Abstract
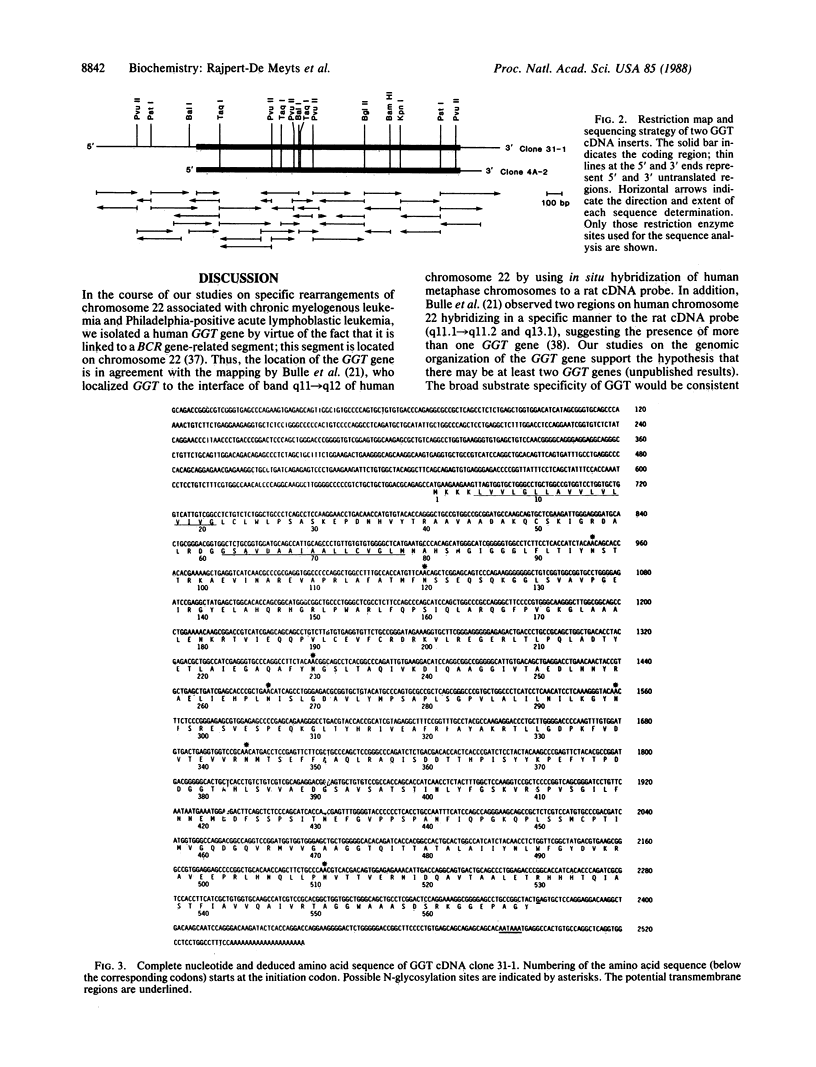
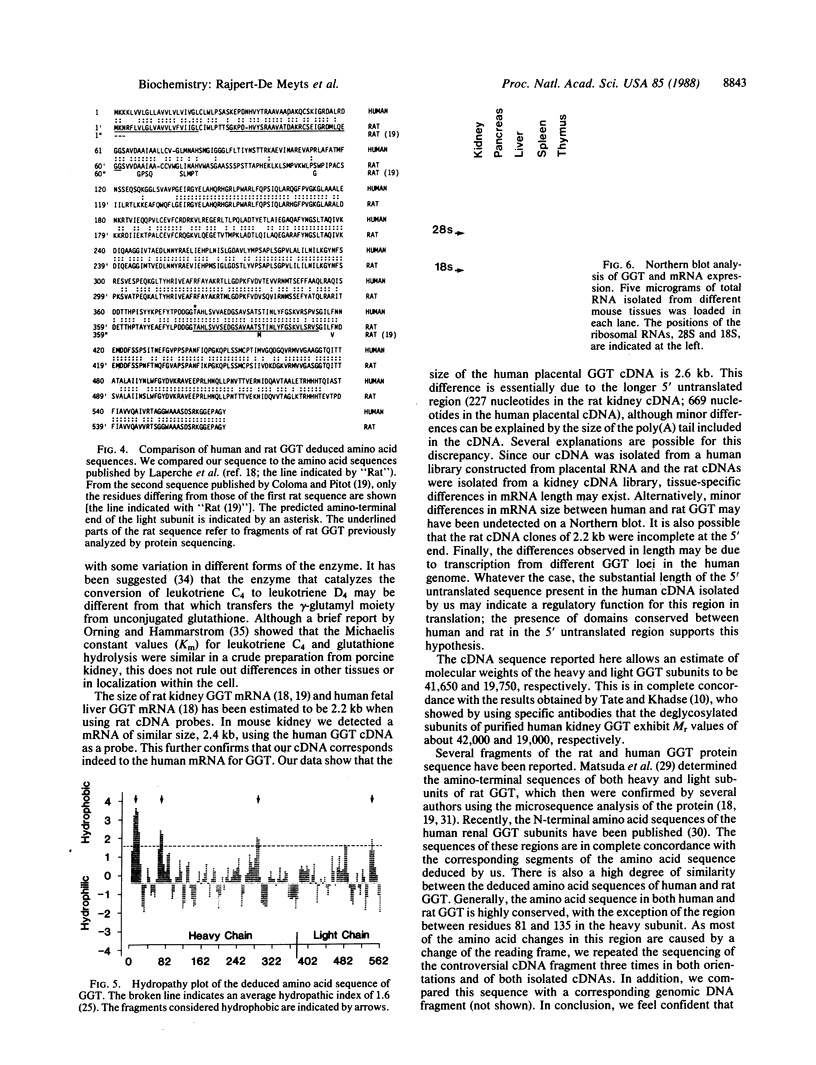
We have identified the gene for human gamma-glutamyl transpeptidase [GGT; glutamine:D-glutamyl-peptide 5-glutamyltransferase (also called gamma-glutamyltransferase), EC 2.3.2.2] in a BCR gene-related region located in band q11----qter of chromosome 22. Two cDNAs complementary to the GGT mRNA have been isolated from a human placental library constructed in phage lambda gt11. The largest cDNA has a size of 2535 base pairs (bp) and an open reading frame of 1707 nucleotides encoding 569 amino acids. By using a probe corresponding to this cDNA, a mRNA of approximately 2.4 kilobases was detected by RNA blot-hybridization analysis in mouse kidney RNA. The GGT precursor encoded by the coding sequence would have an estimated Mr of 61,400. We compared our nucleotide and deduced amino acid sequences with the published results of rat kidney cDNAs. The human and rat amino acid sequences are similar; however, a considerable discrepancy in nucleotide sequence was found within a 180-bp fragment of the heavy chain, resulting in a completely different amino acid sequence for this region. In addition, the 5' untranslated sequence of the human cDNA (669 bp) is substantially larger than that determined in the rat cDNA (227 bp). Our results may be valuable for further studies on the protein structure of human GGT as well as studies on the regulation of the enzyme.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barouki R., Chobert M. N., Billon M. C., Finidori J., Tsapis R., Hanoune J. Glucocorticoid hormones increase the activity of gamma-glutamyltransferase in a highly differentiated hepatoma cell line. Biochim Biophys Acta. 1982 Sep 13;721(1):11–21. doi: 10.1016/0167-4889(82)90018-0. [DOI] [PubMed] [Google Scholar]
- Barouki R., Chobert M. N., Finidori J., Aggerbeck M., Nalpas B., Hanoune J. Ethanol effects in a rat hepatoma cell line: induction of gamma-glutamyltransferase. Hepatology. 1983 May-Jun;3(3):323–329. doi: 10.1002/hep.1840030308. [DOI] [PubMed] [Google Scholar]
- Barouki R., Finidori J., Chobert M. N., Aggerbeck M., Laperche Y., Hanoune J. Biosynthesis and processing of gamma-glutamyl transpeptidase in hepatoma tissue culture cells. J Biol Chem. 1984 Jun 25;259(12):7970–7974. [PubMed] [Google Scholar]
- Billon M. C., Dupre G., Hanoune J. In vivo modulation of rat hepatic gamma-glutamyltransferase activity by glucocorticoids. Mol Cell Endocrinol. 1980 May;18(2):99–108. doi: 10.1016/0303-7207(80)90085-4. [DOI] [PubMed] [Google Scholar]
