Abstract
A surveillance study was performed in four Singapore public hospitals from 2006 to 2008 to determine the correlation between antibiotic prescription and Gram-negative bacterial antimicrobial resistance. Targeted organisms included ceftriaxone- and ciprofloxacin-resistant Escherichia coli and Klebsiella pneumoniae, as well as imipenem-resistant Pseudomonas aeruginosa and Acinetobacter spp. Antibiotic prescription data were collated in the WHO anatomical therapeutic chemical (ATC)/defined daily dose (DDD) format, while antibiotic resistance was expressed as incidence density adjusted for total inpatient-days every quarter. Individual trends were determined by linear regression, while possible associations between antibiotic prescription and resistance were evaluated via cross-correlation analysis. Results over 3 years indicated significantly rising incidence densities of ceftriaxone- and ciprofloxacin-resistant E. coli and imipenem-resistant Acinetobacter spp. (blood isolates only). Antimicrobial-resistant Klebsiella pneumoniae rates declined. The prescription rates of piperacillin-tazobactam, ertapenem, meropenem, ciprofloxacin, and levofloxacin increased significantly, while imipenem and moxifloxacin prescription decreased. Cross-correlation analysis demonstrated possible associations between prescription of fluoroquinolones and ciprofloxacin-resistant E. coli (R2 = 0.46), fluoroquinolones and ceftriaxone-resistant E. coli (R2 = 0.47), and carbapenems and imipenem-resistant Acinetobacter spp. (R2 = 0.48), all at zero time lag. Changes in meropenem prescription were associated with a similar trend in imipenem-resistant Acinetobacter blood isolates after a 3-month time lag. No correlation was found between cephalosporin use and resistance. In conclusion, our data demonstrated correlation between prescription of and Gram-negative bacterial resistance to several, but not all, key antimicrobial agents in Singapore hospitals. In areas where Gram-negative bacterial resistance is endemic and prescription of broad-spectrum antimicrobial agents is high, factors other than antimicrobial usage may be equally important in maintaining high resistance rates.
Antimicrobial resistance is an escalating global public health threat (3, 19). Gram-negative bacterial resistance is of particular importance as there is a dearth of novel antibiotics directed against these organisms (1), which are increasing in prevalence worldwide (8, 12, 15). Logically, an association should exist between antibiotic consumption and Gram-negative bacterial resistance, and this was demonstrated in multiple studies in both the hospital and community settings (2, 7, 10, 13, 18). Causal relationships have been difficult to establish, however, and there are studies that have failed to show any interdependence (1, 6). Even in several supportive studies, the association between antimicrobial consumption and resistance was not universal among all the antibiotics and organisms tested (2, 10, 18). This has been attributed to various reasons, including failure to control for confounding factors such as infection control measures, study biases, and lack of uniformity in susceptibility testing methods and definitions of resistance (14).
A review of various individual hospital studies highlighted the general trend of progressively increasing prevalence of quinolone-, cephalosporin-, and carbapenem-resistant Gram-negative bacilli in Singapore hospitals over the past 3 decades (11). No attempt had previously been made to investigate the association between antimicrobial consumption and Gram-negative bacterial resistance locally. Since 2006, the Network for Antimicrobial Resistance Surveillance (Singapore) has conducted laboratory- and pharmacy-based surveillance of antibiotic resistance and prescription in local public hospitals. The aim of this study is to report surveillance results over the past 3 years and to investigate the relationship between Gram-negative bacterial antimicrobial resistance and broad-spectrum antibiotic prescription in local institutions. This is the first systematic evaluation of the problem of antimicrobial resistance and broad-spectrum antibiotic prescription in Singapore.
MATERIALS AND METHODS
Study sites and period.
Four of six Singapore public hospitals participated in the study. Hospital 1 is a 1,500-bed tertiary hospital; hospitals 2 through 4 are secondary general hospitals with 1,200, 900, and 400 beds, respectively. Data from January 2006 to December 2008 were analyzed on a quarterly basis for the purposes of the study. Denominator data in the form of hospital inpatient-days, i.e., the sum of each daily inpatient census every quarter, were obtained from the hospitals' administrative records.
Antibiotic prescription.
