Abstract
Human cytomegalovirus (HCMV) remains a serious threat for immunocompromised individuals, including transplant recipients and newborns. To date, all drugs licensed for the treatment of HCMV infection and disease target the viral DNA polymerase. Although these drugs are effective, several drawbacks are associated with their use, including toxicity and emergence of drug resistance. Hence, new and improved antivirals with novel molecular targets are urgently needed. Here we report on the antiviral properties of AIC246, a representative of a novel class of low-molecular-weight compounds that is currently undergoing clinical phase II studies. The anti-HCMV activity of AIC246 was evaluated in vitro and in vivo using various cell culture assays and an engineered mouse xenograft model. In addition, antiviral properties of the drug were characterized in comparison to the current gold standard ganciclovir. We demonstrate that AIC246 exhibits excellent in vitro inhibitory activity against HCMV laboratory strains and clinical isolates, retains activity against ganciclovir-resistant viruses, is well tolerated in different cell types (median selectivity index, 18,000), and exerts a potent in vivo efficacy in a mouse xenograft model. Moreover, we show that the antiviral block induced by AIC246 is reversible and the efficacy of the drug is not significantly affected by cell culture variations such as cell type or multiplicity of infection. Finally, initial mode-of-action analyses reveal that AIC246 targets a process in the viral replication cycle that occurs later than DNA synthesis. Thus, AIC246 acts via a mode of action that differs from that of polymerase inhibitors like ganciclovir.
Human cytomegalovirus (HCMV) is a widespread opportunistic pathogen in immunocompromised individuals, including transplant recipients and tumor or AIDS patients, and remains the leading viral cause of birth defects (1, 9, 12, 17, 29). To date, a limited number of drugs are licensed for the systemic treatment of HCMV infection and disease: ganciclovir (GCV) (Cymevene; Roche), its oral prodrug valganciclovir (VGCV) (Valcyte; Roche), cidofovir (CDF) (Vistide; Gilead), and foscarnet (FOS) (Foscavir; Astra-Zeneca). In addition, valaciclovir (VACV) (Valtrex; GlaxoSmithKline), a drug that has been primarily developed for the treatment of herpes simplex virus (HSV) and varicella-zoster virus (VZV) infection, has gained marketing approval in certain countries for prophylaxis of HCMV infections in transplant patients. Although GCV, VGCV, CDF, and FOS are effective, several drawbacks are associated with the use of these drugs, including toxicity, poor oral bioavailability (except VGCV), and emergence of drug resistance (3, 20). The active forms of GCV, CDF, and FOS share the same molecular target, the viral polymerase UL54. Consequently, drug-resistant strains of HCMV encoding UL54 mutations have been found for all three compounds, and the emergence of cross-resistant strains has been described in clinical settings. In addition, resistance to GCV is also associated with mutations in the viral protein kinase UL97 leading to a lack of synthesis of GCV-triphosphate, the active form of the drug (15, 18). Given this, there is an urgent need to develop new, safe, and efficacious antiviral drugs with molecular targets not shared with those currently in use. In line with this, recent attempts to identify novel anti-HCMV compounds mainly concentrated on two promising novel drug targets, the viral terminase complex and the viral protein kinase UL97 (reviewed in references 3, 20, 23, and 24 ). The HCMV terminase complex is a two-subunit enzyme that catalyzes cleavage and packaging of viral DNA (8). Different molecular entities targeting this enzyme have been discovered (e.g., BDCRB, GW275175X, and BAY 38-4766) but so far no “terminase inhibitor” has attained phase II clinical development (reviewed in reference 20). Maribavir, an agent targeting the viral UL97 kinase, an enzyme that is involved in viral DNA synthesis and egress of viral capsids from cell nuclei, was under investigation in phase III clinical trials (20). However, it has been reported that maribavir failed in a recent pivotal phase III study of bone marrow transplant patients who were treated prophylactically. Moreover, since a parallel phase III trial in liver-transplanted patients was stopped, the future of this program is uncertain (34, 35).
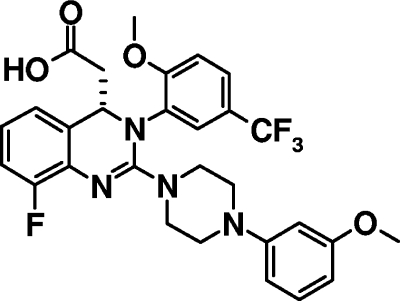
In our attempt to discover novel anti-HCMV compounds that could potentially yield new therapeutic agents, we identified 3,4-dihydro-quinazoline-4-yl-acetic acid derivatives as a novel class of compounds with anti-HCMV activity by screening a compound library in a high-throughput manner. Hit-to-lead optimization activities, including extensive structure-activity relationship studies and pharmacological analyses (unpublished data), led to the discovery of AIC246 (C29H28F4N4O4) (Fig. 1). Due to an excellent preclinical profile with respect to efficacy, safety, tolerability, and pharmacokinetics, AIC246 was chosen as a development candidate out of this new class of anti-HCMV drugs and is currently undergoing phase II evaluations (to be published elsewhere). Here we report on the antiviral properties of AIC246 in vitro and in vivo using different HCMV laboratory strains, different clinical isolates, GCV-resistant viruses, and a mouse xenograft model. Moreover, we monitored the effects of drug removal and of time of drug addition on compound efficacy. Taken together, the studies presented here demonstrate that the novel compound AIC246 exhibits excellent anti-HCMV activity both in vitro and in vivo and suggest that the mode of action of AIC246 differs from that of polymerase inhibitors like GCV.
FIG. 1.
Chemical structure of AIC246.
MATERIALS AND METHODS
Cells, cell culture, and viruses.
Normal human dermal fibroblast (NHDF) cells (catalog no. CC-2511), normal human lung fibroblast (NHLF) cells (catalog no. CC-2512), human foreskin fibroblast (HFF) cells (Hs27, catalog no. CRL-1634), human lung fibroblast cells (MRC5, catalog no. CCL-171), and human embryonic lung fibroblast (HELF) cells (HEL299, catalog no. 87042207) were purchased from Clonetics, the American Type Culture Collection (ATCC), and the European Collection of Cell Cultures (ECACC). All cells were cultured at 37°C with 5% CO2 as described previously (16, 28). The HCMV laboratory strains AD169 and Davis were purchased from ATCC (ATCC accession no. VR 538 and VR-807). The green fluorescent protein (GFP)-expressing recombinant AD169 strain RV-HG was reconstituted from the HCMV-BACmid pHG kindly provided by E. Borst and M. Messerle (10); the recombinant AD169 strain HCMV-GFP was obtained from T. Stamminger (22). HCMV clinical isolates were provided by A. Eis (University of Bonn), D. Michel and T. Mertens (University of Ulm), and C. Sinzger (University of Tübingen). The ganciclovir-resistant virus mutant AD169 rGCV was selected in cell culture as described earlier (28). Briefly, the HCMV strain AD169 was serially passaged in NHDF cells in the presence of increasing concentrations of ganciclovir, starting at a drug concentration of 2 μM (the approximate 50% effective concentration [EC50]). The resultant GCV-resistant progeny virus was plaque purified and then analyzed. Sequencing of the open reading frames (ORFs) UL97 and UL54 revealed the UL97 M460I mutation that is known to confer 5- to 6-fold resistance against GCV (15). HCMV virus stocks were propagated using NHDF cells and titrated by means of IE1p72 fluorescence exactly as described previously (4, 21).