- Bulle F., Mattei M. G., Siegrist S., Pawlak A., Passage E., Chobert M. N., Laperche Y., Guellaën G. Assignment of the human gamma-glutamyl transferase gene to the long arm of chromosome 22. Hum Genet. 1987 Jul;76(3):283–286. doi: 10.1007/BF00283624. [DOI] [PubMed] [Google Scholar]
- Capraro M. A., Hughey R. P. Processing of the propeptide form of rat renal gamma-glutamyltranspeptidase. FEBS Lett. 1983 Jun 27;157(1):139–143. doi: 10.1016/0014-5793(83)81132-6. [DOI] [PubMed] [Google Scholar]
- Coloma J., Pitot H. C. Characterization and sequence of a cDNA clone of gamma-glutamyltranspeptidase. Nucleic Acids Res. 1986 Feb 11;14(3):1393–1403. doi: 10.1093/nar/14.3.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curthoys N. P. Role of gamma-glutamyltranspeptidase in the renal metabolism of glutathione. Miner Electrolyte Metab. 1983;9(4-6):236–245. [PubMed] [Google Scholar]
- Finidori J., Laperche Y., Haguenauer-Tsapis R., Barouki R., Guellaen G., Hanoune J. In vitro biosynthesis and membrane insertion of gamma-glutamyl transpeptidase. J Biol Chem. 1984 Apr 25;259(8):4687–4690. [PubMed] [Google Scholar]
- Groffen J., Stephenson J. R., Heisterkamp N., de Klein A., Bartram C. R., Grosveld G. Philadelphia chromosomal breakpoints are clustered within a limited region, bcr, on chromosome 22. Cell. 1984 Jan;36(1):93–99. doi: 10.1016/0092-8674(84)90077-1. [DOI] [PubMed] [Google Scholar]
- Grosveld G., Verwoerd T., van Agthoven T., de Klein A., Ramachandran K. L., Heisterkamp N., Stam K., Groffen J. The chronic myelocytic cell line K562 contains a breakpoint in bcr and produces a chimeric bcr/c-abl transcript. Mol Cell Biol. 1986 Feb;6(2):607–616. doi: 10.1128/mcb.6.2.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanigan M. H., Pitot H. C. Gamma-glutamyl transpeptidase--its role in hepatocarcinogenesis. Carcinogenesis. 1985 Feb;6(2):165–172. doi: 10.1093/carcin/6.2.165. [DOI] [PubMed] [Google Scholar]
- Hart G. W., Brew K., Grant G. A., Bradshaw R. A., Lennarz W. J. Primary structural requirements for the enzymatic formation of the N-glycosidic bond in glycoproteins. Studies with natural and synthetic peptides. J Biol Chem. 1979 Oct 10;254(19):9747–9753. [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J. Duplication of the bcr and gamma-glutamyl transpeptidase genes. Nucleic Acids Res. 1988 Aug 25;16(16):8045–8056. doi: 10.1093/nar/16.16.8045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heisterkamp N., Stam K., Groffen J., de Klein A., Grosveld G. Structural organization of the bcr gene and its role in the Ph' translocation. 1985 Jun 27-Jul 3Nature. 315(6022):758–761. doi: 10.1038/315758a0. [DOI] [PubMed] [Google Scholar]
