Abstract
We describe the acquisition of flucytosine, azole, and caspofungin resistance in sequential Candida glabrata bloodstream isolates collected from a bone marrow transplant patient with clinical failure. Point mutations in C. glabrata FUR1 (CgFUR1) and CgFKS2 and overexpression of CgCDR1 and CgCDR2 were observed in resistant isolates.
Candida glabrata has emerged as an important fungal pathogen, particularly in patients with hematologic malignancies (8). This Candida species is known to have intrinsic low-level fluconazole (FLC) resistance but can easily acquire high-level resistance and cross-resistance to other triazoles (16). Echinocandins appeared as a therapeutic advance in the management of C. glabrata infections (12), and clinical failures associated with resistance remain rare events (4, 5, 6, 9, 10, 13, 20). Here we report a fatal case of a hematopoietic stem cell transplant (HSCT) recipient with recurrent C. glabrata candidemia with isolates developing resistance to flucytosine (5FC), azoles, and caspofungin (CSF) following exposure to these drugs.
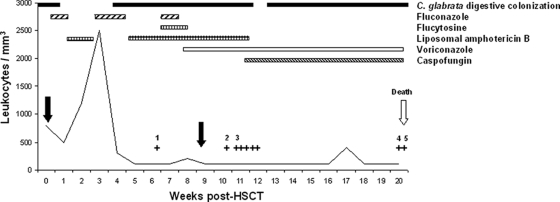
A 9-year-old girl suffering from Fanconi anemia underwent an unrelated HSCT on 25 October 2001 (Fig. 1). At this time, she had digestive colonization with C. glabrata and received FLC prophylaxis from 22 October to 31 October and from 12 November to 24 November. From 1 November to 12 November and from 24 November to 10 December, she received broad-spectrum antibiotics in combination with liposomal amphotericin B (LAMB) as empirical therapy. The first blood culture grew positive for C. glabrata on 8 December (isolate 1) (ID32C strips; bioMérieux, France). The patient was treated with intravenous FLC in combination with intravenous 5FC and LAMB, and the central venous catheter was removed. After an 8-day treatment, use of FLC and 5FC was stopped and intravenous voriconazole (VRC) was introduced on 21 December. Because there was no sustained engraftment, a second HSCT was performed on 26 December without any granulocyte recovery. Despite antifungal combination therapy, C. glabrata was again recovered from blood cultures on 3 January (isolate 2), 7 January (isolate 3), 8 January, 9 January, 10 January, and 11 January. LAMB was replaced by CSF on January 12. On 14 March (isolate 4) and 16 March (isolate 5), two blood cultures grew positive for C. glabrata. Use of all therapeutics was stopped on 14 March, and the patient died on 17 March.
FIG. 1.
Schematic representation of Candida glabrata bloodstream infection in an allogeneic HSCT recipient. Day 0 was the day of the first HSCT. The two HSCTs are represented with black arrows. Each plus symbol represents a C. glabrata-positive blood culture. Each isolate studied is indicated by a number. Dosages of antifungal drugs were as follows: FLC at 200 mg/day for the first two periods and 600 mg/day for the last period, 5FC at 150 mg/kg of body weight/day, LAMB at 3 mg/kg/day, CSF at 50 mg/day, and VRC at 300 mg/day.
C. glabrata MICs are summarized in Table 1. Molecular typing of the C. glabrata isolates was performed as previously described (2). Briefly, DNA from each isolate was subjected to PCR for 8 microsatellite-containing regions by using fluorescent primers. With an internal fluorescent ladder, fragment size analysis revealed similar genotypes for all 5 isolates, with respective sizes of 114, 115, 117, 171, 169, 224, 175, and 226 bp for microsatellites 2bis to 9. The genetic basis of 5FC resistance was determined by sequencing the C. glabrata FCY1 (CgFCY1) gene (GenBank accession no. XM_445483), encoding cytosine deaminase, and the CgFUR1 gene (GenBank accession no. XM_447193), encoding uracil phosphoribosyltransferase, in the clinical isolates and the CBS138 strain (with primer pairs 5Fcy1/3Fcy1 and 5Fur1/3Fur1; see the supplemental material). All isolates displayed a CgFCY1 sequence similar to that of the CBS138 strain. In contrast, a missense point mutation in CgFUR1 conferring a G190D substitution in Fur1p was detected only in isolates 2 and 3. To investigate azole resistance, the CgERG11 gene (GenBank accession no. XP445876), encoding the cytochrome P450 C14 α-demethylase, was sequenced in the clinical isolates and in the CBS138 strain (with primer pair FC14/RC14; see the supplemental material). Levels of expression of CgERG11 in the CBS138 strain and in clinical isolates were compared by semiquantitative reverse transcription (RT)-PCR (primer pair RTFC14/RTRC14; see the supplemental material). The sequences of CgERG11, as well as its expression levels, in clinical isolates were identical to that of the CBS138 strain.