Antibiotic prescription data were extracted from the electronic pharmacy records from each hospital. These figures comprise actual prescription data rather than purchase data. Antimicrobial agents tracked include carbapenems (imipenem, meropenem, and ertapenem), cephalosporins (ceftriaxone, ceftazidime, and cefepime only), fluoroquinolones (ciprofloxacin, levofloxacin, and moxifloxacin), and piperacillin-tazobactam. Defined daily dose (DDD) per 1,000 inpatient-days for each drug or drug category prescribed every quarter was calculated following the World Health Organization (WHO) anatomical therapeutic chemical (ATC) classification system of 2009 (20).
Antimicrobial resistance.
Microbiologic data were extracted from the laboratory information system of each participating hospital and converted centrally into a standard format using WHONET 5.4 (WHO, Geneva, Switzerland), with duplicates eliminated according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI) (4). The organisms tracked include ceftriaxone- and/or ciprofloxacin-resistant Escherichia coli and Klebsiella pneumoniae and imipenem-resistant Pseudomonas aeruginosa and Acinetobacter spp. Data were expressed as incidence density per 1,000 inpatient-days for every quarter. All hospital laboratories performed antimicrobial susceptibility testing predominantly through disk susceptibility testing, supplemented by the Vitek 2 system (bioMérieux, Marcy l'Etoile, France), following CLSI guidelines (5).
Statistical analysis.
Each antibiotic prescription and resistance series was first explored independently for trend over time by linear regression. If a statistically significant (P ≤ 0.05; R2 > 0.3) trend was found, the presence or absence of associations between selected clinically meaningful antibiotic resistance and prescription series was further explored in pairs using cross-correlation analysis, where quarterly time lags of up to 1 year in both directions (i.e., time lag of −4 to 4) were applied to the antimicrobial resistance series during the analysis. An association between antibiotic prescription and resistance was deemed to be present and significant if the coefficient of determination (R2) was >0.3 at any one time lag, and the highest correlation coefficient for each pair determined the most likely time lag where antibiotic prescription affected resistance or vice versa for that particular pair. A negative time lag for any result meant that antimicrobial resistance had preceded antibiotic prescription and vice versa for a positive time lag. All statistical analyses were performed using Systat version 12.0 (Systat Software, Inc., Point Richmond, CA).
RESULTS
Antibiotic prescription.
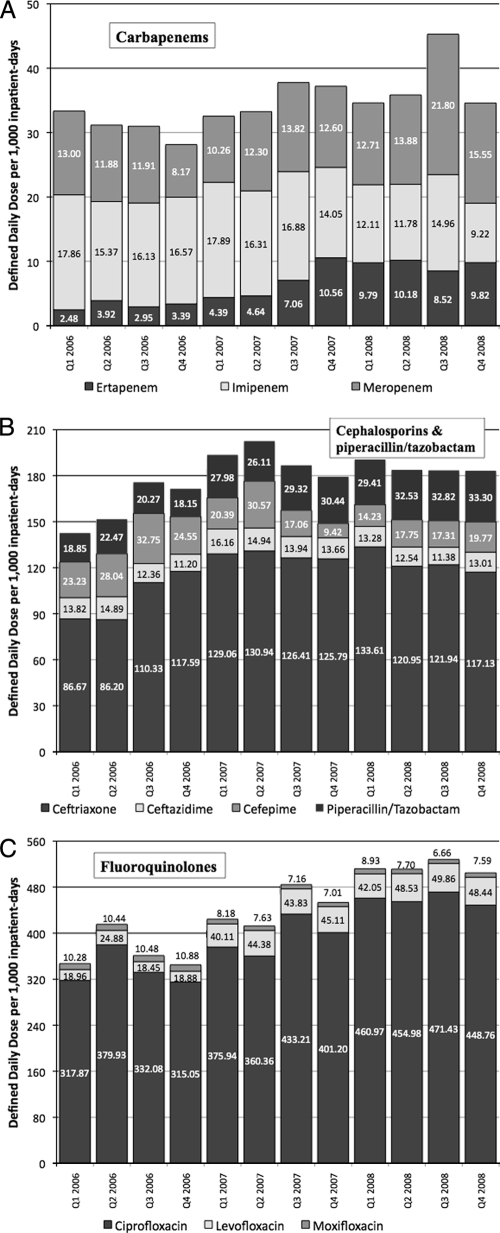
Overall prescription of the different antimicrobial agents tracked is charted in Fig. 1, while the statistical trend for each antibiotic over 3 years is shown in Table 1. A significant increase in prescription was seen for the fluoroquinolones and carbapenems. However, the coefficient of determination for the carbapenems was poor (R2 < 0.5). Cephalosporin prescription did not significantly increase over 3 years.