Animals.
Fox Chase NOD SCID mice (Taconic M&B A/S, Ry, Denmark) were housed under sterile conditions in individually ventilated cages with filter top lids (Tecniplast, Hohenpeißenberg, Germany). All necessary handling was done under laminar flow hoods. Animals were fed a diet of irradiated mouse and rat feed (Kliba “Provimi”) and received autoclaved water ad libitum. Animal care and use were conducted according to federal guidelines.
Antiviral compounds.
AIC246 and BAY 38-4766 were synthesized at the medical chemistry department of Bayer Schering Pharma AG, Wuppertal, Germany, and stored as a 50 mM stock solution in dimethyl sulfoxide (DMSO) for in vitro use. Ganciclovir (ganciclovir sodium; Roche, Grenzach, Germany) was used as a reference drug as a 50 mM solution in 0.9% saline, the intravenous formulation of Cymevene. For animal experiments, commercially available valgancyclovir (VGCV) (Valcyte, Roche) was used. VGCV and AIC246 were formulated in 2% DMSO in 0.5% methylcellulose-99.5% phosphate-buffered saline (PBS).
HCMV cytopathic effect reduction assay (CPE-RA).
CPE-RAs were performed basically as described elsewhere (HCMV replication assay) (28). In brief, the addition of 2 μl test compound of 50, 5, 0.5, 0.05, 0.005, and 0.0005 mM DMSO stock solutions to 100 μl cell culture medium in duplicates was followed by serial 2-fold dilutions in 96-well microtiter plates. Each well was supplemented with 150 μl of either a suspension of 1 × 104 NHDF cells mixed with cell-free HCMV (multiplicity of infection [MOI], 0.03) or a suspension of 1 × 104 to 3 × 104 HCMV-infected and uninfected NHDF cells (MOI, 0.001 to 0.002). Noninfected and nontreated cells served as controls on each plate. Final compound concentrations ranged between 250 and 0.00005 μM. Plates were incubated for 6 to 7 days at 37°C or until the virus control reached 100% CPE. A mixture containing 20% Giemsa stain (Merck) and 5% formalin solution (Merck) was added to the wells for fixation and staining. After extensive washing, plates were dried at 56°C followed by visual evaluation using an overhead microscope (plaque magnifier; Tecnorama Zürich). Each assay was performed at least in triplicate, and standard deviations were calculated. The assay plate data were used to calculate the EC50 (CPE-RA), i.e., the concentration of drug that inhibits the CPE by 50% compared with an untreated virus-infected control.
HCMV plaque assay.
HCMV plaque assays were performed as described previously (28). NHDF cells (1 × 105 to 2 × 105) seeded in 24-well tissue culture plates were infected by inoculating 0.1 ml of serial log dilutions of a suspension of infected and uninfected cells. After a 16-h adsorption period, the cell culture supernatant was replaced by 1 ml of a methylcellulose (MC) overlay medium (0.5% MC-Dulbecco modified Eagle medium [DMEM]-10% fetal calf serum [FCS]). Cultures were incubated for 7 to 14 days. Plates were fixed and stained as described above. Subsequently, plates were visually evaluated by counting plaques. Results are expressed as PFU per ml titrated cell suspension.
HCMV fluorescence reduction assay (GFP-RA).
The susceptibilities of recombinant HCMV laboratory strains expressing GFP were determined by a GFP-based fluorescence reduction assay. For standard assays, 1.5 × 104 NHDF cells/well were cultured in black 96-well plates (Greiner Bio-One, Germany) and infected with either HCMV-GFP (MOI, 0.1) or RV-HG (MOI, 0.2 to 0.5). After virus adsorption, the virus inoculum was replaced with 200 μl fresh medium. Thereafter, 100 μl medium containing the test compounds was added to wells of horizontal row G, followed by serial 3-fold dilutions up to row A. All drug concentrations were tested at least in duplicate. Wells of the horizontal row H served as the virus control. Plates were incubated at 37°C for 7 days. The medium was replaced by 200 μl PBS, and GFP units (GFPU) were determined by a charge-coupled-device-camera-based fluorescence detector (FluoBox; Bayer Technology Services GmbH, Leverkusen, Germany) which captures 96 images simultaneously. Drug effects were calculated as a percentage of reduction in GFPU in the presence of each drug concentration compared to the GFPU determined in the absence of drug. EC50 and EC90 values (drug concentrations producing 50% and 90% reduction in GFPU) were determined as described in “Statistical analysis.” To evaluate the antiviral activity of AIC246 in different cell types or as a function of the amount of input virus, the assay was performed essentially in the same way, but either different fibroblast cells (cell-type dependency) or increasing multiplicities of infection of HCMV AD169-GFP (MOI, 0.003 to 1; MOI dependency) were used for infection. All assays were performed at least in triplicate, and standard deviations were calculated.
Cytotoxicity assay.
The influence of antiviral drugs on the viability of different fibroblast cells during a 7-day incubation period was evaluated using the alamarBlue viability assay (Biosource Europe, Nivelle, Belgium), as described by the manufacturer. Briefly, 96-well microtiter plates were seeded with 1.5 × 104 cells/well and incubated overnight. Drugs were added to the wells in 3-fold serial dilutions starting from 0.33 mM (the DMSO concentration was kept constant at 0.66% throughout the whole plate). After a 7-day incubation period, alamarBlue solution was added to each well and the fluorescence signal was measured using a SpectraFluor Plus fluorescence reader (Tecan Deutschland GmbH, Crailsheim, Germany). The relative fluorescence units of treated wells were expressed as percentages of untreated cell control wells and plotted against the logarithm of drug concentrations. Drug concentrations reducing cell viability by 50% (CC50s) were determined from dose-response curves as described in “Statistical analysis.” The assays were performed at least three times with duplicate samples. CC50 values were used to calculate the selectivity index (SI = CC50/EC50) for individual substances.
Focus expansion assay.
Focus expansion assays were performed essentially as described previously (30). Briefly, late-stage-infected fibroblasts were cocultured with an excess of uninfected fibroblasts for 5 days in the presence or the absence of 50 nM AIC246 (∼10 × EC50). Cells were fixed with ice-cold methanol, and HCMV immediate-early (IE) antigen was detected by indirect immunofluorescence analysis using monoclonal antibody E13 (Biosoft, Cambridge, United Kingdom) and a Cy3-coupled secondary antibody. Cell nuclei were counterstained using DAPI (4′,6-diamidino-2-phenylindole), and dishes were analyzed with a Zeiss Axiovert 135 microscope. All tests were done in quadruplicate, and the average number of infected cells per focus was quantified.
