Abstract
Porphyromonas gingivalis is a major pathogen of chronic periodontitis and exists in a biofilm on the surface of the tooth root. Oxantel, a cholinergic anthelmintic and fumarate reductase inhibitor, significantly inhibited biofilm formation by P. gingivalis and disrupted established biofilms at concentrations below its MIC against planktonic cells. Oxantel was more effective against P. gingivalis in biofilm than metronidazole, a commonly used antibiotic for periodontitis.
Periodontitis is a chronic inflammatory disease of the supporting tissues of the teeth and is estimated to affect around 30% of the adult population, with severe forms affecting 5 to 10% (19). Porphyromonas gingivalis, a Gram-negative, asaccharolytic anaerobe that relies on the catabolism of amino acids for the production of metabolic energy, is considered to be a major pathogen in chronic periodontitis. P. gingivalis and other oral bacterial species exist in vivo as a biofilm called subgingival plaque that is accreted to the surface of the tooth root. Sessile P. gingivalis cells can release antigens, toxins, and hydrolytic enzymes, such as lipopolysaccharide, proteinases, and hemagglutinins, that stimulate a host immune response. However, the host response is not very effective at eliminating bacteria within biofilms, and a chronic inflammatory response results in tissue destruction and ultimately tooth loss (13).
Fumarate respiration is the most widespread type of anaerobic respiration (12). In a previous comparative proteomic analysis of P. gingivalis, 2.9- and 4.0-fold reductions of two components of the trimeric P. gingivalis fumarate reductase (Frd) complex (FrdA and FrdB, respectively) were observed during heme-limited growth of the bacterium (6). The lower abundance of the Frd complex correlated with the diminished growth (6). Smith et al. (22) showed that the Frd activity of the anaerobe Campylobacter jejuni was higher in cultures growing exponentially than in cultures that had entered the stationary growth phase. The Frd enzyme complex is required for the growth of Bacteroides fragilis in heme-limited media and to enable colonization of murine stomachs by Helicobacter pylori (1, 2, 8). Together, these findings suggest that Frd activity may limit bacterial growth, which could make it an attractive new therapeutic target to control P. gingivalis infection, especially as the Frd complex is absent in humans (11, 23).
Cholinergic anthelmintics, such as oxantel, thiabendazole, and morantel, which are used for the treatment of intestinal parasites like the whipworm Trichocephalus trichiurus, are known fumarate reductase inhibitors (5, 7, 10, 21). In this study, we determined the inhibitory effects of these anthelmintics on the planktonic and biofilm growth of P. gingivalis.
Effect of anthelmintics on P. gingivalis planktonic growth.
MICs of the anthelmintics on planktonic P. gingivalis were determined in a 96-well plate assay with a starting inoculum of ∼5.0 × 107 CFU per well essentially as described previously (14). Two strains of P. gingivalis were used for the planktonic growth inhibition assays, ATCC 33277, a fimbriated strain that readily forms biofilms, and strain W50, an afimbriated strain which forms biofilms poorly. Oxantel pamoate had the most significant effect of the three inhibitors on planktonic growth of P. gingivalis. The MIC of oxantel was 125 μM for P. gingivalis 33277 and 112 μM for P. gingivalis W50 (Table 1). There was a significant inhibitory effect of oxantel on the growth of P. gingivalis strains 33277 and W50 at concentrations as low as 31.25 μM. There was also a correlation of increasing oxantel concentration with longer mean generation time at sub-MICs (Table 1). The planktonic MICs of oxantel for the P. gingivalis strains reported here were more than six times lower than those reported for H. pylori and C. jejuni, even though the cell numbers used for the P. gingivalis MIC determinations were ∼10 times higher than those used with H. pylori (15-17). The MIC of morantel citrate for P. gingivalis was similar to that reported for H. pylori, whereas there was minimal P. gingivalis growth inhibition with thiabendazole, which was possibly related to the drug's extremely low solubility (18).
TABLE 1.
Effects of anthelmintics on planktonic growth of P. gingivalisa
| Drug and strain | MIC (μM) | MGT (h−1) with indicated concn of drug |
||
|---|---|---|---|---|
| DMSO only | 31.25 μM | 62.5 μM | ||
| Oxantel | ||||
| P. gingivalis W50 | 112 | 6.6b | 19.2c | 23.8d |
| P. gingivalis 33277 | 125 | 6.6b | 10.0c | 24.0d |
| Morantel | ||||
| P. gingivalis W50 | 2,800 | 6.8b | 11.0c | 11.3c |
| Thiabendazole | ||||
| P. gingivalis W50 | >3,000 | 6.3 | 6.8 | 6.2 |
Growth data were statistically analyzed using a one-way classification analysis of variance with a Scheffe multiple comparison. MGT, mean generation time. The superscript letters b, c, and d indicate values significantly different from other MGT values in the same row that are not similarly marked (P < 0.05).
Effects of oxantel on P. gingivalis biofilm formation.