FIG. 1.
Antibiotic prescription volumes in DDD/1,000 inpatient-days from four public Singapore hospitals over 3 years. (A) Carbapenems. (B) Cephalosporins and piperacillin-tazobactam. (C) Fluoroquinolones.
TABLE 1.
Trends in antibiotic prescription in Singapore hospitals, 2006 to 2008
| Antibiotic(s) | Gradient (DDD/1,000 inpatient-days per quarter) | R2 | P value | 95% CIa | Trend |
|---|---|---|---|---|---|
| Cephalosporins | 0.147 | 0.138 | 0.23 | −0.111-0.407 | Stable |
| Ceftriaxoneb | 0.279 | 0.406 | 0.03 | 0.041-0.516 | Increasing |
| Ceftazidime | −0.013 | 0.104 | 0.31 | −0.040-0.141 | Stable |
| Cefepimeb | −0.118 | 0.385 | 0.03 | −0.223-−0.013 | Decreasing |
| Piperacillin-tazobactamb | 0.142 | 0.845 | <0.01 | 0.099-0.184 | Increasing |
| Fluoroquinolonesb | 1.677 | 0.807 | <0.01 | 1.098-2.255 | Increasing |
| Ciprofloxacinb | 1.399 | 0.772 | <0.01 | 0.864-1.935 | Increasing |
| Levofloxacinb | 0.311 | 0.780 | <0.01 | 0.195-0.428 | Increasing |
| Moxifloxacinb | −0.034 | 0.621 | <0.01 | −0.053-−0.015 | Decreasing |
| Carbapenemsb | 0.079 | 0.431 | 0.02 | 0.015-0.143 | Increasing |
| Imipenemb | −0.057 | 0.587 | <0.01 | −0.090-−0.023 | Decreasing |
| Meropenemb | 0.057 | 0.387 | 0.03 | 0.006-0.107 | Increasing |
| Ertapenemb | 0.079 | 0.815 | <0.01 | 0.052-0.105 | Increasing |
95% CI, 95% confidence interval.
Results where R2 was >0.3 and P was <0.05.
In terms of individual antimicrobial agents, there was increased prescription of ceftriaxone, ciprofloxacin, levofloxacin, meropenem, ertapenem, and piperacillin-tazobactam over this period, whereas cefepime, moxifloxacin, and imipenem prescription decreased significantly.
Antimicrobial resistance.
The total number of organisms tested along with the percentage and incidence density of antimicrobial-resistant organisms over the 3-year period is shown in Table 2. The statistical trend for the incidence density of each antimicrobial-resistant organism is shown in Table 3. Over the study period, ciprofloxacin-resistant E. coli was the most prevalent antimicrobial-resistant organism. Virtually half of all Acinetobacter spp. were resistant to imipenem, compared to a far lower percentage of P. aeruginosa. However, the incidence densities of imipenem-resistant Acinetobacter spp. and P. aeruginosa were similar in view of the more frequent isolation of the latter. Ceftriaxone resistance of both Enterobacteriaceae tested was also common.
TABLE 2.
Incidence density and percentage of antimicrobial-resistant Gram-negative bacteria in Singapore hospitals, 2006 to 2008
| Organism(s), drug susceptibility, and isolate type | No. of resistant isolates | % Resistance | Median incidence density of resistant isolates/1,000 inpatient-days (range) |
|---|---|---|---|
| Escherichia coli | |||
| Ceftriaxone | |||
| All isolates | 6,629 | 20.0 | 1.87 (1.61-2.17) |
| Blood isolates | 854 | 21.7 | 0.24 (0.18-0.30) |
| Ciprofloxacin | |||
| All isolates | 12,081 | 38.7 | 3.37 (3.18-3.74) |
| Blood isolates | 1,285 | 31.0 | 0.36 (0.31-0.40) |
| Klebsiella pneumoniae | |||
| Ceftriaxone | |||
| All isolates | 6,321 | 32.3 | 1.76 (1.42-2.27) |
| Blood isolates | 685 | 27.4 | 0.19 (0.15-0.24) |
| Ciprofloxacin | |||
| All isolates | 6,285 | 30.1 | 1.72 (1.32-2.39) |
| Blood isolates | 610 | 24.0 | 0.16 (0.13-0.25) |
| Acinetobacter spp., imipenem | |||
| All isolates | 2,000 | 46.2 | 0.56 (0.43-0.72) |
| Blood isolates | 184 | 50.0 | 0.05 (0.03-0.08) |
| Pseudomonas aeruginosa, imipenem | |||
| All isolates | 1,139 | 7.5 | 0.32 (0.24-0.41) |
| Blood isolates | 119 | 12.8 | 0.03 (0.02-0.07) |
TABLE 3.