Kinetic block release assay.
NHDF cells (3 × 105/well) grown in six-well plates were infected with AD169 at an MOI of 0.1. After virus adsorption, cells were treated with either GCV (20 μM) or AIC246 (50 nM) for 96 h (∼10 × EC50). One well was kept untreated and served as a virus control. After the 96-h incubation period, supernatants of the untreated virus control and of one drug-treated well were harvested and stored at −80°C. All other cultures were washed five times with PBS and then incubated in drug-free medium. Cell culture supernatants of infected cells were collected at 24, 48, 72, and 96 h after removal (hpr) of the antiviral compound and stored at −80°C. Finally, virus titers of each collected cell culture supernatant were quantified via IE1p72 fluorescence titration as described above. Duplicate samples were used for all drug block release studies.
Time-of-addition assay.
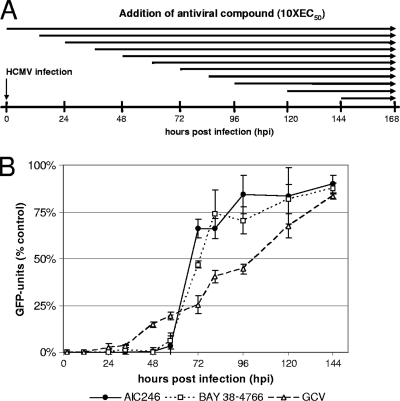
NHDF cells (1.5 × 104/well) were cultured in black 96-well plates and infected with AD169-GFP at an MOI of 0.1. Subsequently, fresh media or media containing fixed virus inhibitory concentrations (∼10 × EC50) of AIC246 (50 nM), GCV (20 μM), and BAY 38-4766 (11 μM) were added to the infected cells at 0, 11, 24, 33, 48, 57, 72, 81, 96, 120, and 144 h postinfection (hpi). At 7 days (168 h) postinfection (pi), media were replaced by PBS and GFP units (GFPU) of the infected cells were quantified as described above. Drug effects were calculated as a percentage of reduction in GFPU in the presence of each drug compared to the GFPU determined in the absence of drug. GFPU determined in wells incubated with the respective inhibitor from 0 hpi onwards were used for background correction. Each drug was tested in duplicate at each time point.
Mouse xenograft model.
The in vivo antiviral activity of AIC246 was assessed using a HCMV xenograft mouse model as described by Chong et al. (14). Briefly, Gelfoam hemostyptic gelatin devices (Upjohn) were cut aseptically into 1-cm2 pieces. These implants were soaked in NHDF cell culture growth medium (GM), and sponges were brought to 37°C in a CO2 incubator. NHDF cells were infected with cell-free HCMV strain Davis at an MOI of 0.03. After 4 h, cells were collected by trypsinization followed by centrifugation at room temperature for 10 min at 800 × g. Cells were resuspended in GM and counted using a hemocytometer. Each Gelfoam implant was seeded with a suspension of 1 × 106 infected cells by pipetting the cells onto the sponges. Human cells were allowed to adhere to the collagen sponges for at least 3 to 4 h at 37°C. To enhance vascularization of the implant, 250 ng recombinant human basic fibroblast growth factor (Calbiochem) was pipetted onto each implant 1 h prior to transplantation. Mice (18 to 25 g body weight) were anesthetized, and the Gelfoam sponges were implanted subcutaneously in the dorsoscapular area. After transplantation, mice were randomized and grouped in ∼10 animals per treatment group. Starting 4 h after transplantation, mice were treated once daily with the indicated compounds for nine consecutive days. Drugs were applied per os by oral gavage. Total administration volume was 10 ml/kg. Mice were sacrificed after 9 days of treatment, and the Gelfoam implants were removed and digested with collagenase (Calbiochem) at 37°C. After 2 to 3 h, human cells were recovered by centrifugation and resuspended in GM. Subsequently, the isolated cell suspensions were serially diluted and mixed with uninfected NHDF indicator cells and PFU were determined by plaque assays as described above. Virus titers determined from isolated cells are given as PFU/ml.
Statistical analysis.
For statistical analysis, data were examined using one-way analysis of variance (ANOVA) with Bonferroni's post test. P values were confirmed using an unpaired Student t test.
Fifty percent effective dose (ED50), ED90, and CC50 values were calculated using nonlinear regression curve fit with a variable slope. GraphPad Prism 3.02 or 4.0 software (GraphPad Software Inc., La Jolla, CA) was used for all analyses.
RESULTS
Antiviral activity of AIC246 in cell culture.
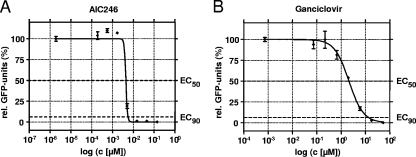
The antiviral efficacy of AIC246 was compared to that of the reference compound ganciclovir (GCV) in a classical CPE reduction assay (CPE-RA) and a GFP-based fluorescent reduction assay (GFP-RA) using different HCMV laboratory strains. The results are summarized in Table 1 and demonstrate that AIC246 exhibited very potent in vitro antiviral activity as measured by the two independent assays. In fact, the inhibitory potency of AIC246 surpasses the current gold standard GCV by more than 400-fold with respect to EC50s (mean, ∼4.5 nM versus ∼2 μM) and by more than 2,000-fold with respect to EC90 values (mean, ∼6.1 nM versus ∼14.5 μM). NHDF monolayers showed no microscopically apparent cytotoxic effects at AIC246 concentrations of <33 μM when observed during antiviral assays. Note that the EC50s of GCV obtained in both assays are in agreement with previously reported results (6, 27, 28). A typical example for a nonlinear regression curve of AIC246 or GCV is shown in Fig. 2. It should be noted that the antiviral activity of AIC246 is characterized by a very steep dose-response curve and that this accounts for only slight differences between EC50 and EC90 values (median slope: AIC246, ∼10; GCV, ∼1.2).
TABLE 1.
Sensitivities of different HCMV laboratory strains to AIC246 and GCV in fibroblast cells
| Assay | HCMV strain | EC50(μM)a |
EC90 (μM)a |
No. of independent expts | ||
|---|---|---|---|---|---|---|
| AIC246 | Ganciclovir | AIC246 | Ganciclovir | |||
| CPE-RAb | Davis | 0.0040 ± 0.0010 | 2.70 ± 0.70 | NDd | ND | 7 |
| AD169 | 0.0050 ± 0.0010 | 4.30 ± 1.80 | ND | ND | 13 | |
| GFP-RAc | AD169-GFP | 0.0038 ± 0.0009 | 1.73 ± 0.93 | 0.0051 ± 0.0014 | 10.7 ± 2.5 | 18 |
| RV-HG | 0.0049 ± 0.0009 | 2.33 ± 2.75 | 0.0071 ± 0.0025 | 18.3 ± 22.7 | 5 | |
EC50 and EC90 values were determined by the indicated antiviral assay. Nonlinear regression analysis was performed, and the resulting graphs were used to calculate the respective values. Results are expressed as means ± standard deviations.
