Abstract
We compared the kinetics of amphotericin B (AMB) lung accumulation and fungal clearance by liposomal amphotericin B (L-AMB) and amphotericin B lipid complex (ABLC) in a neutropenic murine model of invasive pulmonary mucormycosis (IPM). Immunosuppressed BALB/c mice were inoculated with 1 × 106 Rhizopus oryzae spores and administered L-AMB or ABLC at daily intravenous doses of 1, 5, or 10 mg/kg of body weight for 5 days starting 12 h after infection. At a dose of 10 mg/kg/day, both L-AMB and ABLC were effective at reducing the R. oryzae lung fungal burden and achieved lung tissue concentrations exceeding the isolate mean fungicidal concentration (MFC) of 8 μg/ml by 72 h. When ABLC was dosed at 5 mg/kg/day, the ABLC-treated animals had significantly higher AMB lung concentrations than the L-AMB treated animals at 24 h (6.64 and 1.44 μg/g, respectively; P = 0.013) and 72 h (7.49 and 1.03 μg/g, respectively; P = 0.005), and these higher concentrations were associated with improved fungal clearance, as determined by quantitative real-time PCR (mean conidial equivalent of R. oryzae DNA per lung, 4.44 ± 0.44 and 6.57 ± 0.74 log10, respectively; P < 0.001). Analysis of the AMB tissue concentration-response relationships revealed that the suppression of R. oryzae growth in the lung required tissue concentrations that approached the MFC for the infecting isolate (50% effective concentration, 8.19 μg/g [95% confidence interval, 2.81 to 18.1 μg/g]). The rates of survival were similar in the animals treated with L-AMB and ABLC at 10 mg/kg/day. These data suggest that higher initial doses may be required during L-AMB treatment than during ABLC treatment of experimental IPM.
Invasive pulmonary mucormycosis (IPM) is an uncommon but frequently fatal angioinvasive mold infection that has increased in incidence over the last decade, especially in patients with hematological malignancies and recipients of hematopoietic stem cell transplantation (HSCT) (23). In a recent multicenter, prospective observational study of invasive fungal infections in HSCT recipients, mucormycosis was the third most common invasive fungal infection (7.2%), behind invasive aspergillosis (59.2%) and invasive candidiasis (24.8%) (21). Data from the Centers for Disease Control and Prevention Transplant Associated Infection Surveillance Network (TRANSNET) reported that the incidence of mucormycosis in U.S. transplant centers increased nearly sixfold from 2001 to 2004, with Rhizopus being the most frequently isolated genus (22).
Although new diagnostic and treatment options have improved the survival rates in patients with invasive pulmonary aspergillosis (IPA) over the last decade, the prognosis for patients with IPM remains poor, as only one-third of the patients survive beyond 12 weeks after the diagnosis (13, 21, 23). The outcome of IPM is heavily dependent on a timely diagnosis, as the initial clinical manifestations and radiographic appearance of IPM are often indistinguishable from those of IPA, and the first-line antifungals used to treat aspergillosis, such as voriconazole, lack activity against members of the order Mucorales (24). In one case series, 84% of leukemia and HSCT patients were receiving ineffective antifungal therapy at the time of diagnosis of IPM (15). Similarly, we found that delays in the administration of lipid amphotericin B (AMB) formulations as few as 6 days from the time of the initial appearance of symptoms was associated with a doubling of the 12-week mortality rate for IPM (48.6% and 82.9%, respectively; P = 0.029) (6). These data suggest that the rapid delivery to infected organs of antifungals active against Mucorales is critical to suppress fungal proliferation and reduce the potential for angioinvasion and subsequent dissemination (6).
Although no prospective randomized trials have compared antifungals for the primary treatment of IPM, lipid formulations of AMB are considered the first-line treatment during the acute phases of infection due to their spectra of activity and predictable pharmacokinetics (12). Currently, two lipid formulations are frequently prescribed for the treatment of IPM: AMB lipid complex (ABLC) and liposomal AMB (L-AMB). These formulations differ in their compositions, particle sizes, and pharmacokinetic behaviors. L-AMB consists of small unilamellar particles (60 to 70 nm) that avoid uptake by the mononuclear phagocytic system (MPS) (28). Hence, the intravenous administration of L-AMB results in sustained, high concentrations of encapsulated AMB in the bloodstream and a somewhat delayed distribution of free drug into tissue. Conversely, the intravenous administration of the larger-particle ABLC formulation (1,600 to 11,000 nm) results in relatively lower AMB bloodstream concentrations due to the rapid uptake and distribution to tissues rich in mononuclear phagocytic cells, including lung tissue (9, 18, 19). The clinical relevance of these pharmacokinetic differences between L-AMB and ABLC, however, remains unknown.
In a previous study, we examined how pharmacokinetic differences between L-AMB and ABLC affected the rate and extent of fungal clearance in a neutropenic murine model of IPA (16). At daily doses of <10 mg/kg of body weight per day, ABLC treatment achieved significantly higher concentrations of AMB in lung tissue at earlier time points of therapy, and the higher concentrations were associated with the more rapid clearance of Aspergillus fumigatus. However, both formulations were effective after 5 days of therapy for the treatment of this AMB-susceptible isolate. Nevertheless, formulation-dependent differences in antifungal pharmacokinetics may be more critical in the treatment of pulmonary infections caused by more angioinvasive, amphotericin B-tolerant pathogens, such as Rhizopus orzyae. Therefore, we examined whether the differences in the pharmacokinetics between L-AMB and ABLC may require the use of different dosing approaches for the treatment of mucormycosis. To explore this question, we compared (i) the patterns of AMB accumulation in lung tissue following intravenous treatment with L-AMB or ABLC and (ii) the dose-dependent patterns of Rhizopus oryzae clearance from the lung in an experimental model of IPM.
