Abstract
Topical therapy is an attractive approach for the treatment of Leishmania major cutaneous leishmaniasis (CL). WR279396, an expanded-spectrum aminoglycoside ointment, is now in phase 3 trials. Because the application of a cream is easier than the injection of pentavalent antimony, many patients with CL will likely be treated with WR279396 soon after the onset of a lesion. However, this new therapeutic approach may impair the acquisition of immunity. We evaluated the impact of early topical therapy on acquired immunity in an optimized mouse model of L. major-induced CL. The efficacy of the WR279396 ointment in this model has been established previously. Acquired immunity was defined as the absence of lesions upon reinoculation of the same parasite isolate at a different skin site. Bioluminescence-based follow-up of luciferase-expressing L. major loads was also performed. In this model, the control of L. major loads at the initial inoculation site and the acquisition of immunity are simultaneous (day 22 postinoculation). The clinical and parasitological efficacies of WR279396 applied as early as day 11 postinoculation, i.e., during the L. major multiplication phase, did not impair the acquisition of immunity to a second L. major challenge. This is reassuring from the perspective of the wide deployment of WR279396-based therapy in foci where L. major is endemic.
Cutaneous leishmaniasis (CL) is a dermatological disease caused by protozoan Leishmania parasites that are transmitted to their mammalian hosts by sand fly bites. The topical treatment of CL is a practical and safe therapeutic option (5). One strong argument for the topical treatment of CL is the simpler implementation of application procedures at primary health care centers, thus, allowing the earlier treatment of the majority of patients.
WR279396, a formulation of paromomycin and gentamicin in a hydrophilic base, is efficacious in patients with Leishmania major CL and has a satisfactory safety profile (3, 18). A phase 3 study is ongoing in Tunisia. To identify the parameters that influence the efficacy of WR279396, we have optimized a C57BL/6 mouse-based model of CL that mimics important features of Leishmania metacyclic promastigote transmission to mammals, including humans (10, 12). Real-time bioluminescence-based determination of the parasitic load in mice treated with WR279396 showed an accelerated and greater than 2-log reduction of the parasitic load during a 10-day topical application. These curative applications resulted in a long-term stable cure, with a very low number of parasites persisting in the healed skin (12).
Questions about the risk of secondary episodes in topically treated and cured patients, when and if those patients are reexposed to Leishmania isolates of the same species, persist. The optimized C57BL/6 mouse-based model (12) offers a relevant experimental system with which to study the impact of early therapeutic intervention against CL on the development of skin-protective processes (referred to here for simplicity as acquired immunity), which spontaneously develop in the absence of therapeutic intervention (14). The clinical and parasitological efficacies of WR279396 applied as early as day 11 postinoculation, i.e., during the multiplication phase of infection, did not impair the acquisition of immunity to a second L. major challenge.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice (age, 5 weeks) and Swiss nu/nu mice were purchased from Charles River (Saint Germain-sur-l'Arbresle, France) and were housed according to the institutional guidelines of the A3 animal facility at the Institut Pasteur (Paris, France; http://webcampus.pasteur.fr/jcms/c_87141/documents).
Generation of luciferase-transgenic Leishmania major.
A 1.66-kbp firefly luciferase-coding region was cut with NcoI-EagI from pGL3 basic (Promega, Madison, WI) and was subsequently cloned into the Leishmania expression vector pF4X1.HYG (Jenabioscience, Jena, Germany), as described previously (10, 12). Following transfection and electroporation, L. major promastigotes were incubated for 24 h in drug-free medium and plated on semisolid medium containing 100 μg/ml of hygromycin B (7).
Leishmania inoculum preparation, inoculation in the ear dermis, and monitoring of parasite-loaded ear features.
Luciferase-transgenic L. major strain NIH 173 (MHOM/IR/−/173) amastigotes were isolated from parasitized Swiss nude mice (10). The promastigote developmental stage was grown at 26°C in M199 medium, as described previously (7), and mammal infective-stage metacyclic promastigotes were isolated from stationary-phase cultures (age, 6 days) by density gradient centrifugation (19). C57BL/6 mice were anesthetized by the intraperitoneal administration of a mixture of ketamine (120 mg/kg of body weight; Imalgene 1000; Merial, France) and xylazine (2% 4 mg/kg; Rompun; Bayer, Leverkusen, Germany). Ten thousand metacyclic promastigotes were injected in the right ear dermis. Ear thickness was measured by using a direct-reading vernier caliper (Thomas Scientific, Swedesboro, NJ).