CPE reduction assay.
Fluorescence reduction assay.
ND, not determined.
FIG. 2.
Representative in vitro dose-response curves of AIC246 (A) and ganciclovir (B) determined by the GFP-RA. Error bars represent standard deviations of results for duplicate samples.
Previous publications reported that the developmental anti-HCMV drug maribavir exhibits widely differing EC50s for the same HCMV strain (AD169) infecting different cell types. While good antiviral activity was observed in lung fibroblasts, only reduced or poor efficacy was measured in skin fibroblasts (6, 16, 36). In light of these findings it was important to assess the activity of our new candidate molecule against a variety of HCMV permissive cells and to compare the results to those obtained for the approved reference compound GCV (Table 2). Since the cell tropism of HCMV laboratory strains is restricted to fibroblasts, normal dermal fibroblasts (NHDF), foreskin fibroblasts (HS27), normal lung fibroblasts (NHLF, MRC5), and embryonic lung fibroblasts (HEL299) were included in this evaluation. CC50 values were determined in parallel as described in Materials and Methods, and selectivity indices (SIs) were calculated. As depicted in Table 2, the antiviral activity of AIC246 was virtually unchanged in all cell types tested. In contrast, we recognized a moderate variability of GCV EC50s (maximum, 7-fold) with respect to the fibroblast cells used for infection. Importantly, concurrent determinations of cytotoxicity in an alamarBlue assay demonstrated that AIC246 was well tolerated in all cell types used, thus leading to SIs of at least 12,000 (median SI, 18,000) (Table 2).
TABLE 2.
Antiviral activity of AIC246 determined for various cell types
| Cell type | Drug | EC50a (μM) | CC50b (μM) | SIc |
|---|---|---|---|---|
| HS27 | AIC246 | 0.0056 ± 0.0004 (4) | 107 (2) | 19,107 |
| Ganciclovir | 0.32 ± 0.11 (6) | >333 (2) | >1,044 | |
| NHDF | AIC246 | 0.0035 ± 0.0010 (6) | 91 (2) | 25,899 |
| Ganciclovir | 1.91 ± 1.30 (6) | >333 (2) | >174 | |
| HELF | AIC246 | 0.0035 ± 0.0013 (5) | 63 (2) | 17,877 |
| Ganciclovir | 2.39 ± 1.03 (5) | >333 (2) | >139 | |
| NHLF | AIC246 | 0.0050 ± 0.0020 (5) | 64 (2) | 12,903 |
| Ganciclovir | 2.07 ± 1.01 (5) | >333 (2) | >161 | |
| MRC5 | AIC246 | 0.0045 ± 0.0004 (3) | 127 (2) | 28,015 |
| Ganciclovir | 1.65 ± 0.56 (3) | >333 (2) | >202 |
EC50s determined by the fluorescence reduction assay expressed as mean ± standard deviation. The number of independent experiments is shown in parentheses.
CC50 values determined by the alamarBlue viability assay as described in Materials and Methods. Values with a > sign represent the highest concentration tested.
Selectivity index (SI) = CC50/EC50.
Effect of multiplicity of infection on the inhibition of HCMV by AIC246.
A defining feature of an antiviral compound is its multiplicity of infection (MOI) dependency, meaning that the antiviral potency of certain drugs decreases as the ratio of input virus to cells increases. In order to investigate the MOI dependence of AIC246, GFP-RAs in the presence of increasing virus titers were carried out. GCV was included in these experiments as a reference compound. As shown in Table 3, the efficacy of AIC246 was only moderately influenced by an ∼333-fold titer increase in virus inoculum leading to an ∼3-fold increase in the respective EC50. In contrast, GCV EC50s increased up to ∼6-fold in the μM range in parallel experiments. These results suggest that AIC246 is sufficiently potent to combat even high-MOI infections.
TABLE 3.
MOI dependency of AIC246
| MOIa | EC50b (μM) |
|
|---|---|---|
| AIC246 | Ganciclovir | |
| 0.003 | 0.0013 ± 0.0004 (4) | 0.99 ± 0.58 (5) |
| 0.01 | 0.0015 ± 0.0004 (2) | 0.68 ± 0.30 (4) |
| 0.03 | 0.0029 ± 0.0012 (5) | 1.74 ± 1.27 (6) |
| 0.1 | 0.0034 ± 0.0009 (8) | 2.21 ± 1.23 (8) |
| 0.3 | 0.0036 ± 0.0009 (4) | 6.51 ± 2.26 (4) |
| 1 | 0.0042 ± 0.0018 (8) | 5.26 ± 2.13 (8) |
Multiplicity of infection (MOI) of HCMV strain AD169-GFP.
EC50values determined by the fluorescence reduction assay are expressed as means ± standard deviations. The number of independent experiments is shown in parentheses.
Antiviral activity of AIC246 against GCV-resistant virus strains and clinical HCMV isolates.
Next, we sought (i) to prove that the antiviral activity of AIC246 is not restricted to HCMV laboratory strains and (ii) to assess the potential utility of the drug in the treatment of GCV-resistant infections. For this, the efficacy of AIC246 was evaluated against a GCV-resistant AD169 mutant and a panel of seven different clinical HCMV isolates also including GCV-resistant viruses (Table 4). The mutations conferring resistance to GCV of strain AD169 rGCV, I-E17251S and I-1974R, were mapped to the viral protein kinase UL97 (M460I and 2× C603W, respectively). The identified UL97 mutations are well characterized and have been found earlier to confer resistance to GCV (15). All viruses tested were sensitive to AIC246, with EC50s in the CPE-RA ranging from 1.8 nM to 6.1 nM. Thus, GCV-resistant viruses retained sensitivity to AIC246, which suggests that the target site or the inhibitory mechanism of AIC246 is different from that of GCV.
TABLE 4.
Susceptibility of HCMV laboratory strains and clinical isolates to AIC246 and GCV
| HCMV strain | EC50a (μM) |
|
|---|---|---|
| AIC246 | Ganciclovir | |
| Laboratory strains | ||
| AD169 | 0.0051 ± 0.0012 | 2.4 ± 2.5 |
| AD169 rGCVb | 0.0039 ± 0.0011 | 12 ± 0.7 |
| Clinical isolates | ||
| I-Se | 0.0031 ± 0.0038 | NDc |
| I-Ba | 0.0058 ± 0.0001 | ND |
| I-La | 0.0034 ± 0.0035 | ND |
| I-472 | 0.0018 ± 0.0016 | 5.0 ± 3.90 |
| I-E16415Sd | 0.0026 ± 0.0016 | 1.1 ± 0.45 |
| GCV-resistant clinical isolates | ||
| I-E17251Sd,e | 0.0061 ± 0.0044 | 14 ± 2.5 |
| I-1947Rd,e | 0.0023 ± 0.0023 | 15 ± 13 |
EC50values were determined by the CPE reduction assay as described in Materials and Methods. Data are means of at least two independent experiments and are expressed ± standard deviations.
