Abstract
Neisseria meningitidis has been relatively slow to acquire resistance to penicillin. We previously reported an increase in the incidence of invasive meningococcal disease (IMD) strains with decreased susceptibility to penicillin (DSP) in Ontario. Our objectives were to evaluate trends in IMD with DSP, to identify case-level predictors of IMD with DSP, and to evaluate the relationship among DSP, bacterial phenotype, and the likelihood of a fatal outcome. All IMD isolates received in Ontario between 2000 and 2006 were submitted to the Public Health Laboratories, Toronto, for confirmation of the species, serogroup determination, and susceptibility testing. Isolates were considered to be IMD strains with DSP if the penicillin MIC was ≥0.125 μg/ml. Temporal trends were evaluated using multivariable Poisson regression models. Correlates of diminished susceptibility and fatal outcome were evaluated with multivariable logistic regression models. The overall rate of IMD caused by strains with DSP in Ontario was approximately 1.20 cases per million population annually (95% confidence interval [95% CI], 0.99 to 1.46). Seventy-nine strains (21.7%) were IMD strains with DSP. There was no year-to-year trend in the incidence of IMD with DSP. IMD with DSP was strongly associated with strains of serogroups Y (odds ratio [OR], 6.3; 95% CI, 3.6 to 11.1) and W-135 (OR, 8.2; 95% CI, 4.0 to 16.7). Infection with serogroup B or C strains was associated with a marked increase in the risk of mortality (OR, 3.07; 95% CI, 1.39 to 6.75); however, no association between IMD with DSP and mortality was observed. In contrast to trends of the 1990s, the incidence of IMD with DSP was stable in Ontario between 2000 and 2006. In Ontario, the serogroup rather than the penicillin MIC is the microbiological parameter most predictive of mortality.
Neisseria meningitidis is the causative agent of invasive meningococcal disease (IMD), an important cause of bacterial meningitis and septicemia (3, 28, 32). IMD is endemic in Canada, with most cases caused by isolates from B, C, Y, and W-135 serogroups (23, 25, 32, 33). Although effective vaccines against some strains of N. meningitidis (serogroups A, C, Y, and W-135) are now available, they do not include all serogroups of public health concern, and management of IMD is accomplished through the use of antibiotics for both chemoprophylactic and therapeutic treatment (19, 25). While most strains of N. meningitidis are susceptible to a range of antimicrobials, sporadic resistance to members of nearly all antimicrobial classes has been reported previously and resistance to sulfonamides and tetracycline is not uncommon (3, 17). Despite widespread penicillin resistance among some bacterial pathogens, N. meningitidis has been slow to acquire resistance to penicillin (30, 37) and remains one of the few serious bacterial pathogens for which penicillin is the drug of choice (3, 28, 37).
In the 1980s, meningococci exhibiting decreased susceptibility to penicillin (DSP) were observed in the United Kingdom, Spain, and South Africa (6, 17, 29, 36). Since then, DSP has been reported worldwide, with the first case of infection caused by a strain with DSP reported in Ontario, Canada, in 1989 (27). In Ontario, growing concern over resistance has prompted routine susceptibility testing of all bacterial IMD isolates. Between January 1997 and December 2000, the percentage of meningococcal isolates in Ontario demonstrating DSP increased more than twofold (7).
Despite similar national surveillance efforts, the lack of international consensus on MIC breakpoints has prevented a comprehensive evaluation of the incidence and implications of DSP. Prior to 2005, the interpretation of susceptibility to penicillin was based on breakpoints published in the literature, and isolates for which the MIC was ≥0.125 μg/ml were considered to have DSP. The adoption of the Clinical and Laboratory Standards Institute (CLSI) MIC interpretative standards (10, 11) in 2005 allowed standardized, and more nuanced, identification of N. meningitidis specimens as susceptible, intermediately susceptible, or resistant to antimicrobials of interest.
The clinical implications of diminished susceptibility to penicillin have yet to be investigated on a large scale, and existing studies have yielded conflicting results (20, 30, 31, 34). We sought to evaluate trends in penicillin susceptibility among clinical meningococcal isolates in Ontario since 1999 using the updated CLSI standards (10, 11), to identify clinical and microbiological correlates of DSP, and to evaluate the association among DSP, bacterial phenotype, and the likelihood of a fatal outcome in IMD cases. Given the importance of chemotherapy and prophylaxis for the management of IMD, we also evaluated the susceptibilities of strains to other antimicrobials, including chloramphenicol, ceftriaxone, and rifampin.
MATERIALS AND METHODS
Laboratory methods.