Estimation of L. major load by limiting-dilution analysis.
The number of parasites present in L. major-infected tissues/organs was estimated as described previously (11). Briefly, ear and ear-draining lymph node homogenates were serially diluted in complete HOSMEM-II medium (3b) and were then distributed in previously prepared 96-well plates containing 3% semisolid agar (Bacto agar; Difco) supplemented with 10% sterile heparinized rabbit blood. After 10 days at 26°C, each well was examined and defined as positive or negative on the basis of the presence or the absence of viable promastigotes. A limiting-dilution analysis was applied to the data to estimate the number of viable Leishmania parasites, expressed as the limiting-dilution assay unit (LDAU) (21). Statistical analysis of the results was based on the maximum-likelihood method (20, 22).
In vivo bioluminescence imaging for determination of Leishmania population size in ear dermis.
A luciferase substrate (d-luciferin potassium salt; Xenogen) was inoculated into the mouse peritoneal cavity at a concentration of 150 mg/kg for 25 min before bioluminescence imaging. The mice were anesthetized in a 2.5% isoflurane atmosphere (Aerane; Baxter SA, Maurepas, France) for 5 min and maintained in the imaging chamber for analysis. Analysis of the emitted photons with a charge-coupled-device (CCD) camera (IVIS imaging system, 100 series) was performed after definition of a region of interest (ROI) that delimited the surface of the entire ear. The same ROI was applied to every mouse at every time point. The total photon emission was expressed as the number of photons per second per ROI (sometimes referred to as the “bioluminescence value”).
Topical formulation and ointment regimen design.
Topical formulations were prepared at the Walter Reed Army Institute of Research (Washington, DC). WR279396 consists of paromomycin sulfate (15%) plus gentamicin (0.5%) in a hydrophilic base, called the “vehicle,” as described previously (8). The topical ointments were applied at day 11 post-primary inoculation (p.p.i.) on the L. major-loaded ears once every 2 days for 10 days. The preparation was applied directly to the ears to form a thin layer. The ointment was covered with an occlusive dressing, as described previously (12).
Experimental procedure for monitoring the establishment of acquired immunity.
Forty to 60 C57BL/6 mice per experiment were inoculated intradermally in the ear with luciferase-transgenic L. major parasites on day 0. Eleven days later, the total photon emission of each ear was quantified. The mice were selected and distributed into groups according to equal median bioluminescence and standard deviation values. Each experimental group contained 7 to 12 individually tagged mice. To determine the establishment of acquired immunity, different groups of mice were reinoculated in the contralateral ear with the same parasite isolate at day 11, 15, 22, or 31 p.p.i. At each time of reinoculation, a control group of naïve mice also received the same isolate of L. major. This control was used (i) to determine the quality of this inoculum and (ii) to assess the reference conditions for monitoring the establishment of acquired immunity in the other groups. To analyze the influence of early curative treatment on acquired immunity, one group of mice was treated with WR279396 (see above) and was subsequently reinoculated in the contralateral ear at day 47 p.p.i. Two groups were analyzed as controls. For the first group, the ears were left untreated. For the second group, the WR279396 vehicle (without paromomycin or gentamicin) was applied and immediately covered with an occlusive dressing.
Statistics.
Lesion size or logarithm-transformed parasitic loads were analyzed by two-way analysis of variance (ANOVA), as described previously (12), with the two factors being the topical treatment (the vehicle or the WR279396 formulation) and the period of observation (treatment, posttreatment, and end of the observation period under study) in the R (programming language) statistical environment. A probability (P) level of <0.05 was accepted for the purpose of declaring that the treatments had statistically significant effects.
RESULTS
Parasitic load control at the initial inoculation site and acquisition of immunity are simultaneous.