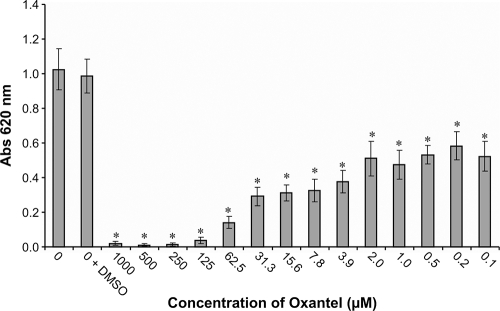
Biofilm formation over 24 h in a static 96-well model was conducted essentially as described previously using crystal violet to quantitate biofilm mass (3, 20). All concentrations of oxantel tested significantly reduced the biofilm biomass after 24 h, and oxantel concentrations above 125 μM effectively abolished biofilm formation (Fig. 1). Oxantel concentrations as low as 0.1 μM significantly reduced the biofilm mass at 24 h.
FIG. 1.
Effects of oxantel on P. gingivalis ATCC 33277 biofilm formation and growth in a 96-well microtiter static assay. The biofilms were quantified at 24 h, and the results represent the means of 12 replicates. *, significantly different (P < 0.001) from controls (0 and 0 + dimethyl sulfoxide [DMSO]), evaluated using a one-way classification analysis of variance with a Scheffe multiple comparison.
Flow cell biofilm culture and CLSM analysis.
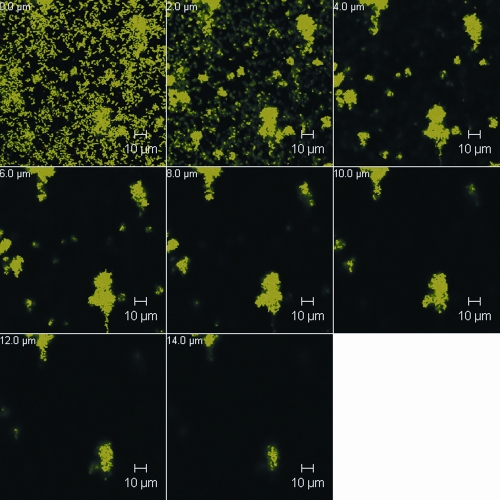
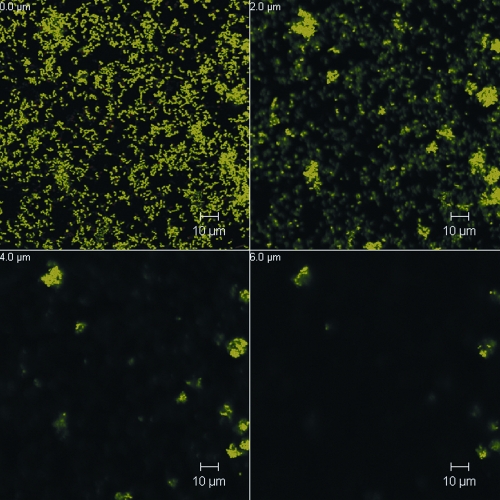
The biofilm culture of P. gingivalis ATCC 33277 in flow cells was similar to that described by Chen et al. (4) based on a three-channel flow cell system (Stovall Life Science, Greensboro, NC). The system was inoculated with 1 ml of an exponentially growing P. gingivalis culture diluted to 5 × 108 cells/ml and incubated for 1 h prior to a constant flow (0.2 ml/min) of 5× diluted supplemented brain heart infusion broth. To determine the effect of oxantel on an established P. gingivalis biofilm, 1 ml of 125 or 12.5 μM oxantel pamoate dissolved in sterile water or sterile water alone (control) was injected into each channel of the system 18 h after inoculation and the mixture was incubated for 30 min. The flow of medium was then resumed for another 10 min to wash off any unbound cells. Confocal laser scanning microscopy (CLSM) of the bacterial biofilms was carried out on a Meta 510 confocal microscope with an inverted stage (Zeiss). BacLight stain (Molecular Probes) was used to stain the biofilms in situ. The biometric parameters of the biofilm were determined from five images at random positions from each of the biological replicates obtained at wavelengths of 488 nm and 568 nm. All images obtained were analyzed using COMSTAT software (9). After 18 h of incubation in the flow cell P. gingivalis ATCC 33277 produced a structured biofilm that featured many microcolonies that formed “towers” or “mushrooms” that had a maximum height of ∼14 μm, and over 40% of the surface area of the substratum was colonized by bacterial cells (Fig. 2). A single oxantel treatment at both tested concentrations caused significant reductions in both biovolume and average thickness of the biofilm. Oxantel treatment affected the structure of the P. gingivalis biofilms, as seen by the significant increases in the ratio of the surface area of the biofilm to the biovolume (relative to the control), the decrease in the size of microcolonies, and the decrease in area of the surface of the substratum that had attached cells at the higher oxantel concentration (Table 2; Fig. 3). The confocal images of the biofilms showed a decreased tower height (or maximum biofilm thickness) after treatment with 12.5 μM oxantel to below 10 μm, whereas 125 μM oxantel treatment reduced tower height to below 6 μm, which is commensurate with the changes in the surface area of biofilm/biovolume ratio and microcolony size (Fig. 3; Table 2).