(This work was previously presented at the 48th Interscience Conference on Antimicrobial Agents and Chemotherapy [15a].)
MATERIALS AND METHODS
Reagents.
Cortisone acetate, cyclophosphamide, and AMB powder were obtained from Sigma-Aldrich (St. Louis, MO). Commercial formulations of L-AMB (Ambisome; Astellas Inc., Deerfield, IL) and ABLC (Abelcet; Enzon Inc., Bridgewater, NJ) were obtained from the respective manufacturers. Antifungals were reconstituted according to the manufacturers' instructions and diluted in 5% dextrose before intravenous administration to animals. Cyclophosphamide and cortisone were prepared and administered as described previously (17).
Animals.
Eight-week-old BALB/c mice (weight, 18 to 22 g; Harlan Laboratories) were used for all experiments. The mice were housed in HEPA-filtered cage systems containing sterilized food and 5% dextrose-water that was provided ad libitum. All mice were cared for in accordance with the highest standards of humane and ethical care, as approved by The University of Texas M. D. Anderson Cancer Center and the University of Houston Institutional Animal Care and Use Committees.
Inoculum preparation.
A clinical strain of Rhizopus oryzae isolated from resected lung tissue from a leukemia patient with IPM was used for all experiments. The isolate was cultivated on yeast agar (YAG) plates (0.5% yeast extract, 1.0% dextrose, 0.2% vitamin mix, 0.1% trace elements, 1.5% agar, 1% MgSO4) for 96 h before the zygospores were harvested by flooding the plate with phosphate-buffered saline (PBS)-0.1% Tween 20. The suspension was then passed through a 40-μm-pore-size filter (BD Biosciences, San Jose, CA) to remove the hyphal elements. The spore suspension was then centrifuged at 15,000 × g for 5 min, the supernatant was discarded, and the concentration was adjusted to 3 × 107 spores/ml by use of a hemocytometer. The harvested spores were >99% viable when serial dilutions were plated on YAG plates. The AMB MICs and the mean fungicidal concentration (MFC) were determined for the test isolate in triplicate by using amphotericin B epsilometer strips (AB Biodisk, Solna, Sweden) and published methods for filamentous fungi (8).
Immunosuppression and infection.
Mice were immunosuppressed with 150-mg/kg intraperitoneal injections of cyclophosphamide on days −4 and −1 prior to inoculation. This regimen results in the total reduction of peripheral polymorphonuclear cells for up to 96 h after infection (17). A repeat dose of cyclophosphamide (100 mg/kg) was administered at day +3 after animal infection to maintain neutropenia until the animals were euthanized at day +5. A single dose of cortisone acetate (300 mg/kg administered subcutaneously) was administered 1 day prior to infection to suppress pulmonary alveolar macrophage function. Doxycycline HCl (Sigma) was supplemented in the animal drinking water as antibacterial prophylaxis. Overall, this immunosuppression regimen resulted in <15% changes in animal body weight and no mortality among uninfected animals.
Before inoculation, the animals were rendered unconscious with aerosolized 10% isoflurane-oxygen delivered in a small-animal anesthesia chamber. An inoculum of 1 × 106 R. oryzae spores was then administered by the slow instillation of 35-μl droplets into both nares. The mice, which were in an upright position, were allowed to inhale the inoculum until normal breathing resumed and the animal regained consciousness. This protocol was found in previous studies to result in reproducible pulmonary mucormycosis that is fatal by 4 to 7 days after inhalation if the animals do not receive antifungal treatment (14).
Antifungal treatment and sample collection.
Groups of immunosuppressed infected mice (n = 40 per treatment group) received intravenous antifungal therapy with L-AMB or ABLC at a dose of 1, 5, or 10 mg/kg of body weight diluted in sterile 5% dextrose-water, which was administered once daily by injection into the lateral tail vein with a 30-gauge needle. All antifungal regimens were started 12 h after inoculation and continued until day +4 (a total of five doses). Control animals were administered 5% dextrose-water alone. At serial time points after infection (0, 24, 72, and 120 h), groups of 10 mice each were euthanized prior to the next scheduled drug dose by CO2 narcosis, and the lungs were aseptically removed and stored at −80°C until analysis. Animals exhibiting two or more signs of morbidity (i.e., ruffled fur, hunched posture, limited ambulation, hypothermia, or respiratory difficulty) before the planned time points for euthanasia were euthanized, and data for those mice were included with the data for the subsequent time point of analysis.
Tissue fungal burden.
The lung tissue R. oryzae fungal burden was determined by real-time quantitative PCR by previously reported methods (10). Briefly, DNA isolated from an aliquot (80 μl) of weighed lung homogenized in 1 ml of PBS was assayed in duplicate with an ABI Prism 7000 sequence detection system (Applied Biosystems, Foster City, CA) with PCR primers and a dually labeled fluorescent hybridization probe specific for the R. oryzae 18S rRNA gene (10). The cycle threshold of each sample was interpolated from a six-point standard curve prepared by spiking uninfected mouse lung tissue with known concentrations of R. oryzae conidia (102 to 107). A plasmid internal standard was amplified in separate reactions to correct for the percent differences in DNA recovery (26-28). The results are reported as the conidial equivalent (CE) of R. oryzae DNA per lung.
Histologic assessment of fungal burden.