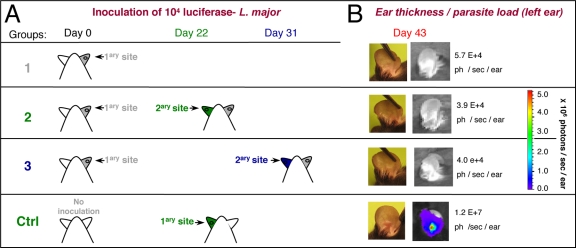
All intradermal parasite inoculations (104 L. major metacyclic promastigotes) were performed as described previously (12). Control (Ctrl) mice were left uninoculated (Fig. 1A). At day 11 postinoculation (p.i.), the mice were distributed into three groups (Fig. 1A) on the basis of equal median bioluminescence values: 5 × 105 ± 3 × 105 photons/s/ear. The mice in group 1 were not reinoculated; the mice in groups 2 and 3 received a second identical inoculum in the contralateral (left) ear (secondary site) on days 22 and 31, respectively (Fig. 1A). As a control for the second inoculation, a group of naïve mice (Fig. 1A, Ctrl, i.e., no prior inoculation at day 0) were inoculated only on day 22.
FIG. 1.
Primary inoculation of C57BL/6 mice with L. major in the ear dermis. (A) Defining the different mouse groups (n = 10 animals per group). At day 0, 104 luciferase-expressing NIH 173 metacyclic promastigotes were inoculated into the right ear dermis (primary [1ary] site) of C57BL/6 mice in groups 1, 2, and 3. At days 22 and 31 p.p.i., 104 luciferase-expressing NIH 173 metacyclic promastigotes were inoculated into the contralateral (left) ears (secondary [2ary] inoculation) of the mice in groups 2 and 3. As controls (Ctrl), a group of naïve mice received 104 parasites in the left ear at day 22 after the primary inoculation of the test group. (B) Analyses of the left ears of the mice from the different groups at day 43 p.p.i. Pictures of representative ears are shown. The clinical features (clinical signs) were established by macroscopic observations. The parasitic load was assessed from the bioluminescence of the luciferase-expressing parasites. The number of photons (ph) per second per ear is indicated.
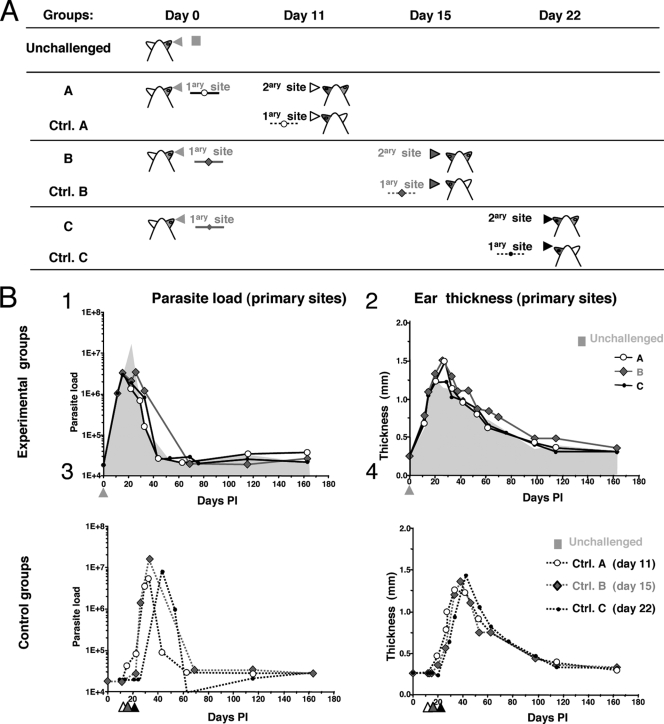
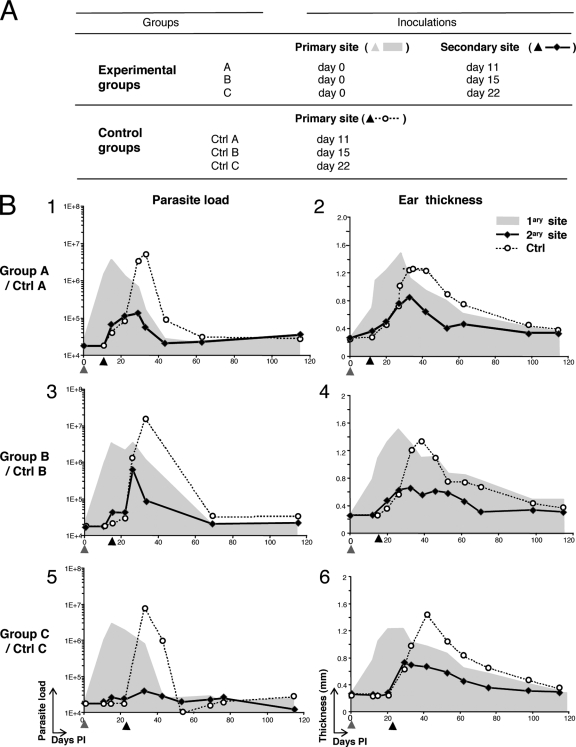
The mice in groups 2 and 3 (which were reinoculated on days 22 and 31, respectively) did not display any bioluminescence signals at the secondary site of inoculation (see the pictures of the left ear taken at day 43 p.p.i. in Fig. 1B) or sustained ear lesions. In contrast, in the mice first inoculated on day 22, the parasitic loads increased markedly in the left ear at day 43 (Fig. 1B, Ctrl). Thus, acquired immunity resulting in the control of the parasitic load at the site of reinoculation was established at least as early as 22 days p.p.i. To determine whether the acquisition of immunity could occur even earlier, i.e., during the expansion phase of the parasite population, the mice were inoculated at day 0 and were then reinoculated 11, 15, or 22 days later. Seven experimental groups were studied (Fig. 2A and 3A). Over the time of observation postinoculation, the bioluminescence values and ear thickness at the primary site of the challenged groups (groups A to C) and unchallenged groups (Fig. 2B, panels 1 and 2, gray area) displayed similar profiles. Control groups (Ctrl groups A, B, and C) also had similar clinical and parasitological profiles (Fig. 2B, panels 3 and 4). Additional parasite inoculation