- Hermans A., Heisterkamp N., von Linden M., van Baal S., Meijer D., van der Plas D., Wiedemann L. M., Groffen J., Bootsma D., Grosveld G. Unique fusion of bcr and c-abl genes in Philadelphia chromosome positive acute lymphoblastic leukemia. Cell. 1987 Oct 9;51(1):33–40. doi: 10.1016/0092-8674(87)90007-9. [DOI] [PubMed] [Google Scholar]
- Hughey R. P., Coyle P. J., Curthoys N. P. Comparison of the association and orientation of gamma-glutamyltranspeptidase in lecithin vesicles and in native membranes. J Biol Chem. 1979 Feb 25;254(4):1124–1128. [PubMed] [Google Scholar]
- Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol Rev. 1983 Mar;47(1):1–45. doi: 10.1128/mr.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laperche Y., Bulle F., Aissani T., Chobert M. N., Aggerbeck M., Hanoune J., Guellaën G. Molecular cloning and nucleotide sequence of rat kidney gamma-glutamyl transpeptidase cDNA. Proc Natl Acad Sci U S A. 1986 Feb;83(4):937–941. doi: 10.1073/pnas.83.4.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y., Tsuji A., Katunuma N. Studies on the structure of gamma-glutamyltranspeptidase. III. Evidence that the amino terminus of the heavy subunit is the membrane binding segment. J Biochem. 1983 May;93(5):1427–1433. doi: 10.1093/oxfordjournals.jbchem.a134278. [DOI] [PubMed] [Google Scholar]
- Meister A., Anderson M. E. Glutathione. Annu Rev Biochem. 1983;52:711–760. doi: 10.1146/annurev.bi.52.070183.003431. [DOI] [PubMed] [Google Scholar]
- Nash B., Tate S. S. Biosynthesis of rat renal gamma-glutamyl transpeptidase. Evidence for a common precursor of the two subunits. J Biol Chem. 1982 Jan 25;257(2):585–588. [PubMed] [Google Scholar]
- Nash B., Tate S. S. In vitro translation and processing of rat kidney gamma-glutamyl transpeptidase. J Biol Chem. 1984 Jan 10;259(1):678–685. [PubMed] [Google Scholar]
- Norrander J., Kempe T., Messing J. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983 Dec;26(1):101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- Orning L., Hammarström S. Kinetics of the conversion of leukotriene C by gamma-glutamyl transpeptidase. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1304–1309. doi: 10.1016/0006-291x(82)91255-4. [DOI] [PubMed] [Google Scholar]