TABLE 1.
MICs of consecutive C. glabrata isolates
| Drug | MIC (μg/ml) for isolate (day/mo/yr isolated)a |
||||
|---|---|---|---|---|---|
| 1 (08.12.2001) | 2 (03.01.2002) | 3 (07.01.2002) | 4 (14.03.2002) | 5 (16.03.2002) | |
| Amphotericin B | 1 | 2 | 0.75 | 0.75 | 0.75 |
| Flucytosine | 0.004 | >32 | >32 | 0.004 | 0.004 |
| Fluconazole | 8 | 2 | 1 | >256 | >256 |
| Voriconazole | 0.25 | 0.125 | 0.047 | 12 | 16 |
| Caspofungin | 0.19 | 0.125 | 0.125 | >32 | >32 |
Susceptibility testing was performed using the Etest method (AB-Biodisk, Sweden) according to the manufacturer's instructions. C. parapsilosis ATCC 22019 served as the control strain. MIC interpretative criteria for the susceptibility to FLC, VRC, and 5FC were as previously published by the CLSI (14, 17). The susceptibility breakpoint of ≤2 mg/liter was applied for CSF (15).
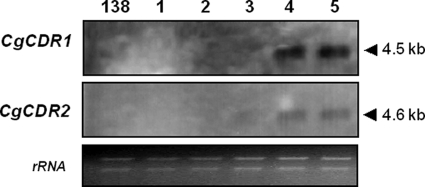
Expression of the C. glabrata genes for ATP-binding cassette (ABC) transporters Cdr1p and Cdr2p was studied by Northern blot analysis of the clinical isolates and the CBS138 strain by using a previously described method (3) (amplification of labeled DNA probes with primer pairs CG1S/CG1R and CG2S/CG2R; see the supplemental material). Basal CgCDR1 (GenBank accession no. AAF05069) and CgCDR2 (GenBank accession no. AF046120) expression levels in the CBS138 strain and in isolates 1, 2, and 3 were too low to be detected, but higher levels of CgCDR1 and, to a lesser extent, CgCDR2 expression were detected in isolates 4 and 5 (Fig. 2). Finally, a region of CgFKS1 and CgFKS2, encoding ß-glucan synthase and previously shown to be a hot spot of mutation associated with echinocandin resistance (13), in the CBS138 strain and clinical isolates was sequenced (primer pairs Cg-FKS1F/Cg-FKS1R and Cg-FKS2F/Cg-FKS2R; see the supplemental material). The CgFKS1 fragment sequences were similar for all isolates, but a nonsynonymous nucleotide mutation (T1988C) in CgFKS2, leading to an S663P amino acid substitution in Fks2p, was detected only in isolates 4 and 5.
FIG. 2.
Expression analysis of C. glabrata CgCDR1 and CgCDR2 genes in clinical isolates and in wild-type strain CBS138 (138). Northern blot analysis of total RNA from the clinical isolates 1, 2, 3, 4, and 5 and from strain CBS138 hybridized with CgCDR1 and CgCDR2 RNA probes. The ethidium bromide-stained 28S/18S rRNAs are shown as a control for loading.