In the province of Ontario, all bacterial IMD isolates were submitted to the Public Health Laboratories, Toronto, for confirmation of the species, serogrouping, and molecular characterization, and culture isolates were subjected to antimicrobial susceptibility testing (AST). Confirmation of the species as N. meningitidis was accomplished by standard laboratory protocols (Gram reaction, cellular and colonial morphology, and biochemical and carbohydrate reaction analyses). A preliminary serological group was identified by bacterial agglutination with rabbit antisera against capsule polysaccharide antigens of eight serogroups, A, B, C, 29E, W-135, X, Y, and Z, with antisera provided by the National Microbiology Laboratory, Winnipeg, Manitoba, Canada.
Specimens which were negative by culture were simultaneously identified and serologically grouped by real-time PCR using the protocol previously published by Mothershed et al. (21). N. meningitidis DNA was extracted from specimens by using the QIAamp DNA minikit (Qiagen Inc., Mississauga, Ontario, Canada). Detection of meningococcal DNA was conducted using N. meningitidis-specific primers targeting the capsular transport gene ctrA and serogroup-specific primers targeting sacB (for serogroup A strains), siaD (for serogroup B and C strains), synG (for serogroup W-135 strains), synF (for serogroup Y strains), and xcbB (for serogroup X strains) (21).
Following identification, all isolates were sent to the National Microbiology Laboratory, Public Health Agency of Canada, for serogroup verification, serotyping, and serosubtyping (performed with an enzyme-linked immunosorbent assay using whole-cell antigens and monoclonal antibodies) (1).
AST of IMD isolates.
With the exception of specimens identified by PCR, all N. meningitidis isolates underwent AST via the CLSI M7-A7 agar dilution method (11). Test inocula were prepared from a direct colony suspension taken from a 20- to 24-h growth on sheep blood agar (incubated at 35°C in 5% CO2). Colonies were suspended in brain heart infusion broth to a 0.5 McFarland standard (108 CFU/ml). A final cell density of approximately 104 CFU/spot was delivered onto Mueller-Hinton agar supplemented with 5% sheep blood by using a replicator, and the agar was incubated at 35°C in 5% CO2 for 24 h prior to determination of MICs. Isolates were tested for susceptibility to penicillin (0.004 to 8.0 μg/ml), chloramphenicol (2 and 4 μg/ml), ceftriaxone (0.125 to 2.0 μg/ml), and rifampin (0.5 and 1.0 μg/ml) at the concentrations noted. Each specimen was then reported to be susceptible, intermediate, or resistant according to CLSI interpretive criteria (10).
Prior to 2005, standardized AST protocols and interpretive breakpoints for N. meningitidis were not available, and AST was performed in accordance with literature breakpoints (17). To standardize MIC interpretation, N. meningitidis isolates from 2000 to 2005 were retested in 2007 according to the current CLSI-recommended standards and interpretive criteria (10, 11). Beta-lactamase production was also tested using the chromogenic cephalosporin nitrocefin and a code SR0112 kit (Oxoid Limited, Basingstoke, Hampshire, England).
Identification of variables associated with an increased risk of death from IMD.
Case demographics, sources of specimen isolation, dates of specimen submission, and disease outcomes were obtained from patient requisitions, the Reportable Disease Information System (RDIS), and the Integrated Public Health Information System (iPHIS). Cases of IMD were reported in Ontario if they met the following national criteria for a confirmed case (24): isolation of N. meningitidis from a normally sterile site (blood, cerebrospinal fluid, or joint, pleural, or pericardial fluid) or demonstration of the presence of N. meningitidis DNA in a specimen from a normally sterile site by an appropriately validated nucleic acid test.
Statistical methods.
We performed descriptive analyses of trends in IMD and in individual N. meningitidis serogroups in Ontario for the province as a whole and for groupings by patient age, gender, and region and the season and year of bacterial isolation. Subject age, gender, and health region were missing for 5.1, 3.1, and 0.7% of the study sample, respectively. Missing values for age were replaced with random age values drawn from a log-normal distribution with a mean and standard deviation identical to those of the known ages. Missing gender designations and health regions were randomly replaced in proportion to known distributions. Rates per 100,000 person-years were calculated using population denominators from Statistics Canada community profiles (available in the CANSIM database [http://cansim2.statcan.ca/cgi-win/cnsmcgi.exe?CANSIMFile=CII/CII_1_E.HTM&RootDir=CII/&LANG=E]). The geographic origins of specimens were coded using Ontario health regions as defined by the Association of Public Health Epidemiologists of Ontario (APHEO) (4).
