of holo-ACP was present in the AcpH-overproducing strain 3 h after treatment with N5-Pan, whereas in the empty vector control, the predominant species was ethyldethia-ACP. The ACP pools of the acpH deletion strain JT13 did not differ markedly from the parental strain (data not shown).
Purified ethyldethia-ACP was isolated as described previously (5), with the exception that 400 μg/ml N5-Pan was added to the culture that overexpressed ACP. Both ethyldethia-ACP and holo-ACP were hydrolyzed by AcpH in vitro (Fig. 2). Ethyldethia-ACP is therefore a substrate for AcpH both in vivo and in vitro, and AcpH provides an only modestly increased resistance to N5-Pan.
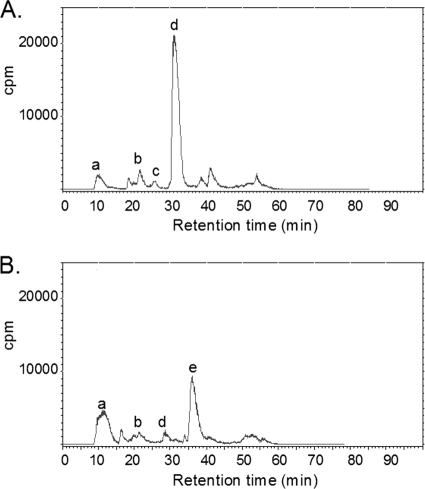
We then examined CoA synthesis in cultures treated with N5-Pan, reasoning that if CoA synthesis was inhibited by the analog or its products in vivo, ethyldethia-CoA would constitute the sole source of cellular ACP prosthetic groups, thereby leading to the observed preferential accumulation of ethyldethia-ACP over holo-ACP. The β-alanine auxotrophic strain JT7 carrying a panD deletion (4, 22) was constructed by transducing NRD45 (UB1005 ΔpanD) to tetracycline resistance by using the strain CAG12184 lysate as described above. The cellular CoA and ACP pools were specifically labeled using tritiated β-alanine (12, 16). A culture of strain JT7 was grown to an optical density of 0.2 in minimal M9 medium containing 0.4% glycerol, 0.1% Casamino Acids, and 4 μM β-[3-3H]alanine (0.25 Ci/mol); the culture was divided in half; and 100 μg/ml N5-Pan was added to one culture. After incubation for a further 90 min (approximately two doublings), the CoA pools were extracted by the method of Iram and Cronan (9) and analyzed by reverse-phase high-performance liquid chromatography (HPLC) on a C18 column. After treatment with the analog, CoA synthesis was severely inhibited, leading to >10-fold decreased levels of the key metabolic intermediate, acetyl-CoA (Fig. 4).
FIG. 4.
Intracellular CoA pools of strain JT7 treated with N5-Pan (B) or left untreated (A). Major peaks determined by internal standards included in each run are as follows: a, a mixture of β-alanine, pantothenate, and malonyl-CoA; b, CoA; c, succinyl-CoA; and d, acetyl-CoA. Peak a includes all labeled compounds that failed to bind to the HPLC column (probably mainly β-alanine and pantothenate that was not removed by the preparatory C18 column) and was highly variable from run to run. Note that malonyl-CoA, which was also included in peak a, is only a trace component of the CoA pools (11, 12). Peak e is an artifact of an unknown structure formed upon addition of N5-Pan to minimal M9 medium containing β-[3-3H]alanine. Its formation occurred in both the presence and the absence of cells.
Zhang and coworkers argued that N5-Pan did not inhibit CoA synthesis, based on the finding that the major effect of depletion of intracellular CoA, inhibition of protein synthesis (11, 13), was not observed (23). However, their studies were done in the presence of exogenous amino acids, which relieve the amino acid starvation caused by CoA depletion (13).
We attempted to determine if a specific step of CoA synthesis was inhibited by N5-Pan by separately overproducing each of the four CoA synthetic enzymes, CoaA, CoaD, CoaE, and Dfp, in the expression vector pBAD24 (8). Overproduction of these enzymes failed to impart N5-Pan resistance and instead caused increased sensitivity to N5-Pan (Fig. 3C), likely due to increased flux of the analog through the synthetic pathway. Identification of the specific enzyme(s) inhibited will require further in vitro analysis.
Inhibition by pantothenamides of the CoA synthetic enzyme pantothenate kinase, CoaA, was reported previously (2, 10, 18, 21), but because neither overexpression of CoaA (this work) nor supplementation of the medium with pantothenate (23) relieved growth inhibition, it is uncertain whether inhibition of this enzyme is sufficient to account for the toxicity of N5-Pan. Inhibition of the fatty acid synthetic enzymes by ethyldethia-ACP may also be responsible for part of the toxic effects of the analog. However, supplementation of Streptococcus pneumoniae with exogenous fatty acids was insufficient to restore growth (23). Thus, the most likely scenario seems to be that the mode of action of the pantothenamide analogs is through combined inhibition of CoA and fatty acid synthetic enzymes and perhaps of enzymes that utilize CoA or acetyl-CoA.
Acknowledgments
This work was supported by NIH research grant AI15650 from the National Institute of Allergy and Infectious Diseases.
Footnotes
Published ahead of print on 4 January 2010.