We described the molecular mechanisms likely responsible for the development of multiple resistances in C. glabrata isolates during therapy. If mechanisms of secondary azole resistance in C. glabrata are mostly associated with increased expression of the CgCDR1 and CgCDR2 genes (18, 19), as in our isolates, mechanisms of 5FC resistance are based upon mutations resulting in enzyme deficiency (1). It was recently demonstrated with C. lusitaniae that a fur1 null mutant was resistant to both 5FC and 5-fluorouracil (11). Isolates 2 and 3 were also fully cross-resistant to 5-fluorouracil (data not shown), supporting the idea that a defective Fur1p bearing the G190D substitution was very likely responsible for the fluoropyrimidine resistance phenotypes. The apparent loss of 5FC resistance in isolates resistant to azoles and caspofungin is probably the consequence of the presence of different subpopulations derived from a common progenitor. Here, resistance to echinocandins was associated with the Fks2p mutation S663P, as previously described (7). Considering the fact that neither azoles nor AMB is the best alternative for treating C. glabrata infection, the occurrence of echinocandin resistance represents a real therapeutic challenge. Furthermore, these results show that C. glabrata can acquire resistance to multiple antifungal drugs through successive independent genetic events. The recovery of different isolates exhibiting clonality for microsatellite markers but genetic diversity for antifungal resistance markers demonstrates the high propensity of C. glabrata to readily mutate in vivo in a single patient. Expression of resistance in C. glabrata is facilitated by the haploid nature of the genome. Such a number of events over a rather small time scale (5 months) suggests that this organism could divide actively in a severe neutropenia context despite antifungal treatment. This report also demonstrates the importance of susceptibility testing in patients with recurrent isolation of Candida spp. while receiving or having previously been exposed to antifungal treatments.
Supplementary Material
Footnotes
Published ahead of print on 28 December 2009.
Supplemental material for this article may be found at http://aac.asm.org/.
REFERENCES
- 1.Balkis, M. M., S. D. Leidich, P. K. Mukherjee, and M. A. Ghannoum. 2002. Mechanisms of fungal resistance: an overview. Drugs 62:1025-1040. [DOI] [PubMed] [Google Scholar]
- 2.Brisse, S., C. Pannier, A. Angoulvant, T. de Meeus, L. Diancourt, O. Faure, H. Muller, J. Peman, M. A. Viviani, R. Grillot, B. Dujon, C. Fairhead, and C. Hennequin. 2009. Uneven distribution of mating types among genotypes of Candida glabrata isolates from clinical samples. Eukaryot. Cell 8:287-295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapeland-Leclerc, F., J. Bouchoux, A. Goumar, C. Chastin, J. Villard, and T. Noël. 2005. Inactivation of the FCY2 gene encoding purine-cytosine permease promotes cross-resistance to flucytosine and fluconazole in Candida lusitaniae. Antimicrob. Agents Chemother. 49:3101-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cleary, J. D., G. Garcia-Effron, S. W. Chapman, and D. S. Perlin. 2008. Reduced Candida glabrata susceptibility secondary to an FKS1 mutation developed during candidemia treatment. Antimicrob. Agents Chemother. 52:2263-2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daneman, N., A. K. Chan, S. M. Poutanen, R. Rennie, C. Sand, and S. Porter. 2006. The emergence of caspofungin resistance during treatment of recurrent Candida glabrata candidemia. Clin. Microbiol. Infect. 12(Suppl. 4):P1204. [Google Scholar]
- 6.Dodgson, K. J., A. R. Dodgson, C. Pujol, S. A. Messer, D. R. Soll, and M. A. Pfaller. 2005. Caspofungin resistant Candida glabrata. Clin. Microbiol. Infect. 11(Suppl. 2):P1158. [Google Scholar]