We sought to determine whether rates of meningococcal disease, meningococcal disease due to specific serotypes, or meningococcal disease due to drug-resistant isolates were changing significantly over time. The significance of temporal trends was evaluated using Poisson regression models, with year terms centered around 2003, incorporating sine and cosine terms to control for seasonal oscillation. Confidence intervals were calculated using nonlinear prediction methods. Similar analyses were repeated for meningococcal isolates with DSP, but regression models evaluating temporal trends also controlled for the underlying rate of IMD in Ontario.
We evaluated differences in penicillin susceptibility by region and by gender and age group of patients both through calculation of geometric mean titers and through statistical testing of log-transformed MIC values. Differences in the log MIC among groups and regions were evaluated using one- and two-way analyses of variance, with stepwise unpaired t tests used to further evaluate between-group differences. We also evaluated trends in the log-transformed MIC by using linear regression models.
We sought to determine whether characteristics of patients or infecting isolates or geographical information predicted an elevated penicillin MIC. Associations between an elevated penicillin MIC and isolate and patient characteristics were evaluated using univariable and multivariable logistic regression models. Multivariable models incorporated sources of specimens, patient age and gender, and factors with an association with resistance at a P value of <0.15. Univariable and multivariable logistic models were also used to evaluate the relationship between an elevated MIC of penicillin and outcome, with and without controlling for patient characteristics and the bacterial phenotype.
RESULTS
AST.
Of the 389 isolates received at Central Public Health Laboratories, Toronto, between January 2000 and December 2006, 363 (93.3%) underwent AST. Seventy-nine isolates (21.7%) demonstrated intermediate susceptibility to penicillin (MIC, 0.125 to 0.25 μg/ml). No isolates were resistant to penicillin, and accordingly, none exhibited beta-lactamase activity. All isolates were susceptible to ceftriaxone (MIC, ≤0.12 μg/ml), rifampin (MIC, ≤0.5 μg/ml), and chloramphenicol (MIC, ≤2 μg/ml), with the exception of three isolates from 2006 which demonstrated intermediate susceptibility to chloramphenicol (MIC, 4 μg/ml). Production of chloramphenicol acetyltransferases was not evaluated.
Incidence of IMD.
The overall rate of IMD in Ontario from 2000 to 2006 was estimated to be 4.38 cases per million population (95% confidence interval [95% CI], 3.96 to 4.85). The majority of cases were caused by serogroup B (37.5%) or serogroup C (29.6%), with the next largest proportions of cases caused by serogroup Y (22.1%) and serogroup W-135 (10.8%). Only two cases were caused by serogroup A strains. The most common bacterial serotype was 2a, which comprised 80% of serogroup C strains.
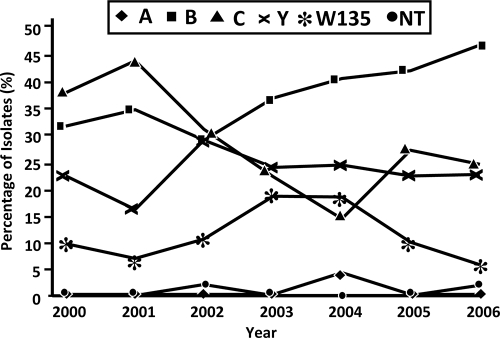
As expected, IMD occurred with distinct wintertime seasonality (P value for seasonal oscillation with combined sine and cosine terms, 0.003). The incidence was highest in children <1 year of age (incidence rate ratio [IRR], 9.44; 95% CI, 6.67 to 13.37), those aged 1 to 4 years (IRR, 2.83; 95% CI, 2.07 to 3.87), individuals aged 15 to 24 years (IRR, 2.03; 95% CI, 1.61 to 2.58), and individuals aged 75 years or more (IRR, 1.48; 95% CI, 1.09 to 2.00). IMD rates were lower in the central east and central west regions of Ontario than elsewhere in the province (IRR, 0.52; 95% CI, 0.41 to 0.67). Overall, a significant downward trend in the incidence of IMD during the study period was seen (IRR per year, 0.93; 95% CI, 0.89 to 0.98), with most of this decline occurring in disease cases caused by serogroup C strains (IRR per year, 0.82; 95% CI, 0.79 to 0.90). As a result of this decline, there was a significant increase in the proportion of IMD cases due to serogroup B (odds ratio [OR] per year, 1.11; 95% CI, 1.01 to 1.24) (Fig. 1).
FIG. 1.