To further evaluate the dose-dependent differences in the clearance of R. orzyae, we compared the histological patterns of fungal invasion in groups of five mice each infected and treated as described above. At 120 h, the mice were euthanized and their lungs were fixed in 10% (vol/vol) formaldehyde before they were embedded in paraffin wax. Matched sections were then stained with Grocott's methenamine silver nitrate. Images were acquired at ×100 and ×400 magnifications by light microscopy from three separate areas of lung infiltration in each of the five mice per treatment group. The hyphal length was then calculated for all discernible hyphal structures (average, 25 to 50) per infiltrate field by the use of calibrated computerized image analysis software (Macnification, version 1.5; Orbicule BVBA, Heverlee, Belgium). The median differences in hyphal lengths were then compared for each treatment group by the Kruskal-Wallis test with Dunn's test for multiple comparisons.
AMB concentration analysis.
Determinations of the total AMB concentrations in the infected lungs were performed by high-performance liquid chromatography, as described previously (16, 27). Briefly, AMB and a spiked internal standard (1-amino-4-nitro-naphthalene [ANNP]) were isolated from tissue homogenates by acetonitrile precipitation of proteins, followed by centrifugation. A 50-μl aliquot of the extracted supernatant was injected through a C18 guard column into a Nova-Pak C18 column (3.9 mm by 150 mm by 4 μm). AMB and ANNP were eluted at flow rate of 1 ml per minute with a gradient program (acetonitrile from 30 to 45% plus 2.5 mM EDTA from 70% to 55% in 8 min) and were detected at 406 nm. The calibration curve was linear over a range of 0.25 to 10 μg/g of tissue. Samples with high off-scale values were diluted to match the standard curve (16, 27). Mean intra- and interassay coefficients of variation over the range of the standard curve were <10%. The lower limit of accurately detectable AMB in tissue was 0.25 μg/g.
Statistical analysis.
All graphical data were expressed as means ± standard deviations and were compared by the Mann-Whitney test or the Kruskal-Wallis test with Dunn's posttest for multiple comparisons, where appropriate. Fungal burden data were log transformed before they were plotted and statistical analysis. Tissue concentrations were expressed as the mean concentrations ± standard deviation of amphotericin B (μg)/lung weight per each treatment group. The differences were considered statistically significant when the P values were ≤0.05. The total amphotericin B concentrations associated with a 50% reduction in the R. oryzae lung tissue fungal burden at 72 h (the 50% effective concentration [EC50]) were determined by fitting the following four-parameter logistic regression model to the experimental data by using computer curve-fitting software (Prism, version 5; GraphPad Software Inc., San Diego, CA): 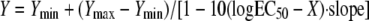 , where Y is response, Ymin is the bottom of the concentration effect curve, Ymax is the top of the concentration effect curve, and X is the logarithm of the AMB concentration in tissue. The goodness of fit was assessed by determining the R2 value and the standard error of the EC50 estimate.
, where Y is response, Ymin is the bottom of the concentration effect curve, Ymax is the top of the concentration effect curve, and X is the logarithm of the AMB concentration in tissue. The goodness of fit was assessed by determining the R2 value and the standard error of the EC50 estimate.
RESULTS
Isolate susceptibility.
Susceptibility testing was performed in triplicate with the R. oryzae test isolate and revealed an AMB MIC of 0.5 μg/ml and an MFC of 8 μg/ml. The MIC and MFC results were consistent throughout the study and with data from previously published in vitro studies that tested the activity of AMB against the same species (7, 12).
Comparative kinetics of fungal burden reduction.
Plots of the R. orzyae tissue fungal burden at the baseline and 24, 72, and 120 h after infection versus the total lung AMB concentrations are presented in Fig. 1. After inoculation, the mean baseline fungal burden in lung tissue for all treatment groups was 5.2 + 0.34 log10 R. oryzae CE DNA. In control animals (treated with 5% dextrose-water), the lung tissue fungal burden increased by an average of 1.5 log10 CE by 120 h. The onset of animal morbidity requiring euthanization occurred in the untreated control groups at between 96 and 120 h after infection.
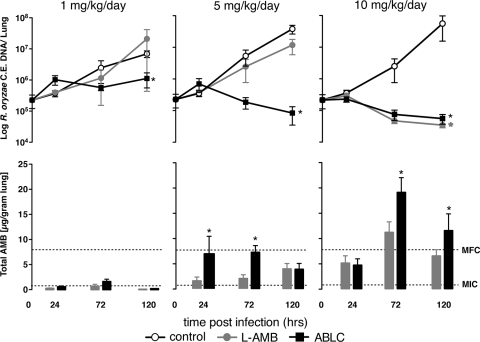
FIG. 1.
L-AMB and ABLC exhibit different dose-dependent patterns of drug delivery and clearance of Rhizopus oryzae in experimental invasive pulmonary mucormycosis in neutropenic mice. (Top row) Relationship of the R. orzyae burden in the different treatment groups versus time. Each datum point represents the mean ± standard deviation of the R. orzyae CE DNA isolated from the lungs of 10 mice assayed by real-time quantitative PCR. (Bottom row) The corresponding mean ± standard deviation total lung tissue amphotericin B concentrations determined by high-performance liquid chromatography analysis of lung tissue homogenates. Concentrations were corrected for the weight of the lung tissue and the volume of the homogenate. *, P < 0.05 by the Kruskal-Wallis test with Dunn's multiple-comparison test. Dotted lines display the MIC and the MFC of the R. oryzae test strain determined by Etest and the CLSI M38-A2 method (6).