- 7.Garcia-Effron, G., S. Lee, S. Park, J. D. Cleary, and D. S. Perlin. 2009. Effect of Candida glabrata FKS1 and FKS2 mutations on echinocandin sensitivity and kinetics of 1,3-β-D-glucan synthase: implication for the existing susceptibility breakpoint. Antimicrob. Agents Chemother. 53:3690-3699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hachem, R., H. Hanna, D. Kontoyiannis, Y. Jiang, and I. Raad. 2008. The changing epidemiology of invasive candidiasis: Candida glabrata and Candida krusei as the leading causes of candidemia in hematologic malignancy. Cancer 112:2493-2499. [DOI] [PubMed] [Google Scholar]
- 9.Katiyar, S., M. Pfaller, and T. Edlind. 2006. Candida albicans and Candida glabrata clinical isolates exhibiting reduced echinocandin susceptibility. Antimicrob. Agents Chemother. 50:2892-2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krogh-Madsen, M., M. C. Arendrup, L. Heslet, and J. D. Knudsen. 2006. Amphotericin B and caspofungin resistance in Candida glabrata isolates recovered from a critically ill patient. Clin. Infect. Dis. 42:938-944. [DOI] [PubMed] [Google Scholar]
- 11.Papon, N., T. Noel, M. Florent, S. Gibot-Leclerc, D. Jean, C. Chastin, J. Villard, and F. Chapeland-Leclerc. 2007. Molecular mechanism of flucytosine resistance in Candida lusitaniae: contribution of the FCY2, FCY1, and FUR1 genes to 5-fluorouracil and fluconazole cross-resistance. Antimicrob. Agents Chemother. 51:369-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pappas, P. G., C. A. Kauffman, D. Andes, D. K. Benjamin, Jr., T. F. Calandra, J. E. Edwards, Jr., S. G. Filler, J. F. Fisher, B. J. Kullberg, L. Ostrosky-Zeichner, A. C. Reboli, J. H. Rex, T. J. Walsh, and J. D. Sobel. 2009. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 48:503-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perlin, D. S. 2007. Resistance to echinocandin-class antifungal drugs. Drug Resist. Updat. 10:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller, M. A., D. J. Diekema, D. L. Gibbs, V. A. Newell, J. F. Meis, I. M. Gould, W. Fu, A. L. Colombo, and E. Rodriguez-Noriega. 2007. Results from the ARTEMIS DISK Global Antifungal Surveillance study, 1997 to 2005: an 8.5-year analysis of susceptibilities of Candida species and other yeast species to fluconazole and voriconazole determined by CLSI standardized disk diffusion testing. J. Clin. Microbiol. 45:1735-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pfaller, M. A., D. J. Diekema, L. Ostrosky-Zeichner, J. H. Rex, B. D. Alexander, D. Andes, S. D. Brown, V. Chaturvedi, M. A. Ghannoum, C. C. Knapp, D. J. Sheehan, and T. J. Walsh. 2008. Correlation of MIC with outcome for Candida species tested against caspofungin, anidulafungin, and micafungin: analysis and proposal for interpretive MIC breakpoints. J. Clin. Microbiol. 46:2620-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pfaller, M. A., S. A. Messer, L. Boyken, R. J. Hollis, C. Rice, S. Tendolkar, and D. J. Diekema. 2004. In vitro activities of voriconazole, posaconazole, and fluconazole against 4,169 clinical isolates of Candida spp. and Cryptococcus neoformans collected during 2001 and 2002 in the ARTEMIS global antifungal surveillance program. Diagn. Microbiol. Infect. Dis. 48:201-205. [DOI] [PubMed] [Google Scholar]
- 17.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 18.Sanguinetti, M., B. Posteraro, B. Fiori, S. Ranno, R. Torelli, and G. Fadda. 2005. Mechanisms of azole resistance in clinical isolates of Candida glabrata collected during a hospital survey of antifungal resistance. Antimicrob. Agents Chemother. 49:668-679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shin, J. H., M. J. Chae, J. W. Song, S. I. Jung, D. Cho, S. J. Kee, S. H. Kim, M. G. Shin, S. P. Suh, and D. W. Ryang. 2007. Changes in karyotype and azole susceptibility of sequential bloodstream isolates from patients with Candida glabrata candidemia. J. Clin. Microbiol. 45:2385-2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thompson, G. R., III, N. P. Wiederhold, A. C. Vallor, N. C. Villareal, J. S. Lewis II, and T. F. Patterson. 2008. Development of caspofungin resistance following prolonged therapy for invasive candidiasis secondary to Candida glabrata infection. Antimicrob. Agents Chemother. 52:3783-3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.