Proportions of N. meningitidis isolates by serogroup in Ontario from 2000 to 2006. Two isolates were nontypeable (NT). Serogroups B and C accounted 66.1% (36.5 and 29.6%, respectively) of the total laboratory-confirmed cases of N. meningitidis infection from 2000 to 2006. During the period from 2000 to 2006, there was a significant decrease in the proportion of cases due to serogroup C (from 37.5% in 2000 to 24.1% in 2006 [P = 0.01]). There was a relative increase in the proportion of cases due to serogroup B during the same time period.
Incidence of IMD caused by strains for which penicillin MICs were elevated.
The approximate incidence of infection with strains with DSP was 1.20 cases per million population annually (95% CI, 0.99 to 1.46). There was no year-to-year trend in the overall incidence of IMD caused by strains with intermediate susceptibility to penicillin (IRR, 0.98; 95% CI, 0.89 to 1.08) (Fig. 2), the incidence adjusted for declining rates of IMD (IRR, 0.95; 95% CI, 0.85 to 1.05), the proportion of isolates with intermediate susceptibility (OR per year, 1.06; 95% CI, 0.95 to 1.18), or the log-transformed average MIC of penicillin (average annual change, −0.01; 95% CI, −0.04 to 0.02).
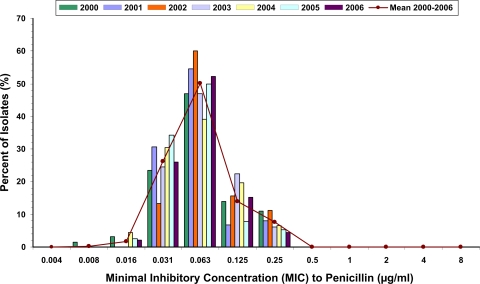
FIG. 2.
Proportions of N. meningitidis isolates by penicillin MIC in Ontario from 2000 to 2006. No difference was seen over time in either the proportion of isolates corresponding to each MIC or the geometric mean MIC, as described in the text. A nonsignificant decrease in the proportion of isolates for which the penicillin MIC was 0.25 μg/ml (from 10.94 to 4.35% [P = 0.21]) between 2000 and 2006 was observed.
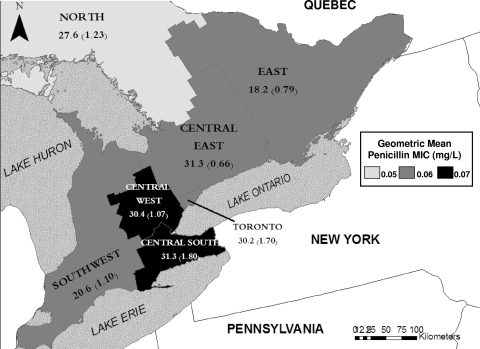
There were no regional differences in rates of IMD caused by strains with DSP after adjustment for overall rates of IMD. However, there was a significant difference in the penicillin MIC between the central west and central south regions of the province and the northern and other central Ontario regions (P = 0.03) (Fig. 3).
FIG. 3.
N. meningitidis isolates with intermediate susceptibility to penicillin by Ontario health region. The map shows six southern Ontario health regions and the southernmost portion of a northern health region as shaded areas. The highest geometric mean penicillin MIC is seen in the central west and central south regions at the western extreme of Lake Ontario. The lowest penicillin MIC is seen in the northern region. Numbers denote the proportion of isolates from each region with intermediate susceptibility to penicillin, and numbers in parentheses denote the number of cases of IMD with intermediate susceptibility per million residents per year.
Significant risk factors for IMD with DSP included infection with serogroup Y (adjusted OR, 6.3; 95% CI, 3.6 to 11.1) or W-135 (adjusted OR, 8.2; 95% CI, 4.0 to 16.7) (Fig. 4) and isolation of the bacteria from cerebrospinal fluid rather than blood (adjusted OR, 2.1; 95% CI, 1.2 to 3.7). No association between the incidence of infection with strains exhibiting DSP and age or gender was identified. Qualitatively similar results were seen when the log-transformed penicillin MIC, rather than the incidence of infection with intermediately susceptible strains, was used as the outcome of interest.
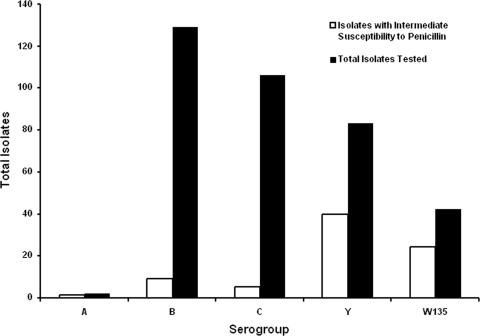
FIG. 4.