Trends in antimicrobial resistance in Singapore hospitals, 2006 to 2008
| Organism(s), drug resistance, and isolate type | Gradient (incidence density/1,000 inpatient-days per quarter) | R2 | P value | 95% CIa | Trend |
|---|---|---|---|---|---|
| Escherichia coli | |||||
| Ceftriaxone | |||||
| All isolatesb | 0.032 | 0.609 | <0.01 | 0.014-0.050 | Increasing |
| Blood isolatesb | 0.007 | 0.572 | <0.01 | 0.003-0.011 | Increasing |
| Ciprofloxacin | |||||
| All isolatesb | 0.031 | 0.424 | 0.02 | 0.005-0.056 | Increasing |
| Blood isolatesb | 0.007 | 0.518 | <0.01 | 0.002-0.011 | Increasing |
| Klebsiella pneumoniae | |||||
| Ceftriaxone | |||||
| All isolatesb | −0.074 | 0.838 | <0.01 | −0.096-−0.051 | Decreasing |
| Blood isolatesb | −0.005 | 0.412 | 0.02 | −0.009-−0.007 | Decreasing |
| Ciprofloxacin | |||||
| All isolatesb | −0.091 | 0.902 | <0.01 | −0.112-−0.070 | Decreasing |
| Blood isolates | −0.004 | 0.264 | 0.08 | −0.009-0.001 | Stable |
| Acinetobacter spp., imipenem | |||||
| All isolates | −0.009 | 0.135 | 0.24 | −0.263-0.007 | Stable |
| Blood isolatesb | 0.003 | 0.394 | 0.03 | 0.003-0.005 | Increasing |
| Pseudomonas aeruginosa, imipenem | |||||
| All isolates | 0.0004 | 0.081 | 0.37 | −0.006-0.014 | Stable |
| Blood isolates | 0.002 | 0.257 | 0.09 | −0.004-0.005 | Stable |
95% CI, 95% confidence interval.
Results where R2 was >0.3 and P was <0.05.
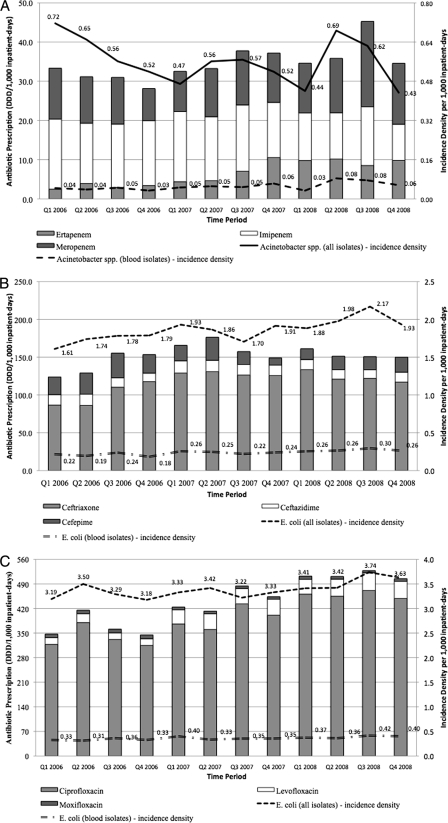
A significant rising trend was found with regard to the incidence densities of ciprofloxacin- and ceftriaxone-resistant E. coli (overall and blood isolates) as well as imipenem-resistant Acinetobacter blood isolates, although the increment was gradual over 3 years. Surprisingly, the incidence density of overall ciprofloxacin-resistant K. pneumoniae fell, with a corresponding trend seen in ceftriaxone-resistant K. pneumoniae (overall and blood isolates). Figure 2 highlights the incidence density of antimicrobial-resistant E. coli and Acinetobacter spp. in conjunction with the prescription of respective classes of antibiotics.
FIG. 2.
Incidence density of antimicrobial-resistant Gram-negative bacteria and respective antibiotic prescription volumes over 3 years. (A) Carbapenem prescription and imipenem-resistant Acinetobacter spp. (B) Cephalosporins and ceftriaxone-resistant E. coli. (C) Fluoroquinolone prescription and ciprofloxacin-resistant E. coli.