in the contralateral ear at day 11 p.p.i. resulted in transient ear lesions, as shown in Fig. 3B, panels 1 and 2 (group A): both the parasitic load and the lesion size increased and peaked at days 25 to 30 p.p.i. This first phase at the secondary site was followed at 25 to 30 days p.p.i. by the establishment of a parasite control phase simultaneously with the parasite control phase at the primary site (Fig. 3B, panel 1). In contrast, the control group was characterized by a sharp increase in the bioluminescent signal at the inoculation site (from 8 × 104 photons/s/ear at day 22 to 5 × 106 photons/s/ear at day 37; Fig. 3B, panel 1). Inoculation in the contralateral ear at day 15 p.p.i. also resulted in increases in both the parasitic load (from 4 × 104 photons/s/ear at day 15 at the secondary site to 5 × 105 photons/s/ear at day 25; Fig. 3B, panel 3) and the ear thickness, with peaks occurring at day 25 to 30 p.p.i. Both the clinical and the parasitological parameters then progressively decreased, as observed at the primary inoculation site. Thus, immunity at the secondary site was not established before the onset of the control of the parasitic load at the primary site (Fig. 3B, panel 3). Consistent with the results of the first experiment, reinoculation on day 22 p.p.i. resulted in control of the parasitic load at the challenge site (3 × 104 photons/s/ear), and no ear lesions were observed. Only a transient increase in ear thickness at between 3 and 10 weeks p.p.i. was observed (Fig. 3B, panel 6).
FIG. 2.
Definition of experimental and control groups and features monitored at primary sites of L. major inoculation. (A) Definition of experimental groups (A to C) and control groups (Ctrl. A to Ctrl. C) (n = 12 mice per group). C57BL/6 mice were first inoculated with 104 luciferase-expressing NIH 173 metacyclic promastigotes in the ear dermis (primary site) and were then reinoculated with 104 luciferase-expressing NIH 173 metacyclic promastigotes in the dermis of the contralateral ear (secondary site) at day 11 p.p.i. (group A), 15 p.p.i. (group B), or 22 p.p.i. (group C). For the control groups, naïve mice received 104 luciferase-expressing NIH 173 metacyclic promastigotes in the dermis on day 11 p.p.i. (Ctrl A), 15 p.p.i. (Ctrl B), or 22 p.p.i. (Ctrl C). Longitudinal follow-up of the parasitic load and the ear thickness was done for up to 163 days p.p.i. (B) Dots represent the median value obtained for each group. Quantification of the parasitic load by determination of the bioluminescence (in photons per second per ear; panels 1 and 3) and ear thickness (in mm; panels 2 and 4) of the primary sites was performed for the experimental groups (panels 1 and 2) and the control groups (panels 3 and 4). The results are depicted as the medians ± standard deviations. Arrowheads indicate the day of parasite inoculation in the unchallenged group and the day of the second inoculation in the experimental and the control groups. The values obtained for the unchallenged group are shown in the gray areas indicated in panels 1 and 2.