Numbers of N. meningitidis isolates with intermediate susceptibility to penicillin by serogroup from 2000 to 2006. Elevated MICs of penicillin were significantly more common for serogroup Y and W-135 strains than for serogroup B or C strains (P < 0.001). A significantly increased risk of death was associated with infection with the B or C serogroup (OR, 3.07; 95% CI, 1.39 to 6.75) but not with an elevated MIC of penicillin, as described in the text.
Impact of intermediate susceptibility to penicillin on clinical outcomes.
No association between patient age or gender or the year, season, or region of specimen collection and the likelihood of mortality was identified. Infection with serogroup B or C strains was associated with a marked increase in the risk of mortality (OR, 3.07; 95% CI, 1.39 to 6.75), and a nonsignificant decrease in mortality was seen in association with serogroup W-135 (OR, 0.33; 95% CI, 0.08 to 1.42) and serogroup Y (OR, 0.44; 95% CI, 0.18 to 1.08). In crude analyses, there was a nonsignificant reduction in the risk of death for individuals with IMD caused by strains with DSP (OR, 0.65; 95% CI, 0.31 to 1.34), but after adjustment for serogroups B and C, this effect was not observed (OR, 1.06; 95% CI, 0.47 to 2.37). In subgroup analyses restricted to serogroup Y, a nonsignificant trend toward increased risk of mortality was seen (OR, 5.53; 95% CI, 0.62 to 49.45), but this effect was not seen in analyses restricted to serogroup W-135 (OR, 0.73; 95% CI, 0.04 to 12.52).
DISCUSSION
Prior to 2000, the incidence of serogroup C IMD cases had increased throughout Canada, and serogroup C was responsible for several localized outbreaks between 1989 and 1993 and in 2000 and 2001 (22, 32). We identified a dramatic reduction in the incidence of serogroup C IMD cases in Ontario, Canada, between 2000 and 2006. This decline overlaps the implementation of the meningococcal serogroup C polysaccharide protein conjugate vaccine into the provincially funded routine childhood immunization program (26). In 2001, the National Advisory Committee on Immunization recommended the vaccine, and in 2003, the vaccine was provincially funded (22, 26). While historically, serogroup B and C infections have displayed complementary cyclical patterns of disease incidence (12, 23), we observed a marked increase in the proportion of serogroup B IMD cases well beyond what would be expected based on historical epidemiological trends (5, 12, 35).
Widespread documentation of clinical N. meningitidis isolates with DSP based on a range of MIC interpretative criteria has warranted an evaluation of current trends in antimicrobial susceptibility. Penicillin remains the therapeutic option of choice for IMD caused by N. meningitidis. In Ontario, the incidence of IMD caused by strains with DSP (1.2 cases per million population) was similar to that observed over a 6-year period in Scotland (1.5 cases per million population) (18). However, the prevalence of IMD caused by strains with DSP varied, with Ontario having a higher prevalence (21.7%) than the United States (3%), sub-Saharan Africa (6%), and the United Kingdom (12.6%) but one similar to those in Sweden (23%), France (31.2%), and Portugal (24.6%) (3, 8, 14, 31).
In contrast to trends observed in the late 1990s in Ontario (7), IMD caused by strains with DSP did not increase in frequency between 2000 and 2006. Recent multiyear studies conducted in Scotland and South Africa have also reported stable prevalences of meningococci with DSP (14, 18). The regional variations in the geometric mean MIC of penicillin in the central west and central south regions may be attributable to differential selective pressures for resistance in these regions. Regional variations in antibiotic resistance are often reflective of selective pressure created by local antibiotic consumption (15); however, a quantitative assessment of differential antibiotic use patterns by region would be required to critically evaluate this relationship.
We previously reported a high prevalence of DSP among serogroup W-135 isolates (7). Our present analysis confirmed this finding, and our data are consistent with the results of earlier studies which have demonstrated high levels of DSP among both serogroup W-135 and serogroup C IMD isolates (3, 7, 8, 31). To our knowledge, this is the first report of serogroup Y IMD isolates demonstrating a high prevalence of intermediate susceptibility; however, this finding may be attributable to an overall increase in serogroup Y IMD cases in Canada during this period (with most of this increase due to Ontario cases) (32, 33).
Earlier investigations have implicated patient age, clinical disease manifestation (e.g., septicemia), and the bacterial phenotype in increasing risk of death from invasive meningococcal infections (13, 30, 31). In our analysis, infection with serogroup B and C strains significantly increased the risk of death from IMD, whereas infection with serogroup Y and W-135 strains was associated with a nonsignificant decrease in the risk of mortality. A few recent studies have also documented the association between a fatal outcome and the meningococcal phenotype; specifically, increases in case fatality rates have been observed for the following serogroup-serotype combinations: C:2a, B:2a, and B:15 (13, 16, 31). Serotype 2a accounted for the vast majority of serogroup C IMD infections during our study period. This phenotype, which emerged and caused several outbreaks in Canada in the 1990s, has demonstrated high levels of virulence and association with poor prognosis (22, 35).