Mice treated with ABLC or L-AMB at daily intravenous doses of 1 mg/kg/day demonstrated patterns of increasing fungal burden similar to those for the control group, with the AMB concentrations in the lung tissue being minimally detectable (medians at 72 h, 0.49 and 0.25 μg/g, respectively; P = 0.14). However, modest suppression of the lung fungal burden was evident in the ABLC-treated animals but not the L-AMB-treated animals by 120 h (change of −0.7 log10 versus the value for the control; P < 0.05).
At 5 mg/kg/day, ABLC-treated animals had significantly higher lung tissue AMB concentrations than the L-AMB-treated animals at 24 h (6.64 and 1.44 μg/g, respectively; P = 0.013) and 72 h (7.49 and 1.03 μg/g, respectively; P = 0.005). Lung tissue AMB concentrations were similar in both treatment groups at 120 h (3.75 and 3.70 μg/g, respectively; P = 1.0). The higher lung tissue AMB concentrations in the ABLC-treated animals were associated with significant reductions in the lung tissue fungal burden by 120 h compared with those for the controls (means, 4.44 ± 0.64 and 7.16 ± 0.76 log10 R. oryzae CE, respectively; P < 0.001) (Fig. 1). In contrast to the results for the ABLC-treated animals, L-AMB at 5 mg/kg/day did not significantly reduce the R. orzyae lung tissue burden compared with that for the controls by 120 h (6.57 ± 0.74 and 7.16 ± 0.76 log10 R. oryzae CE, respectively; P = 0.09) (Fig. 1).
At 10 mg/kg/day, both lipid formulations produced AMB concentrations in lung tissue that surpassed the in vitro MFC of the R. oryzae strain by 72 h (18.6 μg/g ABLC and 9.62 μg/g for L-AMB; P = 0.04). Similarly, both AMB formulations reduced the R. oryzae lung tissue burden by over 2 log10 compared with that in the control animals (4.6 ± 0.42 log10 R. oryzae CE for ABLC versus 4.53 ± 0.23 log10 R. oryzae CE for L-AMB; P = 0.36). Although the increase in the dose from 5 to 10 mg/kg/day resulted in significantly higher lung tissue concentrations for both formulations at all time points tested, a corresponding improvement in antifungal efficacy was observed only for L-AMB (6.57 ± 0.74 and 4.53 ± 0.23 log10 R. oryzae CE, respectively; P < 0.001) and not for ABLC-treated animals (4.44 ± 0.64 and 4.6 ± 0.42 log10 R. oryzae CE, respectively; P = 0.98).
Histological patterns of fungal clearance.
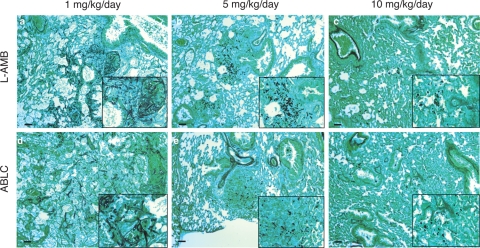
Lung histological sections demonstrated dose-dependent reductions in R. oryzae hyphal length and invasion with increasing L-AMB and ABLC treatment doses. The infiltrates in control animals demonstrated extensive hyphal spreading and angioinvasion, with the median hyphal lengths being 25 μm (Table 1). The hyphal numbers and length were markedly reduced with increasing doses of L-AMB and ABLC (Fig. 2). For L-AMB, the maximal suppression of hyphal invasion was observed as the daily dosage increased from 5 to 10 mg/kg/day (Fig. 2b and c), consistent with the results of the quantitative PCR assay. For ABLC, the maximal suppression of hyphal invasion was observed at daily doses of 5 mg/kg/day (Fig. 2e and f). The median hyphal length per infiltrate field was significantly different for animals treated with L-AMB and ABLC at 5 mg/kg/day (13.3 and 7.4 μm, respectively; P < 0.01) but not 10 mg/kg/day (5.1 and 6.9 μm, respectively; P > 0.05).
TABLE 1.
Median hyphal length in lung histological specimens correlates with dose-dependent differences in the activity of the lipid amphotericin B formulation against experimental acute invasive pulmonary mucormycosis
| Treatment group (dose [mg/kg]) | No. of hyphae measured | Median (range) hyphal length (μm)a | P value for L-AMB vs ABLCb |
|---|---|---|---|
| Control | 365 | 24.9 (3.4-67.7) | |
| L-AMB (1) | 490 | 17.2 (2.2-38.9) | |
| ABLC (1) | 420 | 18.0 (2-55.2) | >0.05 |
| L-AMB (5) | 330 | 13.3 (2-32.9) | |
| ABLC (5) | 245 | 7.4 (2.1-19.1) | <0.01 |
| L-AMB (10) | 250 | 5.1 (2.1-25.2) | |
| ABLC (10) | 285 | 6.9 (2.1-20.6) | >0.05 |
Micrographs were acquired from three separate areas of lung infiltration containing hyphae in groups of five mice. The hyphal length was calculated for all discernible hyphal elements in the infiltrate field by the use of calibrated computerized image analysis software (Macnification, version 1.5; Orbicule BVBA).
The difference in the hyphal length between L-AMB- and ABLC-treated animals at each dose was compared by the Kruskal-Wallis test with Dunn's test for multiple comparisons.
FIG. 2.
L-AMB and ABLC exhibit different histological patterns of dose-dependent control of R. oryzae lung tissue infection burden in experimental IPM. Representative microscopic images of lung histology from groups of five infected mice following 5 days of intravenous therapy with either L-AMB or ABLC at 1, 5, or 10 mg/kg/day are shown. The lungs were excised and fixed in 10% (vol/vol) formaldehyde and embedded in paraffin wax, and matched sections were stained with Grocott's methenamine silver nitrate. Dose-dependent reductions in the numbers of invading hyphae and hyphal length are evident for both formulations. Bars, 25 μm at ×100 magnification; magnifications for inset boxes, ×400.