GCV-resistant AD169 virus (UL97 M460I).
ND, not determined.
Cell-associated viruses (virus-infected cells) were used as viral inocula.
GCV-resistant clinical isolate (UL97 C603W).
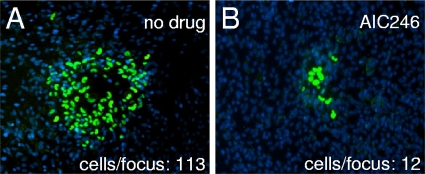
It is well known that in vivo HCMV disseminates predominantly by cell-to-cell spread (26). Given this, we examined the effect of AIC246 on the intracellular dissemination of a strictly cell-associated HCMV isolate in a focus expansion assay (30, 31). Uninfected fibroblast cells were cocultivated with fibroblasts infected with a recent, strictly cell-associated, clinical HCMV isolate. Inhibitory concentrations of AIC246 were added immediately after cocultivation, and the sizes of infectious foci were evaluated 5 days later via detection of HCMV immediate-early (IE) antigen. As shown for one isolate, AIC246 inhibited the focal expansion of a clinical HCMV isolate at high efficiency (cells per focus, 113 [control] versus 12 [treated]) (Fig. 3). The residual expression of IE antigen observed in some AIC246-treated cells is due to the release of preformed infectious virus progeny present in the cytoplasm of the cells used for cocultivation/infection (Fig. 3B). However, this infection was abortive, since no further spread of virus to neighboring cells was observed. This coculture-based assay confirmed the inhibitory efficacy of AIC246 on the replication of clinical HCMV isolates.
FIG. 3.
Effect of AIC246 on focal expansion of a cell-associated HCMV isolate in cell culture. Productively infected fibroblasts were cocultured with an excess of uninfected indicator fibroblasts for 5 days in the absence (A) or presence (B) of AIC246. Cells were fixed and stained for viral immediate-early antigen expression (green signals), and cell nuclei were counterstained with DAPI (blue signals). The number of infected cells per focus is indicated.
Reversibility of antiviral effect.
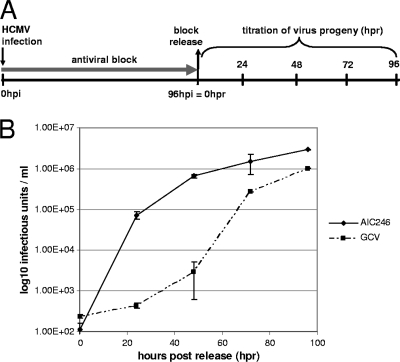
In order to learn more about the properties of this novel small-molecule inhibitor, we next addressed the question of whether the anti-HCMV block induced by AIC246 is reversible. To this end, a kinetic block release experiment was performed (Fig. 4A). AD169-GFP-infected cells were treated with inhibitory concentrations (∼10 × EC50) of AIC246 or the reversible polymerase inhibitor GCV to suppress HCMV replication. Cells were released from the drug block at 96 hpi, and the amount of progeny virus in the supernatant was quantified (Fig. 4B). Following 96 h of inhibitor treatment (96 hpi = 0 h postrelease [hpr]), viral yield measurements revealed an ∼4-log reduction in HCMV titer relative to nontreated infected cells (102 PFU/ml versus 106 PFU/ml; data not shown), demonstrating that both drugs effectively inhibited virus replication at the given concentrations. Upon removal of AIC246 from the culture medium, virus replication rapidly resumed and infectious virus particles were readily detected in the supernatant. In fact, mock-treated virus yield levels (∼106 PFU/ml) were reached as soon as ∼48 h following release of AIC246. In contrast, secretion of virus particles into the supernatant remained depressed for some time after removal of extracellular GCV before it reached mock-treated levels (Fig. 4B). This experiment clearly demonstrated that the antiviral block induced by AIC246 was reversible. The observed delay in the virus rebound kinetics upon GCV withdrawal might be explained by either differences in the intracellular pharmacokinetic half-life of AIC246 and GCV and/or the fact that AIC246 blocks viral replication at a later point within the HCMV replication cycle than the polymerase inhibitor GCV. Given this, it was of interest to locate the stage in the viral replication cycle at which AIC246 exerts its effect.
FIG. 4.
Kinetic block release assay. (A) Schematic representation of the experimental setup. HCMV AD169-infected NHDF cells were treated for 96 h with the indicated inhibitor (antiviral block). At 96 h postinfection (hpi), infected cells were released from the drug block, and the production of progeny virus was analyzed 24 h, 48 h, 72 h, and 96 h following drug release (hpr). (B) Production of progeny virus during antiviral virus block and following block release monitored via virus yield measurements.
Time-of-drug-addition studies.
To determine the process in the virus replication cycle affected by AIC246, a time-of-addition study was employed. Since HCMV replication is characterized by a complex sequence of different phases at which antiviral agents might interfere, two control compounds known to act at early (GCV) or late (BAY 38-4766) phases within the HCMV replication cycle were used in a parallel experiment with AIC246. BAY 38-4766 is a potent HCMV inhibitor that exerts its antiviral activity after viral DNA synthesis by preventing processing and packaging of the DNA (13, 28). Inhibitory concentrations of AIC246, GCV, and BAY 38-4766 were added to AD169-GFP-infected cells at various time points pi. GFP levels in the infected cells were determined at 7 days pi (Fig. 5A). The polymerase inhibitor GCV was found to be effective without loss of activity when added up to 33 hpi (Fig. 5B). In contrast, BAY38-4766 and AIC246 were found to remain active when added as late as 57 hpi. Interestingly, once replication has advanced beyond this point, the efficacy of both drugs drastically decreases. This suggests that AIC246, similarly to BAY 38-4766, targets a process occurring later than DNA synthesis and therefore further supports the idea that the mode of action of AIC246 differs from that of polymerase inhibitors like GCV.
FIG. 5.
Effect of the time of addition of AIC246, BAY 38-4766 or GCV on HCMV replication. (A) Schematic representation of the experimental setup. NHDF cells were infected with AD169-GFP and treated with fixed virus inhibitory concentrations (∼10 × EC50) of GCV, BAY 38-4766, and AIC246 at the indicated time points postinfection (hpi). After 7 days, cell supernatants were replaced by PBS and GFP units (GFPU) were determined. (B) GFPU in compound-treated cells were compared to those in untreated cells, and the percentage of activity is plotted. Results are averages for three experiments carried out in duplicate. Error bars indicate standard deviations.