We demonstrated that intermediate susceptibility to penicillin does not increase the risk of death from meningococcal disease. A similar lack of association between IMD caused by strains with DSP and a fatal outcome in the United Kingdom and sub-Saharan Africa was documented previously (14, 31), while in Australia, penicillin susceptibility was associated with a poor clinical outcome (30). In the context of our results, infection with serogroups displaying a high prevalence of DSP is associated with lower levels of mortality while infection with penicillin-susceptible strains is nonsignificantly associated with higher mortality rates.
Cumulatively, our results suggest that factors other than DSP (e.g., host immune response, rapid diagnosis, and treatment) are more important determinants of IMD outcome (30). Further, we have established that the bacterial phenotype can serve as a marker for intermediate susceptibility to penicillin and risk of mortality, as Trotter et al. have suggested (31).
As resistance of meningococci to ceftriaxone (which has not been reported), rifampin, and chloramphenicol is extremely rare in North America, the susceptibility of our clinical isolates to these antimicrobials is reassuring for their use in prophylaxis. Although chloramphenicol is currently not recommended for the treatment of meningococcal disease in North America, the detection of three strains with intermediate susceptibility is concerning, as chloramphenicol resistance in meningococci has been restricted previously to only 15 cases worldwide (17, 25, 35). Recently, there have been reports documenting fluoroquinolone-resistant meningococci in the United States (9, 38). While meningococcal isolates in Ontario have not been routinely tested with fluoroquinolones, nalidixic acid screening will be implemented as part of the AST surveillance for N. meningitidis. Our data support the continued use of these agents for therapeutic treatment and prophylaxis of IMD; however, continued surveillance of trends in antimicrobial susceptibility is necessary for the rapid identification of emerging resistance and ongoing evaluations of therapy effectiveness in clinical case management.
In contrast to the trends of the late 1990s in Ontario (7), the incidence of IMD caused by strains with DSP did not increase between 2000 and 2006. In Ontario, the bacterial phenotype appears to be the microbiological parameter most predictive of the risk of death from IMD. While further surveillance of ongoing trends in penicillin susceptibility among meningococci will be required, our study emphasizes the importance of AST surveillance for N. meningitidis and continued surveillance, using standardized AST criteria, to assist in the evaluation of IMD risk factors and vaccine effects in the province of Ontario.
Acknowledgments
We thank Raymond Tsang and Averil M. Henderson at the Public Health Agency of Canada's National Microbiology Laboratory in Manitoba, Canada. In addition, we thank the staff of the Reference Identification and Susceptibility Department at the Public Health Laboratories, Toronto, for their technical assistance.
We declare that we have no competing interests and have not received financial support for this study.
Footnotes
Published ahead of print on 19 January 2010.
REFERENCES
- 1.Abdillahi, H., and J. T. Poolman. 1987. Whole-cell ELISA for typing of Neisseria meningitidis with monoclonal antibodies. FEMS Microbiol. Lett. 48:367-371. [PubMed] [Google Scholar]
- 2.Reference deleted.
- 3.Antignac, A., M. Ducos-Galand, A. Guiyoule, R. Pirès, J. Alonso, and M. Taha. 2003. Neisseria meningitidis strains isolated from invasive infections in France (1999-2002): phenotypes and antibiotic susceptibility patterns. Clin. Infect. Dis. 37:912-920. [DOI] [PubMed] [Google Scholar]
- 4.Association of Public Health Epidemiologists of Ontario. 24 June 2008, accession date. Geography in Ontario. Association of Public Health Epidemiologists of Ontario, Ottawa, Canada. http://www.apheo.ca/index.php?pid=195.