Correlation of antimicrobial consumption with resistance.
In general, the rising incidence density of ciprofloxacin-resistant E. coli correlated at zero time lag with rising prescription trends of all quinolones (R2 = 0.46 and 0.42 for all isolates and blood isolates, respectively) as well as ciprofloxacin and levofloxacin individually, piperacillin-tazobactam (R2 = 0.41 and 0.51 for all isolates and blood isolates, respectively), and all carbapenems (R2 = 0.34 and 0.35 for all isolates and blood isolates, respectively) as well as meropenem individually.
The rising ceftriaxone-resistant E. coli trend correlated at zero time lag with prescription trends of quinolones (R2 = 0.47 and 0.52 for all isolates and blood isolates, respectively) including ciprofloxacin and levofloxacin individually, ceftriaxone (R2 = 0.31 for all isolates only), piperacillin-tazobactam (R2 = 0.55 and 0.63 for all isolates and blood isolates, respectively), and the carbapenems (R2 = 0.41 and 0.51, respectively) including meropenem individually.
The increasing imipenem-resistant blood Acinetobacter spp. trend correlated at zero time lag with prescription trends of levofloxacin (R2 = 0.42), piperacillin-tazobactam (R2 = 0.46), and the carbapenems (R2 = 0.48) including ertapenem individually. The prescription trend of meropenem was associated after a lag of 3 months with the trend of imipenem-resistant Acinetobacter spp. (R2 = 0.58).
No other significant positive correlations between antibiotic resistance and prescription up to a positive time lag of 1 year were present. The rising trend of ceftriaxone-resistant E. coli was associated with the rising trend in ertapenem prescription (R2 = 0.47) after a time lag of 3 months (negative lag of one quarter), but there were no other clinically meaningful associations found for the negative time lag cross-correlation analyses.
DISCUSSION
Our study highlights the extensive prescription of broad-spectrum antimicrobial agents in Singapore hospitals, coupled with high resistance rates among the Gram-negative bacilli surveyed. Carbapenem prescription has increased mostly as a consequence of increasing ertapenem prescription, whereas imipenem prescription may be declining due to increased prescription of both ertapenem and meropenem. It is interesting that meropenem is considerably more expensive than imipenem in Singapore; thus, different marketing practices and changing physician perceptions may have accounted for declining imipenem usage, but this is beyond the scope of the present study.
Compared with previous studies, the prevalence of Gram-negative bacterial antimicrobial resistance in Singapore hospitals has remained relatively stable over the past 2 years (9, 11). Ciprofloxacin and ceftriaxone resistance rates in E. coli continued to increase, although antibiotic-resistant K. pneumoniae incidence densities surprisingly decreased over the study period. The reasons for this divergent trend between the Enterobacteriaceae could not be ascertained in a surveillance study, but it is plausible that an increase in incidence density of community extended-spectrum beta-lactamase (ESBL)-producing and quinolone-resistant E. coli isolates may have contributed to this phenomenon. Pada and coworkers had found that up to 12% of more than 1,000 emergency department attendees without previous health care association at hospital 2 in 2007 were colonized with ESBL-positive Enterobacteriaceae—the vast majority of which were E. coli (16). Unfortunately, we could not reliably distinguish between community- and hospital-associated infections in our study.
As infection control measures in the study hospitals had not changed significantly over the study period, the drop in K. pneumoniae rates against the stable or rising trends of other antibiotic-resistant Gram-negative bacilli is less easy to explain, beyond postulating about the possible decline of a predominant nosocomial K. pneumoniae clone. No molecular typing had been performed on K. pneumoniae over this study period; hence, we are unable to verify or disprove this postulation at this time.
Unlike the majority of published studies, there were few significant correlations between antimicrobial prescription and subsequent resistance or vice versa (2, 7, 10, 13, 18). Overall carbapenem prescription was correlated with significant but slight changes in imipenem resistance in Acinetobacter blood isolates within the same time period. Perhaps the major finding was that up to 58% of the changes in imipenem resistance in Acinetobacter blood isolates might be explained by meropenem prescription following a 3-month interval between prescription and resistance development.