REFERENCES
- 1.Byers, D. M., and H. Gong. 2007. Acyl carrier protein: structure-function relationships in a conserved multifunctional protein family. Biochem. Cell Biol. 85:649-662. [DOI] [PubMed] [Google Scholar]
- 2.Clifton, G., S. R. Bryant, and C. G. Skinner. 1970. N′-(substituted) pantothenamides, antimetabolites of pantothenic acid. Arch. Biochem. Biophys. 137:523-528. [DOI] [PubMed] [Google Scholar]
- 3.Cronan, J. E. 2006. A family of arabinose-inducible Escherichia coli expression vectors having pBR322 copy control. Plasmid 55:152-157. [DOI] [PubMed] [Google Scholar]
- 4.Cronan, J. E., Jr. 1980. β-Alanine synthesis in Escherichia coli. J. Bacteriol. 141:1291-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cronan, J. E., and J. Thomas. 2009. Bacterial fatty acid synthesis and its relationships with polyketide synthetic pathways. Methods Enzymol. 459:395-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Lay, N. R., and J. E. Cronan. 2006. Gene-specific random mutagenesis of Escherichia coli in vivo: isolation of temperature-sensitive mutations in the acyl carrier protein of fatty acid synthesis. J. Bacteriol. 188:287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Flugel, R. S., Y. Hwangbo, R. H. Lambalot, J. E. Cronan, Jr., and C. T. Walsh. 2000. Holo-(acyl carrier protein) synthase and phosphopantetheinyl transfer in Escherichia coli. J. Biol. Chem. 275:959-968. [DOI] [PubMed] [Google Scholar]
- 8.Guzman, L. M., D. Belin, M. J. Carson, and J. Beckwith. 1995. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J. Bacteriol. 177:4121-4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iram, S. H., and J. E. Cronan. 2006. The β-oxidation systems of Escherichia coli and Salmonella enterica are not functionally equivalent. J. Bacteriol. 188:599-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivey, R. A., Y. M. Zhang, K. G. Virga, K. Hevener, R. E. Lee, C. O. Rock, S. Jackowski, and H. W. Park. 2004. The structure of the pantothenate kinase-ADP-pantothenate ternary complex reveals the relationship between the binding sites for substrate, allosteric regulator, and antimetabolites. J. Biol. Chem. 279:35622-35629. [DOI] [PubMed] [Google Scholar]
- 11.Jackowski, S., and C. O. Rock. 1986. Consequences of reduced intracellular coenzyme A content in Escherichia coli. J. Bacteriol. 166:866-871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackowski, S., and C. O. Rock. 1981. Regulation of coenzyme A biosynthesis. J. Bacteriol. 148:926-932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keating, D. H., Y. Zhang, and J. E. Cronan, Jr. 1996. The apparent coupling between synthesis and posttranslational modification of Escherichia coli acyl carrier protein is due to inhibition of amino acid biosynthesis. J. Bacteriol. 178:2662-2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meier, J. L., A. C. Mercer, H. Rivera, Jr., and M. D. Burkart. 2006. Synthesis and evaluation of bioorthogonal pantetheine analogues for in vivo protein modification. J. Am. Chem. Soc. 128:12174-12184. [DOI] [PubMed] [Google Scholar]
- 15.Polacco, M. L., and J. E. Cronan, Jr. 1981. A mutant of Escherichia coli conditionally defective in the synthesis of holo-[acyl carrier protein]. J. Biol. Chem. 256:5750-5754. [PubMed] [Google Scholar]
- 16.Rock, C. O., and J. E. Cronan, Jr. 1981. Acyl carrier protein from Escherichia coli. Methods Enzymol. 71(Pt C):341-351. [DOI] [PubMed] [Google Scholar]
- 17.Singer, M., T. A. Baker, G. Schnitzler, S. M. Deischel, M. Goel, W. Dove, K. J. Jaacks, A. D. Grossman, J. W. Erickson, and C. A. Gross. 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 53:1-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strauss, E., and T. P. Begley. 2002. The antibiotic activity of N-pentylpantothenamide results from its conversion to ethyldethia-coenzyme A, a coenzyme A antimetabolite. J. Biol. Chem. 277:48205-48209. [DOI] [PubMed] [Google Scholar]
- 19.Thomas, J., and J. E. Cronan. 2005. The enigmatic acyl carrier protein phosphodiesterase of Escherichia coli: genetic and enzymological characterization. J. Biol. Chem. 280:34675-34683. [DOI] [PubMed] [Google Scholar]
- 20.Vagelos, P. R., and A. R. Larrabee. 1967. Acyl carrier protein. IX. Acyl carrier protein hydrolase. J. Biol. Chem. 242:1776-1781. [PubMed] [Google Scholar]
- 21.Virga, K. G., Y. M. Zhang, R. Leonardi, R. A. Ivey, K. Hevener, H. W. Park, S. Jackowski, C. O. Rock, and R. E. Lee. 2006. Structure-activity relationships and enzyme inhibition of pantothenamide-type pantothenate kinase inhibitors. Bioorg. Med. Chem. 14:1007-1020. [DOI] [PubMed] [Google Scholar]
- 22.Williamson, J. M., and G. M. Brown. 1979. Purification and properties of l-aspartate-alpha-decarboxylase, an enzyme that catalyzes the formation of β-alanine in Escherichia coli. J. Biol. Chem. 254:8074-8082. [PubMed] [Google Scholar]
- 23.Zhang, Y. M., M. W. Frank, K. G. Virga, R. E. Lee, C. O. Rock, and S. Jackowski. 2004. Acyl carrier protein is a cellular target for the antibacterial action of the pantothenamide class of pantothenate antimetabolites. J. Biol. Chem. 279:50969-50975. [DOI] [PubMed] [Google Scholar]