FIG. 3.
C57BL/6 mice receiving a primary inoculation with L. major in the ear dermis develop acquired immunity. (A) Definition of experimental and control groups (n = 12 mice per group). (B) Monitoring of the parasitic load (in photons per second per ear; panels 1, 3, and 5) and ear thickness (in mm; panels 2, 4, and 6) in groups A to C and their corresponding controls (Ctrl A to C). Gray areas correspond to median bioluminescence values and lesion development from the primary site (right ear) for the experimental groups. Arrowheads indicate the time of inoculation at the primary site (shaded arrowheads) or the secondary site (black arrowheads). The acquisition of immunity can be assessed by comparison of the profiles displayed at the secondary sites (plain lines, experimental groups) and the primary sites (dotted lines, corresponding control groups).
Early curative applications of WR279396 (day 11) did not impair the acquisition of immunity.
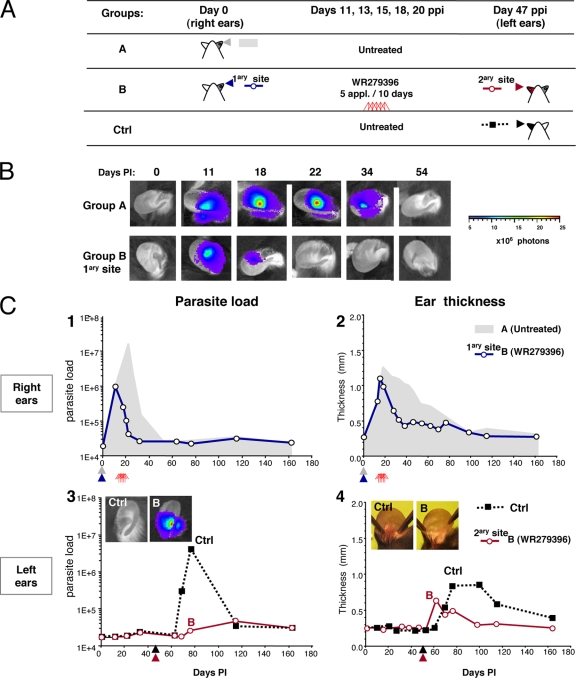
The mice were first inoculated in the right ear (day 0) and were then treated (or not treated [untreated control group]) with the WR279396 topical ointment (Fig. 4A, groups A and B). At day 47 p.p.i., the mice were reinoculated in the contralateral left ear. One control group received the same inoculum only on day 47 (i.e., no prior inoculation at day 0) (Fig. 4A, Ctrl group). The kinetics of the parasitic load and lesion formation were simultaneously assessed in the untreated group (group A), WR279396-treated group B, and the control groups. Two-way ANOVA analysis indicated that the topical treatment (P < 0.001) and the period of observation (P < 0.001) had a significant effect on the parasitic load for the entire experimental treatment group, and there was a significant interaction between the treatment and the period effect (P < 0.008). The parasitic load started to decrease after the third application (day 15; Fig. 4B and C, panel 1). WR279396 was significantly more effective than no treatment (untreated group) during the posttreatment period (P = 0.015). One day after the last application, the bioluminescence values were below the threshold value in 100% of the mice (Fig. 4C, panel 1). The decrease in the parasitic loads occurred prior to the decrease in ear thickness, and a significant difference in ear thickness between the treated and the control groups (P < 0.001) was observed starting at day 22 p.i. (Fig. 4C, panels 1 and 2). No further bioluminescent signal could be detected in the ear tissues of the untreated or the WR279396-treated groups (groups A and B; Fig. 4B and C, panel 1) following the complete and stable resolution of the inflammatory processes at the primary inoculation site.
FIG. 4.