Carbapenem prescription showed significant correlation with antibiotic-resistant E. coli incidence densities at no time lag. This is intuitive, as most nosocomial E. coli isolates are resistant to both classes of antibiotics, and infections are most commonly treated with carbapenems in Singapore. However, quinolone and piperacillin-tazobactam prescription also correlated with both ciprofloxacin- and ceftriaxone-resistant E. coli isolates at no time lag, and analyzing the data at shorter monthly time intervals or the individual hospital level (data not shown) failed to demonstrate any significant time lag as well. Given that antibiotic-resistant K. pneumoniae rates failed to correlate positively with antibiotic prescription and coupling this with the potential influx of community quinolone-resistant and/or ESBL-producing E. coli isolates, it is difficult to state that either ciprofloxacin- or ceftriaxone-resistant E. coli rates are significantly associated with antibiotic prescription despite the significant coefficients of determination.
There are several possible explanations for the lack of significant correlation between antibiotic prescription and resistance in our study. As had previously been pointed out, resistance selection pressure occurs at the individual level and calculating antibiotic prescription using DDD measurements does not measure individual exposure to antibiotics (17). A minority of patients are exposed to the majority of broad-spectrum antibiotic prescriptions in the hospital setting, and these are mainly the patients who are susceptible to infections by antibiotic-resistant pathogens. Hence, although DDD measurements are useful for comparison and benchmarking, they may not correlate well with subsequent antibiotic resistance development due to the inherent biases. In our study, although antibiotic prescriptions had fluctuated, the prescription volumes had generally remained high. It is possible that beyond a certain critical threshold of antibiotic use, antibiotic resistance becomes decoupled from prescription. However, such a threshold—if it exists—has not been defined. Finally, other factors besides antibiotic prescription impact on resistance in the hospital setting, including infection control and the presence of predominant bacterial clones. The interplay of these factors with antibiotic prescription has not been worked out.
There are several limitations of this work. First, the study period of 3 years is relatively short, although further data collection is ongoing. Second, differences in infection control measures in the hospitals over this period were not assessed, although anecdotally there had been no significant intensification of infection control efforts with regard to antimicrobial-resistant Gram-negative bacteria during this time. Third, because of the nature of the surveillance, we could not determine individual level or duration of exposure to antibiotics to further correlate prescription with antibiotic resistance.
In conclusion, the incidence of Gram-negative bacterial antibiotic resistance and broad-spectrum antibiotic prescription is high in Singapore hospitals. This first major attempt at correlating resistance with prescription in Singapore yielded several significant correlations over the 3-year study period.
Acknowledgments
Other members of the Network for Antimicrobial Resistance Surveillance (Singapore) include Prabha Krishnan, Yoke-Fong Chiew, Roland Jureen, and Nancy Tee.
We thank the many dedicated staff who have helped with data collection and technical issues, in particular Mee-Lee Tan, Winnie Lee, Wan-Peng Lim, Hong-Yin Liew, and Siok-Ying Lee.
This work was funded by the following grants: SingHealth Foundation Grant 2006, Ministry of Health Healthcare Quality Improvement Fund 2006, and educational grants from Pfizer Singapore, Wyeth, Janssen-Cilag, Merck Sharpe & Dohme, and AstraZeneca.
Footnotes
The authors have paid a fee to allow immediate free access to this article.
Published ahead of print on 11 January 2010.
REFERENCES
- 1.Bartoloni, A., F. Bartalesi, A. Mantella, E. Dell'Amico, M. Roselli, M. Strohmeyer, H. G. Barahona, V. P. Barron, F. Paradisi, and G. M. Rossolini. 2004. High prevalence of acquired antimicrobial resistance unrelated to heavy antimicrobial consumption. J. Infect. Dis. 189:1291-1294. [DOI] [PubMed] [Google Scholar]
- 2.Bergman, M., S. T. Nyberg, P. Huovinen, P. Paakkari, A. J. Hakanen, and the Finnish Study Group for Antimicrobial Resistance. 2009. Association between antimicrobial consumption and resistance in Escherichia coli. Antimicrob. Agents Chemother. 53:913-917. [Google Scholar]
- 3.Boucher, H. W., G. H. Talbot, J. S. Bradley, J. E. Edwards, D. Gilbert, L. B. Rice, M. Scheld, B. Spellberg, and J. Bartlett. 2009. Bad bugs need drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 48:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing. Supplement M100-S14, vol. 24, no. 1. Clinical and Laboratory Standards Institute, Wayne, PA.