In vivo antiviral activity of AIC246 in a mouse xenotransplant model.
Having shown that AIC246 is a potent, robust, and specific inhibitor of HCMV replication in cell culture that acts via a mode of action distinct from that of GCV, we then investigated the ability of AIC246 to inhibit HCMV replication in an in vivo animal model. Notably, rodent pathogenicity models could not be applied, since HCMV selectively infects human cells and cell culture experiments revealed that AIC246 was not active against rodent CMV strains, including mouse and guinea pig cytomegalovirus (data not shown). Therefore, in vivo efficacy was assessed using an engineered mouse xenograft model. For this, mice transplanted with a Gelfoam sponge carrying infected human cells were treated once daily with AIC246 via oral gavage. After a 9-day treatment period, Gelfoam sponges were explanted and the number of HCMV PFU was determined after processing of samples. Placebo- and VGCV (Valcyte)-treated animals served as treatment controls.
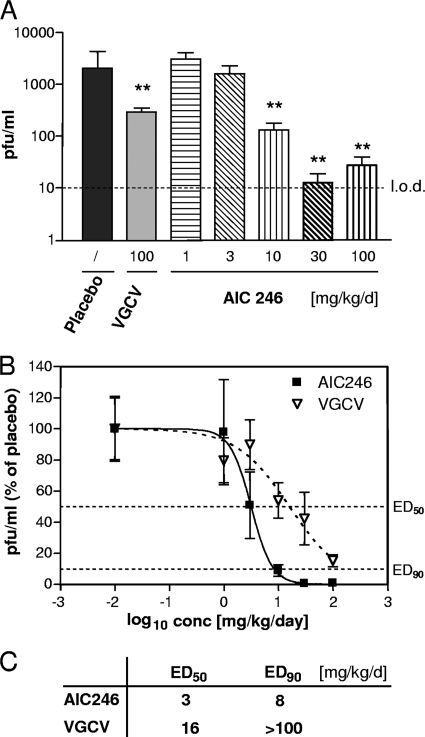
AIC246 treatment led to a dose-dependent reduction of the HCMV titer in transplanted cells compared to that of the placebo-treated control group using the mouse xenograft model (Fig. 6A). Statistical analysis revealed significant antiviral effects for the 10-, 30-, and 100-mg/kg/day treatment groups of AIC246 as well as for the 100-mg/kg/day VGCV control group (P < 0.006). Interestingly, treatment with AIC246 yielded a >2-log PFU reduction in the highest-dose groups compared to an ∼1-log PFU reduction in VGCV-treated animals, thus confirming the excellent antiviral potency of AIC246 as previously shown in cell culture. The slight increase in PFU values at the 100-mg/kg/day dose group was interpreted as assay-based variation close to the detection limit.
FIG. 6.
Effect of VGCV or AIC246 therapy on HCMV replication in a mouse xenograft model. (A) Viral titers of HCMV-infected Gelfoam sponges harvested from transplanted mice receiving antiviral treatment with the indicated doses of placebo, VGCV, or AIC246. Drugs were given once daily per os for 9 days. Results are expressed as means ± standard errors of the means (SEM). Placebo, n = 24; VGCV, n = 20; AIC246 (1 mg/kg/day), n = 10, (3 mg/kg/day) n = 11, (10 mg/kg/day) n = 21, (30 mg/kg/day) n = 21, (100 mg/kg/day) n = 11. **, P value < 0.006 (unpaired t test); l.o.d., limit of detection; PFU, PFU. Data were derived from two additional experiments. (B) In vivo dose-response curves of VGCV and AIC246 therapy. AIC246 data were derived from animals described for panel A. VGCV data were derived from results shown in panel A and two separate experiments using mice receiving antiviral treatment with placebo and 1, 3, 10, 30, and 100 mg/kg VGCV given per os once daily. Results are expressed as means ± standard deviations (SD). For clarification, ED50 and ED90 values (50% or 10% of mean placebo values, respectively) are indicated by a dotted line. (C) ED50 and ED90 values calculated from the dose-response curves depicted in panel B. Since ED90 was not reached in the VGCV treatment group, the ED90 was set to >100 mg/kg/day.
ED50 and ED90 values were calculated from dose-response curves of the respective AIC246 treatment groups and compared to the corresponding once-daily VGCV values that were acquired in independent experiments (Fig. 6B and C); the ED50 and ED90 of AIC246 were determined to be 3 mg/kg/day and 8 mg/kg/day, respectively. For the control compound VGCV, an ED50 of 16 mg/kg/day was obtained (Fig. 6C). Importantly the ED90 of VGCV was not reached within this experiment, not even with the highest dose applied (100 mg/kg/day). Furthermore, the steep dose-response curve of AIC246 observed in vitro was mirrored in this in vivo experiment (Fig. 6B), thus confirming the outstanding AIC246 potency at EC90.
In summary, these data demonstrate that the in vivo activity of AIC246 is at least comparable to the current gold standard at the ED50 level and surpasses that of VGCV by a factor of more than 12 with respect to ED90.
DISCUSSION
HCMV is a leading viral cause of birth defects and life-threatening disease in immunocompromised patients. To date, all approved drugs target the viral DNA polymerase and are associated with severe toxicity issues and emergence of drug resistance. In spite of this, no new drug classes with improved properties and a new mode of action have reached the market for more than two decades (2, 3, 5, 20, 24).
Our attempt to identify novel drugs with a mode of action different from inhibition of DNA polymerase led to the discovery of dihydroquinazolinyl-acetic acids as a new class of HCMV inhibitors. Due to excellent antiviral activity and a favorable toxicological and pharmacological profile in preclinical investigations, AIC246 was chosen from this novel class as a new lead compound (Fig. 1). Phase I trials were initiated and demonstrated that the drug had a favorable pharmacokinetic profile and was safe and generally well tolerated. Consequently, AIC246 is currently being clinically evaluated in phase II studies as an oral therapeutic for the treatment of HCMV infections in transplant patients (unpublished data).
In the present study, we report on the antiviral properties of AIC246 in vitro and in vivo. By all antiviral assays applied, AIC246 exhibits excellent activity not only against HCMV laboratory strains AD169 and Davis but also against a panel of cell-free and cell-associated clinical HCMV isolates with an average EC50 of 4 ± 1.3 nM. In addition, AIC246 was found to be only marginally toxic in a variety of fibroblast cells, as illustrated by an average CC50 value of 90 ± 28 μM. Combined, these results lead to a favorable median in vitro selectivity index of 18,000 and indicate a good tolerability in vivo. Consequently, we assessed the antiviral efficacy of AIC246 in an engineered mouse xenograft model (14). The Gelfoam xenograft model employed has two major advantages over other animal models: first, the appropriate human virus can be used for in vivo evaluation; second, antiviral compounds that show activity only against human cytomegalovirus can be tested (11). Using this animal model, AIC246 shows excellent antiviral efficacy. In fact, AIC246 appeared to be more effective than the most active compound on the market VGCV, the prodrug of GCV in reducing absolute viral titers, when both drugs were given once daily at a dose of 100 mg/kg/day. In line with this observation we found that our drug surpasses VGCV by a factor of 12 or more at the ED90 level (once-daily treatment). However, since VGCV was not sufficiently potent to reach an ED90 in a once-daily experimental design, we also determined the antiviral effect of GCV in mice that received VGCV twice a day. The ED50 and ED90 values obtained in these experiments were 4.7 mg/kg/day and 34 mg/kg/day, respectively (data not shown). Given these results, we concluded that a once-daily administration of AIC246 appeared to be more effective than either a once- or a twice-daily treatment with the gold standard VGCV in this in vivo model. Notably, this result is in agreement with our in vitro studies and implies that a high efficacy may also be expected in patients.