Amphotericin B lung tissue concentration and survival.
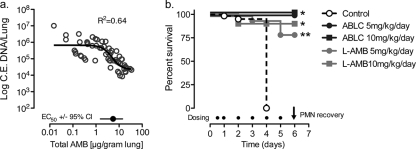
To better explore the relationship between the AMB lung tissue concentrations and an early treatment response, we compared the lung tissue concentrations and R. oryzae fungal burden at 72 h for all L-AMB- and ABLC-treated animals (Fig. 3a). A four-parameter logistic regression model was fitted to the experimental data to estimate the lung tissue EC50 ± 95% confidence interval (CI). The total AMB EC50 in lung tissue was above the MIC and near the MFC for the clinical R. oryzae isolate used to infect the animals (8.19 μg/g; 95% CI, 2.81 to 18.1). This concentration was four- to eightfold higher than the EC50 at 72 h noted for lipid AMB formulations in our previous experimental model of invasive aspergillosis (16).
FIG. 3.
High tissue concentrations of amphotericin B may be required to control fungal growth and improve early survival in neutropenic animals infected with R. oryzae. The relationship of lung fungal burden (conidial equivalent DNA per lung; C.E. DNA) was compared to the amphotericin B tissue concentrations at 72 h for R. oryzae (a). The EC50 in lung tissue was determined by fitting a four-parameter logistic regression model (Hill equation, sigmoidal curve with variable slope) to the fungal burden data by the use of GraphPad Prism (version 5.0) software, as follows: 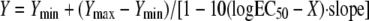 . The broth microdilution MIC and MFC for R. oryzae are 0.5 and 8 μg/ml, respectively. (b) Survival of neutropenic mice (n = 20 per treatment group) following treatment with 5% dextrose (control) or with L-AMB or ABLC at 5 or 10 mg/kg/day. Filled circles, time of dosing. *, P < 0.001 versus the results for the control determined by the log-rank (Mantel-Cox) test; **, P < 0.05 versus the results obtained with ABLC at 5 mg/kg/day determined by the log-rank (Mantel-Cox) test.
. The broth microdilution MIC and MFC for R. oryzae are 0.5 and 8 μg/ml, respectively. (b) Survival of neutropenic mice (n = 20 per treatment group) following treatment with 5% dextrose (control) or with L-AMB or ABLC at 5 or 10 mg/kg/day. Filled circles, time of dosing. *, P < 0.001 versus the results for the control determined by the log-rank (Mantel-Cox) test; **, P < 0.05 versus the results obtained with ABLC at 5 mg/kg/day determined by the log-rank (Mantel-Cox) test.
Although differences in the kinetics of AMB delivery to the lung and the clearance of R. oryzae were observed between the two formulations at 5 mg/kg/day, the impacts of these differences in animal survival from IPM were not clear. Therefore, using the same infection procedures described above, we compared the rates of survival for groups of 20 mice treated with 5% dextrose-water (control) or with L-AMB or ABLC at doses of 5 and 10 mg/kg/day (Fig. 3b). The median survival time (MST) for the control animals was ≤4 days. Both L-AMB and ABLC significantly improved the rate of survival of neutropenic mice infected with R. oryzae compared to that of the controls (MST ≥ 6 days; P < 0.001). By the time of polymorphonuclear leukocyte (PMN) recovery in the model (day +6), a significantly higher proportion of ABLC-treated mice than L-AMB-treated mice treated with 5 mg/kg/day were still alive (100% and 80%, respectively; P = 0.04). With the 10-mg/kg/day dose, the proportions of mice in the ABLC- and L-AMB-treated groups that survived were similar (90% and 100%, respectively; P = 0.15).
DISCUSSION
Lipid AMB formulations continue to be important agents in high-risk patients with suspected or documented fungal pneumonia due to their broad spectrum of activity and predictable pharmacokinetics. The two leading lipid AMB formulations, however, exhibit different pharmacokinetic profiles in humans. Compared to the results obtained with conventional AMB-deoxycholate (AMB-d) dosed at 0.6 mg/kg/day, the administration of L-AMB at 5 mg/kg/day in patients results in sustained high intravascular (bloodstream) concentrations of encapsulated AMB (e.g., maximum concentrations in plasma [Cmaxs], 83 and 1.1 μg/ml, respectively; areas under the concentration-time curves [AUCs], 555 and 17.1 μg·h/ml, respectively) and relatively low volumes of distribution (0.11 and 5 liters/kg, respectively) before the drug enters the tissues (5, 19). In contrast, intravenous ABLC at 5 mg/kg produces bloodstream exposures comparable to those achieved with AMB-d (Cmaxs, 1.7 and 1.1 μg/ml, respectively; AUCs, 14 and 17.1 μg·h/ml, respectively) but has a significantly higher volume of distribution in mononuclear phagocyte systems (MPS)-rich tissue sites (131 and 5 liters/kg, respectively) (5, 19). These pharmacokinetic differences observed in mice, rabbits, and humans would seem to result in different dose-response relationships, depending on the site of infection (tissue) and the susceptibility of the pathogen. However, evidence of clinically significant differences in the dose-response curve for each formulation is lacking and is unlikely to be explored in comparative prospective randomized trials, especially for less common molds such as those that cause mucormycosis.