- 5.Clinical and Laboratory Standards Institute. 2006. Analysis and presentation of cumulative susceptibility test data; approved guideline, 2nd ed. Document M39-A2, vol. 25, no. 28. Clinical and Laboratory Standards Institute, Wayne, PA.
- 6.Colgan, R., J. R. Johnson, M. Kushkowski, and K. Gupta. 2008. Risk factors for trimethoprim-sulfamethoxazole resistance in patients with acute uncomplicated cystitis. Antimicrob. Agents Chemother. 52:846-851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goossens, H., M. Ferech, R. V. Stichele, M. Elseviers, and ESAC Project Group. 2005. Outpatient antibiotic use in Europe and association with resistance: a cross-national database study. Lancet 365:579-587. [DOI] [PubMed] [Google Scholar]
- 8.Hawser, S. P., S. K. Bouchillon, D. J. Hoban, R. E. Badal, P. R. Hsueh, and D. Paterson. 8 June 2009. Emergence of high levels of extended-spectrum beta-lactamase-producing Gram-negative bacilli in Asia/Pacific: data from SMART 2007. Antimicrob. Agents Chemother. doi: 10.1128/AAC.00426-09. [DOI] [PMC free article] [PubMed]
- 9.Hsu, L. Y., T. Y. Tan, R. Jureen, T. H. Koh, P. Krishnan, R. T. P. Lin, N. W. S. Tee, and P. A. Tambyah. 2007. Antimicrobial drug resistance in Singapore hospitals. Emerg. Infect. Dis. 13:1944-1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsueh, P. R., W. H. Chen, and K. T. Luh. 2005. Relationships between antimicrobial use and antimicrobial resistance in Gram-negative bacteria causing nosocomial infections from 1991-2003 at a university hospital in Taiwan. Int. J. Antimicrob. Agents 26:463-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koh, T. H. 2008. Gram-negative resistance in Singapore: a historical perspective. Ann. Acad. Med. Singapore 37:847-854. [PubMed] [Google Scholar]
- 12.Livermore, D. M., R. Hope, G. Brick, M. Lillie, R. Reynolds, and BSAC Working Parties on Resistance Surveillance. 2008. Non-susceptibility trends among Pseudomonas aeruginosa and other non-fermentative Gram-negative bacteria from bacteraemias in the UK and Ireland, 2001-06. J. Antimicrob. Chemother. 62(Suppl. 2):ii55-63. [DOI] [PubMed] [Google Scholar]
- 13.McGowan, J. E., Jr. 1983. Antimicrobial resistance in hospital organisms and its relation to antibiotic use. Rev. Infect. Dis. 5:1033-1048. [DOI] [PubMed] [Google Scholar]
- 14.McGowan, J. E., Jr. 1987. Is antimicrobial resistance in hospital microorganisms related to antibiotic use? Bull. N. Y. Acad. Med. 63:253-268. [PMC free article] [PubMed] [Google Scholar]
- 15.Nicasio, A. M., J. L. Kuti, and D. P. Nicolau. 2008. The current state of multidrug-resistant gram-negative bacilli in North America. Pharmacotherapy 28:235-249. [DOI] [PubMed] [Google Scholar]
- 16.Pada, S., D. C. Lye, P. Krishnan, and Y. S. Leo. 2008. Prevalence and predictors of methicillin-resistant Staphylococcus aureus (MRSA) and extended-spectrum beta-lactamase (ESBL) Gram-negative bacteria at hospital presentation in Singapore, abstr. 69.009. 13th Int. Congr. Infect. Dis., Kuala Lumpur, Malaysia, 19 to 22 June 2008.
- 17.Turnidge, J., and K. Christiansen. 2005. Antibiotic use and resistance—proving the obvious. Lancet 365:548-549. [DOI] [PubMed] [Google Scholar]
- 18.Van de Sande-Bruinsma, N., H. Grundmann, D. Verloo, E. Tiemersma, J. Monen, H. Goossens, M. Ferech, and the European Antimicrobial Resistance Surveillance System Group and European Surveillance of Antimicrobial Consumption Project Group. 2008. Antimicrobial drug use and resistance in Europe. Emerg. Infect. Dis. 14:1722-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. 2001. WHO global strategy for containment of antimicrobial resistance. http://whqlibdoc.who.int/hq/2001/WHO_CDS_CSR_DRS_2001.2.pdf.
- 20.World Health Organization. 2009. WHO Collaborating Centre for Drug Statistics Methodology. ATC/DDD index 2009. http://www.whocc.no/atcddd/.