- 5.Balmer, P., R. Burrow, and E. Miller. 2002. Impact of meningococcal C conjugate vaccine in the UK. J. Med. Microbiol. 51:717-722. [DOI] [PubMed] [Google Scholar]
- 6.Botha, P. 1988. Penicillin-resistant Neisseria meningitidis in southern Africa. Lancet i:54. [DOI] [PubMed] [Google Scholar]
- 7.Brown, S., G. Riley, and F. Jamieson. 2001. Neisseria meningitidis with decreased susceptibility to penicillin in Ontario, Canada 1997-2000. Can. Commun. Dis. Rep. 27:73-75. [PubMed] [Google Scholar]
- 8.Canica, M., R. Dias, B. Nunes, L. Carvalho, and E. Ferreira. 2004. Invasive culture-confirmed Neisseria meningitidis in Portugal: evaluation of serogroups in relation to different variables and antimicrobial susceptibility (2000-2001). J. Med. Microbiol. 53:921-925. [DOI] [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. 2008. Emergence of fluoroquinolone-resistant Neisseria meningitidis—Minnesota and North Dakota, 2007-2008. MMWR Morb. Mortal. Wkly. Rep. 57:173-175. [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute. 2005. Performance standards for antimicrobial susceptibility testing. Fifteenth informational supplement. M100-S15, p.138-139. Clinical and Laboratory Standards Institute, Wayne, PA.
- 11.Clinical and Laboratory Standards Institute. 2005. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7-A7, 6th ed. Clinical and Laboratory Standards Institute, Wayne, PA.
- 12.Diggle, M. A., and S. C. Clarke. 2005. Increased genetic diversity of Neisseria meningitidis isolates after the introduction of the meningococcal serogroup C polysaccharide conjugate vaccines. J. Clin. Microbiol. 43:4649-4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Donalisio, M. R., B. Kemp, M. M. Rocha, and R. M. Ramalheira. 2000. Fatality rate in the epidemiology of meningococcal disease: study in the region of the Campinas, SP, Brazil, 1993 to 1998. Rev. Saude Publica 34:589-595. (In Portuguese.) [DOI] [PubMed] [Google Scholar]
- 14.du Plessis, M., A. von Gottberg, C. Cohen, L. de Gouveia, and K. P. Klugman. 2008. Neisseria meningitidis intermediately resistant to penicillin and causing invasive disease in South Africa in 2001 to 2005. J. Clin. Microbiol. 46:3208-3214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.García-Rey, C., L. Aguilar, F. Baquero, J. Casal, and R. Dal-Ré. 2002. Importance of local variations in antibiotic consumption and geographical differences of erythromycin and penicillin resistance in Streptococcus pneumoniae. J. Clin. Microbiol. 40:159-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jensen, E. S., H. C. Schønheyder, I. Lind, L. Berthelsen, B. Nørgard, and H. T. Sørensen. 2003. Neisseria meningitidis phenotypic markers and septicaemia, disease progress and case-fatality rate of meningococcal disease: a 20-year population based historical follow-up study in a Danish county. J. Med. Microbiol. 52:173-179. [DOI] [PubMed] [Google Scholar]
- 17.Jorgensen, J. H., S. A. Crawford, and K. R. Fiebelkorn. 2005. Susceptibility of Neisseria meningitidis to 16 antimicrobial agents and characterization of resistance mechanisms affecting some agents. J. Clin. Microbiol. 43:3162-3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kyaw, M. H., J. C. Bramley, S. Clark, P. Christie, I. G. Jones, and H. Campbell. 2002. Prevalence of moderate penicillin resistant invasive Neisseria meningitidis infection in Scotland, 1994-9. Epidemiol. Infect. 128:149-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopalco, P. L. 2008. Is there room for improving case management for contacts of meningococcal disease in the European Union? Euro Surveill. 13:pii=8057. [DOI] [PubMed] [Google Scholar]
- 20.Luaces Cubells, C., J. J. García García, J. Roca Martinez, and C. L. Latorre Otin. 1997. Clinical data in children with meningococcal meningitis in a Spanish hospital. Acta Pediatr. 86:26-29. [DOI] [PubMed] [Google Scholar]
- 21.Mothershed, E. A., C. T. Sacchi, A. M. Whitney, G. A. Barnett, G. W. Ajello, S. Schmink, L. W. Mayer, M. Phelan, T. H. Taylor, Jr., S. A. Bernhardt, N. E. Rosenstein, and T. Popovic. 2004. Use of real-time PCR to resolve slide agglutination discrepancies in serogroup identification of Neisseria meningitidis. J. Clin. Microbiol. 42:320-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Advisory Committee on Immunization. 2001. Statement on the recommended use of meningococcal vaccines. Can. Commun. Dis. Rep. 27:1-36. [PubMed] [Google Scholar]
- 23.Navarro, C., S. L. Deeks, A. Medaglia, and R. S. W. Tsang. 2007. Enhanced surveillance of invasive meningococcal disease in Canada: 1 January, 2004, through 31 December, 2005. Can. Commun. Dis. Rep. 33:1-15. [PubMed] [Google Scholar]
- 24.Public Health Agency of Canada. 2000. Case definitions for diseases under national surveillance. Can. Commun. Dis. Rep. 26(S3):1-73. [PubMed] [Google Scholar]
- 25.Public Health Agency of Canada. 2005. Supplement: guidelines for the prevention and control of meningococcal disease. Can. Commun. Dis. Rep. 31(S1):1-20. [PubMed]
- 26.Public Health Agency of Canada. 10 September 2008, accession date. Publicly funded immunization programs in Canada—routine schedule for infants and children (including special programs and catch-up programs). Public Health Agency of Canada, Ottawa, Canada. http://www.phac-aspc.gc.ca/im/ptimprog-progimpt/table-1-eng.php.