To this end, animal models can provide insight into possible formulation-dependent differences in pharmacodynamics for rare molds. In a previous study, we found that treatment with the L-AMB formulation at ≤5 mg/kg/day was associated with the effective, albeit delayed, delivery of AMB to the lungs of neutropenic mice that allowed the proliferation of A. fumigatus for the first 24 to 48 h of treatment (16). In contrast, treatment with ABLC at 5 mg/kg/day achieved concentrations of AMB in the lungs during the first 24 h that surpassed the MFC for the infecting isolate. This result corresponds to early control of the infection, that is, in the first 24 to 72 h. Despite these differences in the two formulations, both formulations produced equivalent reductions in the fungal burden by 120 h and dosages of >5 mg/kg/day did not appear to result in improvements of antifungal activity, a finding that was consistent with the results of a recent prospective randomized trial that failed to show a benefit of 10 mg/kg/day of L-AMB (7).
We hypothesized that the dose-dependent differences in the kinetics of AMB distribution to the lung observed between L-AMB and ABLC may be more important for the control of rapidly angioinvasive molds that are more tolerant of high AMB concentrations, such as R. oryzae. Using a design similar to that which we used in our previous study with A. fumigatus, we found significant dose-dependent differences in the efficacies of L-AMB and ABLC for the treatment of experimental IPM. These differences correlated with the early delivery of AMB to lung tissue during the first 72 h of infection. In L-AMB-treated animals receiving daily doses of ≤5 mg/kg/day, maximal tissue concentrations were not achieved until after 72 h after infection and never surpassed the MFC of the infecting isolate (Fig. 1). Consequently, the proliferation of R. oryzae was similar to that in the control (dextrose-treated) animals. However, when the intravenous dose of L-AMB was increased to 10 mg/kg, lung tissue AMB concentrations surpassed the MFC of the infecting isolate within the first 72 h of infection, resulting in a significantly lower fungal burden compared with that in the control animals. In contrast, ABLC administered at 5 mg/kg/day appeared to deliver within the first 72 h an amount of AMB to the lung that was sufficient to reduce the R. oryzae fungal burden, and no improvement was seen as the dose was increased to 10 mg/kg/day. These differences between the two lipid formulations used at 5 mg/kg/day were supported by the findings of histopathological analysis (Fig. 2; Table 1).
Similar to other studies performed with liposomal carriers and pneumonia models (1), we found that the pattern of amphotericin B delivery to the lung was dose dependent and probably nonlinear at doses of >5 mg/kg/day. This finding is consistent with the pharmacokinetics of escalating dosages of L-AMB in humans, in which changes in disposition processing (e.g., clearance from serum) are observed at doses of >7.5 mg/kg/day, suggestive of a possible first-pass effect in the lungs at higher daily dosages (25). Consistent with the findings of our previous study, we also observed a pattern of decreasing lung tissue L-AMB concentrations with the mycological response by 120 h. The infection inoculum and the degree of damage to the lung tissue have been shown to significantly affect the distribution of these carriers (1); therefore, it seems likely that accumulation of the lipid formulations declines with a decreasing intensity of infection.
To our knowledge, only one other published study has directly compared the efficacy of lipid AMB formulations for the treatment of experimental mucormycosis. Ibrahim and colleagues utilized diabetic ketoacidotic (DKA) and neutropenic murine models to compare the activities of L-AMB and ABLC against disseminated mucormycosis (11). In the DKA model, treatment with ABLC at 7.5 or 15 mg/kg/day was associated with a significantly poorer rate of survival compared to that achieved with a similar L-AMB treatment dose. Interestingly, the difference in survival was evident in the DKA mouse model but not the neutropenic mouse model of mucormycosis. L-AMB also appeared to be more effective at reducing the numbers of CFU in the brain than similar doses of ABLC, although the differences did not correlate with the brain tissue AMB concentrations measured by bioassay. The authors' findings of improved survival with L-AMB treatment compared with that with ABLC treatment is not surprising, given the method of infection (intravenous injection) and the tissue sites that would favor the formulation with higher sustained bloodstream concentrations, i.e., L-AMB. When the findings of those studies are compared with our data, these studies underscore how differences in the efficacies of lipid AMB formulations cannot be extrapolated to all infection sites and immunosuppression backgrounds (11).
Like all animal models of invasive fungal infection, our data should be interpreted with several caveats. First, while our murine model simulates many aspects of IPM in neutropenic patients, the hyperacute infection induced in animals may exaggerate the differences between antifungal agents that are less relevant for human infections with a slower course of progression. Nevertheless, IPM often presents as a rapidly progressing infection in the neutropenic or HSCT patient, and the consequences are dire if effective antifungal therapy is not administered early (6). The validity of measuring total drug concentrations in the tissue homogenate can be questioned for pharmacodynamic studies, especially for antibacterial agents that are primarily active in extracellular compartments (20). However, invasive molds invade both extracellular and intracellular compartments, and in our opinion, the complexity of AMB protein binding (which is concentration dependent and unsaturable) precludes simple estimates of the bioactivity of a drug based on serum pharmacokinetic profiles (4). Parallel analysis of serum amphotericin B pharmacokinetic/pharmacodynamic parameters (i.e., serum Cmax/MIC, AUC/MIC, or the time that the concentration is greater than the MIC) may prove useful and may serve as a surrogate marker for predicting the target tissue concentrations in the lung required to suppress the growth of Rhizopus and Aspergillus. Nevertheless, a number of fixed and dynamic factors influence liposome extravasation from the bloodstream into the lung, including liposome characteristics, drug characteristics, the level of protein binding, and the presence of lung disease or infection (1). Finally, our studies were performed with a single isolate of R. oryzae in one type of immunosuppression background, with treatment with the antifungals being started at a fixed interval (12 h) after infection of the animals. The magnitude of the differences between the two lipid AMB formulations almost certainly varies on the basis of the isolates that are tested, the infection site, the underlying type of immunosuppression, and the time point when antifungal therapy is administered (2, 3).