Effects of WR279396 ointment on development of acquired immunity. (A) Design of the experimental groups (groups A and B) and the corresponding control group (n = 12 mice per group). An initial inoculation of 104 luciferase-expressing NIH 173 metacyclic promastigotes into the right ear dermis (primary site) of C57BL/6 mice was performed at day 0 in groups A (shaded arrowheads) and B (black arrowheads). At day 47 p.p.i., 104 luciferase-expressing L. major metacyclic promastigotes were inoculated in the dermis of the contralateral ears of mice that had received five applications of WR279396 ointment (red triangles) over 10 days (secondary site [red arrowhead], group B). The L. major-inoculated control mice corresponded to naïve mice that were inoculated at day 47 p.p.i. (black arrowhead). (B) The detection of bioluminescence at the primary site is illustrated by the results for a representative mouse within groups A and B. (C) Quantification of the bioluminescence of the parasitic load (in photons per second per ear; panels 1 and 3) and ear thickness (panels 2 and 4) in groups A and B and the control group. (Panels 1 and 2) Gray areas correspond to the bioluminescence and the ear thickness observed in group A. WR279396 treatment (red triangles) induced a significant decrease in both processes in the ear (open circles). (Panels 3 and 4) We compared the parasitic load and monitored the thickness of the ear lesions of the control mice (Ctrl; black squares) and mice that had successively received five applications of the ointment and challenged in the contralateral ear at day 47 (group B; open circles). Pictures of ears from representative mice from the control group and the WR279396-treated group (group B) at day 76 p.i. are presented.
The site of the secondary inoculation (administered in the contralateral left ear on day 47 p.p.i.; Fig. 4A, WR2979396-treated group B) did not display any bioluminescence signals (Fig. 4C, panel 3). Only a slight induration was observed at the secondary site (Fig. 4C, panel 4, and B). In contrast, the first inoculation of mice from the control group resulted in a parasitic load and lesion pattern similar to that observed in group A (untreated group), peaking at 4.7 × 106 photons/s/ear at day 19 after the secondary inoculation (Fig. 4C, panel 3 and Ctrl panel).
Because low parasitic loads (≤5,000 per ear) are not detectable by the use of bioluminescence, we performed two additional experiments following the same design. The mice were killed between days 80 and 96 p.i. The right ears were recovered from both groups and were evaluated by the limiting-dilution assay readout assay. Forty percent of the ears from all groups were positive (≤500 parasites per ear; data not shown).
DISCUSSION
In our optimized experimental model of CL, early curative applications of aminoglycosides (WR279396) did not impair the acquisition of immunity. The application of WR279396 as early as day 11 p.i. on the L. major-loaded ears induced clinical healing and sustained parasitic load control at this primary inoculation site. At the reinoculation site (in the contralateral ear), 7 weeks later, not only were no bioluminescence signals detected but also no lesions were ever observed.
In areas where CL is endemic, patients whose CL lesions heal either spontaneously or following the standard therapy are protected from the occurrence of new skin lesions upon a second natural inoculation of Leishmania from the same species (14). Initial investigations with guinea pigs and laboratory mice demonstrated that host-protective immune processes are detectable during the phase of healing of the primary lesions (4, 15, 16), but the precise timing of the acquisition of the protective process was not determined. In the present study, a bioluminescence-based imaging model adapted from that of Belkaid et al. (1) allowed it to be established that the processes controlling the parasitic load at the reinoculation site were operating as soon as the parasitic load had started to decrease at the primary inoculation site. Early treatment (i.e., treatment that occurs while the parasitic load is increasing) did not impair the activation or the maintenance of parasite-targeting effectors and tissue-protective regulatory leukocytes. Therefore, even decreasing parasitic loads at the lesion site during and after therapy might be sufficient to trigger the development and maturation of these balanced immune processes. Previous experiments with C57BL/6 mice showed that without the L. major expansion phase, the balanced population of immune effectors and regulators accounting for parasite-targeting and skin-protective processes is not established or does not operate at the secondary site (9). Therefore, the phase of expansion of the intracellular amastigote, which is known to be immunologically controlled, is likely necessary for the acquisition of immunity in the absence of therapy.
In the model used in the present study, the processes leading to the long-term persistence of amastigote-hosting cells have recently been sorted out (2, 13). The persisting amastigotes (9) within dendritic cells contribute to the priming and renewal of a mixed and balanced population of Leishmania-reactive effector and regulatory T lymphocytes. Of note, total clearance of the parasites is associated with the disruption of this immune balance, resulting in the loss of immunity (13). Thus, parasite persistence is important for the maintenance of immune protection. In our experiments, despite the curative topical applications of WR279396, low parasitic loads persisted in the lesions of several mice. A similar persistence has recently been observed in the lesions of patients after 10 days of treatment with WR279396 (3a).