Several lines of evidence suggest that the mechanism of AIC246 is different from that of the polymerase inhibitor GCV. First, AIC246 and GCV behave differently in terms of antiviral activity upon withdrawal of the inhibitor from cell culture. While virus replication remained depressed for a longer period after GCV has been removed from the cultures, virus replication rapidly resumed after removal of AIC246. One possible explanation for this phenomenon is that in contrast to GCV, which inhibits synthesis of progeny DNA, AIC246 blocks a process occurring later than viral DNA replication; thus, progeny virus is readily assembled and released upon drug removal. Importantly, this assumption would be consistent with the results of our time-of-addition experiments (see below). However, it is known that GCV has a long intracellular half-life of 12 to 18 h since the intracellular metabolization product of GCV, GCV-triphosphate, cannot freely cross the cell membrane (7). This finding might support an alternative explanation, namely, that the entrapment of GCV-triphosphate within virus-infected cells accounted for the observed prolonged antiviral activity. Second, we have demonstrated that AIC246 was effective against different GCV-resistant viruses. Third, time-of-addition studies place the mode of action of AIC246 at a late stage of viral replication, indicating that AIC246 might interfere with capsid assembly, DNA processing/packaging, or virus egress (25). In this regard, the unexpected finding that the time point of action of AIC246 exactly coincides with that of the control compound BAY 38-4766 was interesting and raised the possibility of a common viral target. BAY 38-4766 is a member of a class of specific HCMV inhibitors that block the cleavage and packaging of HCMV DNA into capsids (13, 28). These processes are conducted by a specific virus-encoded enzyme, the terminase complex. In fact, mutations conferring resistance to BAY 38-4766 have been mapped to the HCMV UL56 and UL89 genes, which encode the two subunits of the viral terminase (13). Interestingly, another unique class of compounds, benzimidazole ribosides, has been shown to inhibit HCMV DNA maturation via the involvement of the UL89 and UL56 gene products, further emphasizing that the viral terminase complex represents a valid and drugable target for anti-HCMV therapy (8, 19, 32, 33). However, since the maturation of herpesviruses is a linked, multistep process that has only partially been characterized, it is conceivable that AIC246 targets other late processes (25). Accordingly, the exact target for the anti-HCMV activity of AIC246 has to be identified. To this end, experiments aiming to isolate and characterize mutant viruses that escape AI246 inhibition are currently in progress.
Altogether, these data demonstrate an outstanding anti-HCMV activity of the novel compound AIC246 in vitro and in vivo and suggest that the drug acts late in the replication cycle via a mechanism that is distinct from that of polymerase inhibitors. The ongoing clinical evaluations will clarify whether AIC246 will overcome some of the problems associated with the currently available anti-HCMV drugs.
Acknowledgments
We thank William Britt and Thomas Stamminger for providing antibodies, Eva Borst and Martin Messerle for HCMV-BAC pHG, and Christian Sinzger for help with the focus expansion assay. The excellent technical assistance of Marion Heidtmann, Kerstin Pixberg, Wiebke Schulze, and Christine Hempel is gratefully acknowledged.
Footnotes
Published ahead of print on 4 January 2010.
REFERENCES
- 1.Adler, S. P., G. Nigro, and L. Pereira. 2007. Recent advances in the prevention and treatment of congenital cytomegalovirus infections. Semin. Perinatol. 31:10-18. [DOI] [PubMed] [Google Scholar]
- 2.Andrei, G., E. De Clercq, and R. Snoeck. 2008. Novel inhibitors of human CMV. Curr. Opin. Investig. Drugs 9:132-145. [PubMed] [Google Scholar]
- 3.Andrei, G., E. De Clercq, and R. Snoeck. 2009. Drug targets in cytomegalovirus infection. Infect. Disord. Drug Targets 9:201-222. [DOI] [PubMed] [Google Scholar]
- 4.Andreoni, M., M. Faircloth, L. Vugler, and W. J. Britt. 1989. A rapid microneutralization assay for the measurement of neutralizing antibody reactive with human cytomegalovirus. J. Virol. Methods 23:157-167. [DOI] [PubMed] [Google Scholar]
- 5.Biron, K. K. 2006. Antiviral drugs for cytomegalovirus diseases. Antiviral Res. 71:154-163. [DOI] [PubMed] [Google Scholar]
- 6.Biron, K. K., R. J. Harvey, S. C. Chamberlain, S. S. Good, A. A. Smith III, M. G. Davis, C. L. Talarico, W. H. Miller, R. Ferris, R. E. Dornsife, S. C. Stanat, J. C. Drach, L. B. Townsend, and G. W. Koszalka. 2002. Potent and selective inhibition of human cytomegalovirus replication by 1263W94, a benzimidazole L-riboside with a unique mode of action. Antimicrob. Agents Chemother. 46:2365-2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biron, K. K., S. C. Stanat, J. B. Sorrell, J. A. Fyfe, P. M. Keller, C. U. Lambe, and D. J. Nelson. 1985. Metabolic activation of the nucleoside analog 9-[(2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine in human diploid fibroblasts infected with human cytomegalovirus. Proc. Natl. Acad. Sci. U. S. A. 82:2473-2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bogner, E. 2002. Human cytomegalovirus terminase as a target for antiviral chemotherapy. Rev. Med. Virol. 12:115-127. [DOI] [PubMed] [Google Scholar]
- 9.Boppana, S. B., K. B. Fowler, R. F. Pass, L. B. Rivera, R. D. Bradford, F. D. Lakeman, and W. J. Britt. 2005. Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. J. Pediatr. 146:817-823. [DOI] [PubMed] [Google Scholar]
- 10.Borst, E. M., K. Wagner, A. Binz, B. Sodeik, and M. Messerle. 2008. The essential human cytomegalovirus gene UL52 is required for cleavage-packaging of the viral genome. J. Virol. 82:2065-2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bravo, F. J., R. D. Cardin, and D. I. Bernstein. 2007. A model of human cytomegalovirus infection in severe combined immunodeficient mice. Antiviral Res. 76:104-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Britt, W. 2008. Manifestations of human cytomegalovirus infection: proposed mechanisms of acute and chronic disease. Curr. Top. Microbiol. Immunol. 325:417-470. [DOI] [PubMed] [Google Scholar]
- 13.Buerger, I., J. Reefschlaeger, W. Bender, P. Eckenberg, A. Popp, O. Weber, S. Graeper, H. D. Klenk, H. Ruebsamen-Waigmann, and S. Hallenberger. 2001. A novel nonnucleoside inhibitor specifically targets cytomegalovirus DNA maturation via the UL89 and UL56 gene products. J. Virol. 75:9077-9086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong, K. T., S. Modiratt, P. Pagano, and R. Hinshaw. 1999. A novel gelfoam mouse model for human cytomegalovirus (HCMV) replication, abstr. 1956, p. 411. Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother. (ICAAC), San Francisco, CA. American Society for Microbiology, Washington, DC.