In summary, our comparative analysis of L-AMB and ABLC for the treatment of acute experimental IPM in neutropenic mice demonstrated significant differences in the dose-dependent activities of the two lipid formulations. The findings obtained with our experimental model were consistent with the concept that the efficacies of antifungals for the treatment of IPM are closely tied to the rapid loading of the target tissues with concentrations of drug sufficient to suppress fungal proliferation and reduce the potential for angioinvasion and subsequent dissemination. Different dosing approaches for L-AMB and ABLC may be required during the initial phase of treatment of IPM to achieve this goal in patients.
Acknowledgments
Enzon Pharmaceuticals provided research support for this study but was not involved with the study design, completion, data analysis, or the conclusions provided in the manuscript.
R.E.L. and D.P.K. received research support and consultancy fees from Merck & Co., Inc., Astellas Inc., and Gilead Inc. R.A.P. received research support from Merck & Co., Inc., Ortho McNeil Inc., Cubist, and Enzon.
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Bakker-Woudenberg, I. A., A. F. Lokerse, M. T. ten Kate, J. W. Mouton, M. C. Woodle, and G. Storm. 1993. Liposomes with prolonged blood circulation and selective localization in Klebsiella pneumoniae-infected lung tissue. J. Infect. Dis. 168:164-171. [DOI] [PubMed] [Google Scholar]
- 2.Balloy, V., M. Huerre, J. P. Latge, and M. Chignard. 2005. Differences in patterns of infection and inflammation for corticosteroid treatment and chemotherapy in experimental invasive pulmonary aspergillosis. Infect. Immun. 73:494-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Becker, M. J., S. de Marie, M. Fens, W. Hop, H. Verbrugh, and I. Bakker-Woudenberg. 2002. Enhanced antifungal efficacy in experimental invasive pulmonary aspergillosis by combination of AmBisome with Fungizone as assessed by several parameters of antifungal response. J. Antimicrob. Chemother. 49:813-820. [DOI] [PubMed] [Google Scholar]
- 4.Bekersky, I., R. M. Fielding, D. E. Dressler, J. W. Lee, D. N. Buell, and T. J. Walsh. 2002. Plasma protein binding of amphotericin B and pharmacokinetics of bound versus unbound amphotericin B after administration of intravenous liposomal amphotericin B (AmBisome) and amphotericin B deoxycholate. Antimicrob. Agents Chemother. 46:834-840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boswell, G. W., D. Buell, and I. Bekersky. 1998. AmBisome (liposomal amphotericin B): a comparative review. J. Clin. Pharmacol. 38:583-592. [DOI] [PubMed] [Google Scholar]
- 6.Chamilos, G., R. E. Lewis, and D. P. Kontoyiannis. 2008. Delaying amphotericin B-based front-line therapy significantly increases mortality in hematologic malignancy patients with zygomycosis. Clin. Infect. Dis. 47:503-509. [DOI] [PubMed] [Google Scholar]
- 7.Cornely, O. A., J. Maertens, M. Bresnik, R. Ebrahimi, A. J. Ullmann, E. Bouza, C. P. Heussel, O. Lortholary, C. Rieger, A. Boehme, M. Aoun, H. A. Horst, A. Thiebaut, M. Ruhnke, D. Reichert, N. Vianelli, S. W. Krause, E. Olavarria, and R. Herbrecht. 2007. Liposomal amphotericin B as initial therapy for invasive mold infection: a randomized trial comparing a high-loading dose regimen with standard dosing (AmBiLoad trial). Clin. Infect. Dis. 44:1289-1297. [DOI] [PubMed] [Google Scholar]
- 8.Espinel-Ingroff, A., V. Chaturvedi, A. Fothergill, and M. G. Rinaldi. 2002. Optimal testing conditions for determining MICs and minimum fungicidal concentrations of new and established antifungal agents for uncommon molds: NCCLS collaborative study. J. Clin. Microbiol. 40:3776-3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Groll, A. H., C. A. Lyman, V. Petraitis, R. Petraitiene, D. Armstrong, D. Mickiene, R. M. Alfaro, R. L. Schaufele, T. Sein, J. Bacher, and T. J. Walsh. 2006. Compartmentalized intrapulmonary pharmacokinetics of amphotericin B and its lipid formulations. Antimicrob. Agents Chemother. 50:3418-3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ibrahim, A. S., J. C. Bowman, V. Avanessian, K. Brown, B. Spellberg, J. E. Edwards, Jr., and C. M. Douglas. 2005. Caspofungin inhibits Rhizopus oryzae 1,3-beta-d-glucan synthase, lowers burden in brain measured by quantitative PCR, and improves survival at a low but not a high dose during murine disseminated zygomycosis. Antimicrob. Agents Chemother. 49:721-727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim, A. S., T. Gebremariam, M. I. Husseiny, D. A. Stevens, Y. Fu, J. E. Edwards, Jr., and B. Spellberg. 2008. Comparison of lipid amphotericin B preparations in treating murine zygomycosis. Antimicrob. Agents Chemother. 52:1573-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kontoyiannis, D. P., and R. E. Lewis. 2006. Invasive zygomycosis: update on pathogenesis, clinical manifestations, and management. Infect. Dis. Clin. North Am. 20:581-607, vi. [DOI] [PubMed] [Google Scholar]
- 13.Kontoyiannis, D. P., M. S. Lionakis, R. E. Lewis, G. Chamilos, M. Healy, C. Perego, A. Safdar, H. Kantarjian, R. Champlin, T. J. Walsh, and I. I. Raad. 2005. Zygomycosis in a tertiary-care cancer center in the era of Aspergillus-active antifungal therapy: a case-control observational study of 27 recent cases. J. Infect. Dis. 191:1350-1360. [DOI] [PubMed] [Google Scholar]
- 14.Lamaris, G. A., R. Ben-Ami, R. E. Lewis, G. Chamilos, G. Samonis, and D. P. Kontoyiannis. 2009. Increased virulence of Zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J. Infect. Dis. 199:1399-1406. [DOI] [PubMed] [Google Scholar]
- 15.Lass-Florl, C., G. Resch, D. Nachbaur, A. Mayr, G. Gastl, J. Auberger, R. Bialek, and M. C. Freund. 2007. The value of computed tomography-guided percutaneous lung biopsy for diagnosis of invasive fungal infection in immunocompromised patients. Clin. Infect. Dis. 45:e101-e104. [DOI] [PubMed] [Google Scholar]
- 15a.Lewis, R. E., N. D. Albert, G. Liao, J. Hou, R. A. Prince, and D. P. Kontoyiannis. 2008. Abstr. 48th Intersci. Conf. Antimicrob. Agents Chemother., abstr. M-2139.