Determination of acquired immunity in WR279396-treated patients (most of whom lived in foci where L. major MON-25 predominates [3]) is not an easy task. It would require the species identification of the Leishmania parasites isolated from a patient during two different natural episodes several months or years apart. L. tropica MON-8 is, indeed, increasingly reported in L. major MON-25 foci where most clinical trials are performed (3). Accurate identification tools exist (6, 17), but the use of appropriate sampling methods under field conditions is inconsistent. In addition, WR279396 ointment is currently used only in the context of clinical trials, in which follow-up does not exceed 1 year. Therefore, reliable data on this issue for humans will be available in 3 years at best and probably longer. Therefore, our experimental approach with mice is currently the most reliable way to explore the acquisition of immunity in WR279396-treated hosts. Even after correction for the possible moderately faster evolution of CL in mice, the observation that curative applications of WR279396 during the phase of the increase in parasitic loads (corresponding to the first days of clinical signs) do not impair the acquisition of immunity is reassuring from the perspective of the wide deployment of this topical treatment in foci where L. major is endemic.
Acknowledgments
We are very grateful to Max Grögl (Walter Reed Army Institute of Research) and Philip Smith (U.S. Army Medical Materiel Development Activity, Fort Detrick, MD) for kindly providing the WR279396 ointment. We also thank Christine Maillet for her excellent technical assistance and Thierry Angelique for his help with taking care of our mice.
This work was supported by the Institut Pasteur.
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Belkaid, Y., S. Mendez, R. Lira, N. Kadambi, G. Milon, and D. Sacks. 2000. A natural model of Leishmania major infection reveals a prolonged “silent” phase of parasite amplification in the skin before the onset of lesion formation and immunity. J. Immunol. 165:969-977. [DOI] [PubMed] [Google Scholar]
- 2.Belkaid, Y., C. A. Piccirillo, S. Mendez, E. M. Shevach, and D. L. Sacks. 2002. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature 420:502-507. [DOI] [PubMed] [Google Scholar]
- 3.Ben Salah, A., P. A. Buffet, G. Morizot, N. Ben Massoud, A. Zaatour, N. Ben Alaya, N. B. Haj Hamida, Z. E. Ahmadi, M. T. Downs, P. L. Smith, K. Dellagi, and M. Grogl. 2009. WR279,396, a third generation aminoglycoside ointment for the treatment of Leishmania major cutaneous leishmaniasis: a phase 2, randomized, double blind, placebo controlled study. PLoS Negl. Trop. Dis. 3:e432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3a.Ben Salah, A., A. Zaatour, N. Ben Messaoud, G. Morizot, K. Dellagi, H. Louzir, P. Smith, M. Grögl, and P. A. Buffet. 2009. Reduction of parasite loads in the superficial and deep dermis in L. major-infected adult patients treated with WR279396, abstr. 298. Abstr. 4th World Congr. Leishmaniasis.
- 3b.Berens, R. L., and J. J. Marr. 1978. An easily prepared defined medium for cultivation of Leishmania donovani promastigotes. J. Parasitol. 64:160. [PubMed] [Google Scholar]
- 4.Bryceson, A. D., R. S. Bray, R. A. Wolstencroft, and D. C. Dumonde. 1970. Immunity in cutaneous leishmaniasis of the guinea-pig. Clin. Exp. Immunol. 7:301-341. [PMC free article] [PubMed] [Google Scholar]
- 5.Buffet, P., E. Caumes, and M. Gentilini. 1994. Treatment of localized cutaneous leishmaniasis. Ann. Dermatol. Venereol. 121:503-511. (In French.) [PubMed] [Google Scholar]
- 6.Foulet, F., F. Botterel, P. Buffet, G. Morizot, D. Rivollet, M. Deniau, F. Pratlong, J. M. Costa, and S. Bretagne. 2007. Detection and identification of Leishmania species from clinical specimens by using a real-time PCR assay and sequencing of the cytochrome b gene. J. Clin. Microbiol. 45:2110-2115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goyard, S., H. Segawa, J. Gordon, M. Showalter, R. Duncan, S. J. Turco, and S. M. Beverley. 2003. An in vitro system for developmental and genetic studies of Leishmania donovani phosphoglycans. Mol. Biochem. Parasitol. 130:31-42. [DOI] [PubMed] [Google Scholar]