- 15.Chou, S. 2008. Cytomegalovirus UL97 mutations in the era of ganciclovir and maribavir. Rev. Med. Virol. 18:233-246. [DOI] [PubMed] [Google Scholar]
- 16.Chou, S., L. C. Van Wechel, and G. I. Marousek. 2006. Effect of cell culture conditions on the anticytomegalovirus activity of maribavir. Antimicrob. Agents Chemother. 50:2557-2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dollard, S. C., S. D. Grosse, and D. S. Ross. 2007. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev. Med. Virol. 17:355-363. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert, C., and G. Boivin. 2005. Human cytomegalovirus resistance to antiviral drugs. Antimicrob. Agents Chemother. 49:873-883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krosky, P. M., M. R. Underwood, S. R. Turk, K. W. Feng, R. K. Jain, R. G. Ptak, A. C. Westerman, K. K. Biron, L. B. Townsend, and J. C. Drach. 1998. Resistance of human cytomegalovirus to benzimidazole ribonucleosides maps to two open reading frames: UL89 and UL56. J. Virol. 72:4721-4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lischka, P., and H. Zimmermann. 2008. Antiviral strategies to combat cytomegalovirus infections in transplant recipients. Curr. Opin. Pharmacol. 8:541-548. [DOI] [PubMed] [Google Scholar]
- 21.Lorz, K., H. Hofmann, A. Berndt, N. Tavalai, R. Mueller, U. Schlotzer-Schrehardt, and T. Stamminger. 2006. Deletion of open reading frame UL26 from the human cytomegalovirus genome results in reduced viral growth, which involves impaired stability of viral particles. J. Virol. 80:5423-5434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marschall, M., M. Freitag, S. Weiler, G. Sorg, and T. Stamminger. 2000. Recombinant green fluorescent protein-expressing human cytomegalovirus as a tool for screening antiviral agents. Antimicrob. Agents Chemother. 44:1588-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marschall, M., and T. Stamminger. 2009. Molecular targets for antiviral therapy of cytomegalovirus infections. Future Microbiol. 4:731-742. [DOI] [PubMed] [Google Scholar]
- 24.Mercorelli, B., E. Sinigalia, A. Loregian, and G. Palu. 2008. Human cytomegalovirus DNA replication: antiviral targets and drugs. Rev. Med. Virol. 18:177-210. [DOI] [PubMed] [Google Scholar]
- 25.Mettenleiter, T. C., B. G. Klupp, and H. Granzow. 2009. Herpesvirus assembly: an update. Virus Res. 143:222-234. [DOI] [PubMed] [Google Scholar]
- 26.Mocarski, E. S., T. Shenk, and R. Pass. 2007. Cytomegalovirus, p. 2702-2772. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 5th ed. Lippincott Williams & Wilkins, Philadelphia, PA.
- 27.Naesens, L., C. E. Stephens, G. Andrei, A. Loregian, L. De Bolle, R. Snoeck, J. W. Sowell, and E. De Clercq. 2006. Antiviral properties of new arylsulfone derivatives with activity against human betaherpesviruses. Antiviral Res. 72:60-67. [DOI] [PubMed] [Google Scholar]
- 28.Reefschlaeger, J., W. Bender, S. Hallenberger, O. Weber, P. Eckenberg, S. Goldmann, M. Haerter, I. Buerger, J. Trappe, J. A. Herrington, D. Haebich, and H. Ruebsamen-Waigmann. 2001. Novel non-nucleoside inhibitors of cytomegaloviruses (BAY 38-4766): in vitro and in vivo antiviral activity and mechanism of action. J. Antimicrob. Chemother. 48:757-767. [DOI] [PubMed] [Google Scholar]
- 29.Sampathkumar, P., and C. Paya. 2007. Pathogenesis in transplant recipients, p. 7:1-7:14. In V. C. Emery (ed.), Human cytomegalovirus. International Medical Press, London, United Kingdom.
- 30.Sinzger, C., J. Knapp, B. Plachter, K. Schmidt, and G. Jahn. 1997. Quantification of replication of clinical cytomegalovirus isolates in cultured endothelial cells and fibroblasts by a focus expansion assay. J. Virol. Methods 63:103-112. [DOI] [PubMed] [Google Scholar]
- 31.Sinzger, C., M. Mangin, C. Weinstock, M. S. Topp, H. Hebart, H. Einsele, and G. Jahn. 2007. Effect of serum and CTL on focal growth of human cytomegalovirus. J. Clin. Virol. 38:112-119. [DOI] [PubMed] [Google Scholar]
- 32.Townsend, L. B., R. V. Devivar, S. R. Turk, M. R. Nassiri, and J. C. Drach. 1995. Design, synthesis, and antiviral activity of certain 2,5,6-trihalo-1-(beta-D-ribofuranosyl)benzimidazoles. J. Med. Chem. 38:4098-4105. [DOI] [PubMed] [Google Scholar]
- 33.Underwood, M. R., R. J. Harvey, S. C. Stanat, M. L. Hemphill, T. Miller, J. C. Drach, L. B. Townsend, and K. K. Biron. 1998. Inhibition of human cytomegalovirus DNA maturation by a benzimidazole ribonucleoside is mediated through the UL89 gene product. J. Virol. 72:717-725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.ViroPharma Inc. 2009. Viropharma announces discontinuation of maribavir phase 3 study in liver transplant patients. Company press release. ViroPharma Inc., Exton, PA.
- 35.ViroPharma Inc. 2009. Viropharma reports results of phase 3 clinical trial for maribavir in bone marrow transplant patients. Company press release. ViroPharma Inc., Exton, PA.
- 36.Williams, S. L., C. B. Hartline, N. L. Kushner, E. A. Harden, D. J. Bidanset, J. C. Drach, L. B. Townsend, M. R. Underwood, K. K. Biron, and E. R. Kern. 2003. In vitro activities of benzimidazole d- and l-ribonucleosides against herpesviruses. Antimicrob. Agents Chemother. 47:2186-2192. [DOI] [PMC free article] [PubMed] [Google Scholar]