- 16.Lewis, R. E., G. Liao, J. Hou, G. Chamilos, R. A. Prince, and D. P. Kontoyiannis. 2007. Comparative analysis of amphotericin B lipid complex and liposomal amphotericin B kinetics of lung accumulation and fungal clearance in a murine model of acute invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 51:1253-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lewis, R. E., and N. P. Wiederhold. 2005. Murine model of invasive aspergillosis. Methods Mol. Med. 118:129-142. [DOI] [PubMed] [Google Scholar]
- 18.Linden, P. K. 2003. Amphotericin B lipid complex for the treatment of invasive fungal infections. Expert Opin. Pharmacother. 4:2099-2110. [DOI] [PubMed] [Google Scholar]
- 19.Matot, I., and R. Pizov. 2000. Pulmonary extraction and accumulation of lipid formulations of amphotericin B. Crit. Care Med. 28:2528-2532. [DOI] [PubMed] [Google Scholar]
- 20.Mouton, J. W., U. Theuretzbacher, W. A. Craig, P. M. Tulkens, H. Derendorf, and O. Cars. 2008. Tissue concentrations: do we ever learn? J. Antimicrob. Chemother. 61:235-237. [DOI] [PubMed] [Google Scholar]
- 21.Neofytos, D., D. Horn, E. Anaissie, W. Steinbach, A. Olyaei, J. Fishman, M. Pfaller, C. Chang, K. Webster, and K. Marr. 2009. Epidemiology and outcome of invasive fungal infection in adult hematopoietic stem cell transplant recipients: analysis of Multicenter Prospective Antifungal Therapy (PATH) Alliance registry. Clin. Infect. Dis. 48:265-273. [DOI] [PubMed] [Google Scholar]
- 22.Park, B. J., P. Pappas, K. Marr, E. J. Anaissie, J. Ito, B. D. Alexander, T. J. Walsh, and D. P. Kontoyiannis. 2007. Recent epidemiology and zygomycosis among organ transplant and stem cell transplant recipients: results from the TRANSNET surveillance network, abstr. M-618, p. 434. Abstr. 47th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 23.Roden, M. M., T. E. Zaoutis, W. L. Buchanan, T. A. Knudsen, T. A. Sarkisova, R. L. Schaufele, M. Sein, T. Sein, C. C. Chiou, J. H. Chu, D. P. Kontoyiannis, and T. J. Walsh. 2005. Epidemiology and outcome of zygomycosis: a review of 929 reported cases. Clin. Infect. Dis. 41:634-653. [DOI] [PubMed] [Google Scholar]
- 24.Walsh, T. J., E. J. Anaissie, D. W. Denning, R. Herbrecht, D. P. Kontoyiannis, K. A. Marr, V. A. Morrison, B. H. Segal, W. J. Steinbach, D. A. Stevens, J. A. van Burik, J. R. Wingard, and T. F. Patterson. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327-360. [DOI] [PubMed] [Google Scholar]
- 25.Walsh, T. J., J. L. Goodman, P. Pappas, I. Bekersky, D. N. Buell, M. Roden, J. Barrett, and E. J. Anaissie. 2001. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob. Agents Chemother. 45:3487-3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wiederhold, N. P., D. P. Kontoyiannis, J. Chi, R. A. Prince, V. H. Tam, and R. E. Lewis. 2004. Pharmacodynamics of caspofungin in a murine model of invasive pulmonary aspergillosis: evidence of concentration-dependent activity. J. Infect. Dis. 190:1464-1471. [DOI] [PubMed] [Google Scholar]
- 27.Wiederhold, N. P., V. H. Tam, J. Chi, R. A. Prince, D. P. Kontoyiannis, and R. E. Lewis. 2006. Pharmacodynamic activity of amphotericin B deoxycholate is associated with peak plasma concentrations in a neutropenic murine model of invasive pulmonary aspergillosis. Antimicrob. Agents Chemother. 50:469-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong-Beringer, A., R. A. Jacobs, and B. J. Guglielmo. 1998. Lipid formulations of amphotericin B: clinical efficacy and toxicities. Clin. Infect. Dis. 27:603-618. [DOI] [PubMed] [Google Scholar]