- 27.Riley, G., S. Brown, and C. Krishnan. 1991. Penicillin resistance in Neisseria meningitidis. N. Engl. J. Med. 324:997. [DOI] [PubMed] [Google Scholar]
- 28.Rosenstein, N. E., B. A. Perkins, D. S. Stephens, T. Popovic, and J. M. Hughes. 2001. Meningococcal disease. N. Engl. J. Med. 344:1378-1388. [DOI] [PubMed] [Google Scholar]
- 29.Sutcliffe, E. M., D. M. Jones, S. el-Sheikh, and A. Percival. 1988. Penicillin-insensitive meningococci in the UK. Lancet i:657-658. [DOI] [PubMed] [Google Scholar]
- 30.Tapsall, J. W., T. Shultz, E. Limnios, R. Munro, J. Mercer, R. Porritt, J. Griffith, G. Hogg, G. Lum, A. Lawrence, D. Hansman, P. Collignon, P. Southwell, K. Ott, M. Gardam, K. J. L. Richardson, J. Bates, D. Murphy, and H. Smith. 2001. Surveillance of antibiotic resistance in invasive isolates of Neisseria meningitidis in Australia 1994-1999. Pathology 33:359-361. [DOI] [PubMed] [Google Scholar]
- 31.Trotter, C. L., A. J. Fox, M. E. Ramsay, F. Sadler, S. J. Gray, R. Mallard, and E. B. Kaczmarski. 2002. Fatal outcome from meningococcal disease—an association with meningococcal phenotype but not with reduced susceptibility to benzylpenicillin. J. Med. Microbiol. 51:855-860. [DOI] [PubMed] [Google Scholar]
- 32.Tsang, R. S. W., S. G. Squires, and T. W. S. Tam. 2003. Characterization of Neisseria meningitidis strains isolated from invasive meningococcal disease cases in Canada in 2001. Can. J. Microbiol. 49:633-638. [DOI] [PubMed] [Google Scholar]
- 33.Tsang, R. S. W., S. G. Squires, W. D. Zollinger, and F. E. Ashton. 2002. Distribution of serogroups of Neisseria meningitidis and antigenic characterization of serogroup Y meningococci in Canada, January 1, 1999 to June 30, 2001. Can. J. Infect. Dis. 13:391-396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Turner, P. C., K. W. Southern, N. J. B. Spencer, and H. Pullen. 1990. Treatment failure in meningococcal meningitis. Lancet 335:732-733. [DOI] [PubMed] [Google Scholar]
- 35.Tzanakaki, G., and P. Mastrantonio. 2007. Aetiology of bacterial meningitis and resistance to antibiotics of causative pathogens in Europe and in the Mediterranean region. Int. J. Antimicrob. Agents 29:621-629. [DOI] [PubMed] [Google Scholar]
- 36.Van Esso, D., D. Fontanals, S. Uriz, M. A. Morera, T. Juncosa, C. Latorre, and M. Duran. 1987. Neisseria meningitidis strains with decreased susceptibility to penicillin. Pediatr. Infect. Dis. J. 6:438-439. [DOI] [PubMed] [Google Scholar]
- 37.Vazquez, J. A. 2001. The resistance of Neisseria meningitidis to the antimicrobial agents: an issue still in evolution. Rev. Med. Microbiol. 12:39-45. [Google Scholar]
- 38.Wu, H. M., B. H. Harcourt, C. P. Hatcher, S. C. Wei, R. T. Novak, X. Wang, B. A. Juni, A. Glennen, D. J. Boxrud, J. Rainbow, S. Schmink, R. D. Mair, M. J. Theodore, M. A. Sander, T. K. Miller, K. Kruger, A. C. Cohn, T. A. Clark, N. E. Messonnier, L. W. Mayer, and R. Lynfield. 2009. Emergence of ciprofloxacin-resistant Neisseria meningitidis in North America. N. Engl. J. Med. 360:886-892. [DOI] [PubMed] [Google Scholar]