- Pawlak A., Lahuna O., Bulle F., Suzuki A., Ferry N., Siegrist S., Chikhi N., Chobert M. N., Guellaen G., Laperche Y. gamma-Glutamyl transpeptidase: a single copy gene in the rat and a multigene family in the human genome. J Biol Chem. 1988 Jul 15;263(20):9913–9916. [PubMed] [Google Scholar]
- Rosalki S. B., Rau D. Serum -glutamyl transpeptidase activity in alcoholism. Clin Chim Acta. 1972 Jun;39(1):41–47. doi: 10.1016/0009-8981(72)90297-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawabu N., Nakagen M., Ozaki K., Wakabayashi T., Toya D., Hattori N., Ishii M. Clinical evaluation of specific gamma-GTP isoenzyme in patients with hepatocellular carcinoma. Cancer. 1983 Jan 15;51(2):327–331. doi: 10.1002/1097-0142(19830115)51:2<327::aid-cncr2820510227>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kumagai H., Echigo T., Tochikura T. Molecular cloning of Escherichia coli K-12 ggt and rapid isolation of gamma-glutamyltranspeptidase. Biochem Biophys Res Commun. 1988 Jan 15;150(1):33–38. doi: 10.1016/0006-291x(88)90482-2. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Khadse V. Renal gamma-glutamyl transpeptidases: influence of glycosylation on the electrophoretic behavior and molecular weights of their subunits. Biochem Biophys Res Commun. 1986 Dec 30;141(3):1189–1194. doi: 10.1016/s0006-291x(86)80170-x. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Khadse V., Wellner D. Renal gamma-glutamyl transpeptidases: structural and immunological studies. Arch Biochem Biophys. 1988 May 1;262(2):397–408. doi: 10.1016/0003-9861(88)90390-6. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Meister A. gamma-Glutamyl transpeptidase: catalytic, structural and functional aspects. Mol Cell Biochem. 1981 Sep 25;39:357–368. doi: 10.1007/BF00232585. [DOI] [PubMed] [Google Scholar]
- Tate S. S., Ross M. E. Human kidney gamma-glutamyl transpeptidase. Catalytic properties, subunit structure, and localization of the gamma-glutamyl binding site on the light subunit. J Biol Chem. 1977 Sep 10;252(17):6042–6045. [PubMed] [Google Scholar]
- Tsuji A., Matsuda Y., Katunuma N. Studies on the structure of gamma-glutamyltranspeptidase. II. Location of the segment anchoring gamma-glutamyltranspeptidase to the membrane. J Biochem. 1980 Jun;87(6):1567–1571. doi: 10.1093/oxfordjournals.jbchem.a132898. [DOI] [PubMed] [Google Scholar]