- 8.Grogl, M., B. G. Schuster, W. Y. Ellis, and J. D. Berman. 1999. Successful topical treatment of murine cutaneous leishmaniasis with a combination of paromomycin (Aminosidine) and gentamicin. J. Parasitol. 85:354-359. [PubMed] [Google Scholar]
- 9.Kebaier, C., J. E. Uzonna, S. M. Beverley, and P. Scott. 2006. Immunization with persistent attenuated delta lpg2 Leishmania major parasites requires adjuvant to provide protective immunity in C57BL/6 mice. Infect. Immun. 74:777-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lang, T., S. Goyard, M. Lebastard, and G. Milon. 2005. Bioluminescent Leishmania expressing luciferase for rapid and high throughput screening of drugs acting on amastigote-harbouring macrophages and for quantitative real-time monitoring of parasitism features in living mice. Cell. Microbiol. 7:383-392. [DOI] [PubMed] [Google Scholar]
- 11.Leclercq, V., M. Lebastard, Y. Belkaid, J. Louis, and G. Milon. 1996. The outcome of the parasitic process initiated by Leishmania infantum in laboratory mice: a tissue-dependent pattern controlled by the Lsh and MHC loci. J. Immunol. 157:4537-4545. [PubMed] [Google Scholar]
- 12.Lecoeur, H., P. Buffet, G. Morizot, S. Goyard, G. Guigon, G. Milon, and T. Lang. 2007. Optimization of topical therapy for Leishmania major localized cutaneous leishmaniasis using a reliable C57BL/6 model. PLoS. Negl. Trop. Dis. 1:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mendez, S., S. K. Reckling, C. A. Piccirillo, D. Sacks, and Y. Belkaid. 2004. Role for CD4(+) CD25(+) regulatory T cells in reactivation of persistent leishmaniasis and control of concomitant immunity. J. Exp. Med. 200:201-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Modabber, F., P. A. Buffet, E. Torreele, G. Milon, and S. L. Croft. 2007. Consultative meeting to develop a strategy for treatment of cutaneous leishmaniasis. Institute Pasteur, Paris. 13-15 June, 2006. Kinetoplastid Biol. Dis. 6:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preston, P. M., R. L. Carter, E. Leuchars, A. J. Davies, and D. C. Dumonde. 1972. Experimental cutaneous leishmaniasis. 3. Effects of thymectomy on the course of infection of CBA mice with Leishmania tropica. Clin. Exp. Immunol. 10:337-357. [PMC free article] [PubMed] [Google Scholar]
- 16.Preston, P. M., and D. C. Dumonde. 1976. Experimental cutaneous leishmaniasis. 5. Protective immunity in subclinical and self-healing infection in mouse. Clin. Exp. Immunol. 23:126-138. [PMC free article] [PubMed] [Google Scholar]
- 17.Reithinger, R., J. C. Dujardin, H. Louzir, C. Pirmez, B. Alexander, and S. Brooker. 2007. Cutaneous leishmaniasis. Lancet Infect. Dis. 7:581-596. [DOI] [PubMed] [Google Scholar]
- 18.Soto, J. M., J. T. Toledo, P. Gutierrez, M. Arboleda, R. S. Nicholls, J. R. Padilla, J. D. Berman, C. K. English, and M. Grogl. 2002. Treatment of cutaneous leishmaniasis with a topical antileishmanial drug (WR279396): phase 2 pilot study. Am. J. Trop. Med. Hyg. 66:147-151. [DOI] [PubMed] [Google Scholar]
- 19.Späth, G. F., and S. M. Beverley. 2001. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Exp. Parasitol. 99:97-103. [DOI] [PubMed] [Google Scholar]
- 20.Strijbosch, L. W., W. A. Buurman, R. J. Does, P. H. Zinken, and G. Groenewegen. 1987. Limiting dilution assays. Experimental design and statistical analysis. J. Immunol. Methods 97:133-140. [DOI] [PubMed] [Google Scholar]
- 21.Sunderkotter, C., M. Kunz, K. Steinbrink, G. Meinardus-Hager, M. Goebeler, H. Bildau, and C. Sorg. 1993. Resistance of mice to experimental leishmaniasis is associated with more rapid appearance of mature macrophages in vitro and in vivo. J. Immunol. 151:4891-4901. [PubMed] [Google Scholar]
- 22.Taswell, C. 1981. Limiting dilution assays for the determination of immunocompetent cell frequencies. I. Data analysis. J. Immunol. 126:1614-1619. [PubMed] [Google Scholar]