FIG. 2.
CLSM images of a representative section of a P. gingivalis ATCC 33277 18-h biofilm grown in a flow cell and stained with BacLight. Horizontal (x-y) optodigital sections, each 2 μm thick over the entire thickness of the biofilm (z), were imaged using a 63× objective at 512 by 512 pixels (0.28 μm per pixel), with each frame at 143.86 μm (x) by 143.86 μm (y).
TABLE 2.
Effects of oxantel treatment on an established 18-h P. gingivalis biofilm cultured in a three-channel flow cell system
| Biofilm parameter | Mean result (% change relative to control)a |
||
|---|---|---|---|
| Control | Oxantel, 12.5 μM | Oxantel, 125 μM | |
| Biovolume (μm3/μm2) | 2.08 ± 0.51 | 1.44 ± 0.18b (−31) | 0.86 ± 0.12b (−59) |
| Avg thickness of biofilm (μm) | 1.42 ± 0.34 | 0.83 ± 0.12b (−42) | 0.39 ± 0.03b (−73) |
| Surface area/biovolume ratio (μm2/μm3) | 1.69 ± 0.19 | 2.31 ± 0.25b (+37) | 2.54 ± 0.24b (+50) |
| Surface area of substratum occupied by cells (%) | 42.2 ± 14.8 | 33.2 ± 6.2 (−21) | 23.8 ± 4.5b (−44) |
| Avg no. of microcoloniesc | 20.9 ± 4.1 | 18.3 ± 0.9 (−12) | 15.6 ± 4.9 (−25) |
| Avg microcolony area (μm2) | 188.4 ± 43.5 | 124.0 ± 21.7 (−34) | 91.2 ± 21.5b (−52) |
As determined using COMSTAT (9) analysis of CLSM images. Data are expressed as the means ± standard deviations of three biological replicates. The percent change compared with the control is shown in parentheses. The biometric data were statistically analyzed using a one-way classification analysis of variance with a Scheffe multiple comparison.
Significantly different from control (P < 0.05).
Microcolonies were defined as clusters of cells with >500 pixel counts.
FIG. 3.
CLSM images of a representative section of a P. gingivalis ATCC 33277 18-h biofilm grown in a flow cell, treated with 125 μM oxantel, and then stained with BacLight. Horizontal (x-y) optodigital sections, each 2 μm thick over the entire thickness of the biofilm (z), were imaged using a 63× objective at 512 by 512 pixels (0.28 μm per pixel), with each frame at 143.86 μm (x) by 143.86 μm (y).
Incorporation of a low oxantel concentration (12.5 μM) into the growth medium in the flow cell also had significant effects on the formation of biofilms by P. gingivalis, decreasing biovolume by 52% and average biofilm thickness by 74% (Table 3). In a similar manner to that seen with the treatment of established biofilms there was a significant increase in the surface area of the biofilm/biovolume ratio (Table 3). Interestingly, there was no significant decrease in the surface area of the substratum that was colonized by bacterial cells, indicating that oxantel doesn't interfere with attachment of the bacterium to the substratum. A current treatment option for refractory chronic periodontitis is the systemic administration of the antibiotic metronidazole. Metronidazole was also incorporated into the growth medium at a concentration of 12.5 μM, and this significantly reduced the biovolume and average thickness of an 18-h P. gingivalis biofilm (Table 3). However, the reductions in biovolume and average thickness were significantly less than those produced by oxantel, and in addition metronidazole had no significant effect on the surface area of the biofilm/biovolume ratio or the number of microcolonies present (Table 3). This suggests that metronidazole only affected P. gingivalis cells at the surface of the biofilm structures, resulting in a reduced biovolume but a structurally similar biofilm. Oxantel at the same concentration caused significantly higher reductions in biovolume and average thickness than metronidazole and significantly increased the surface area of the biofilm/biovolume ratio as well as decreasing the number of microcolonies. These data indicate that oxantel is more effective than metronidazole in inhibiting P. gingivalis biofilms and that its mechanism of action is distinct from that of metronidazole. An advantage of oxantel is its ability to selectively inhibit strictly anaerobic bacterial species (pathogens) but not the commensal aerotolerant anaerobes or aerobic bacteria, as these species lack fumarate reductase.
TABLE 3.
Effects of incorporation of a low concentration (12.5 μM) of oxantel or metronidazole into the growth medium on P. gingivalis biofilm formation for an 18-h culture in a three-channel flow cell system
| Biofilm parameter | Mean result (% change relative to control)a |
||
|---|---|---|---|
| Control | Metronidazole, 12.5 μM | Oxantel, 12.5 μM | |
| Biovolume (μm3/μm2) | 3.03 ± 0.56 | 2.19 ± 0.05b (−38) | 1.18 ± 0.31b,c (−52) |
| Avg thickness of biofilm (μm) | 3.26 ± 0.64 | 2.17 ± 0.66b (−41) | 0.76 ± 0.37b,c (−74) |
| Surface area/biovolume ratio (μm2/μm3) | 1.68 ± 0.32 | 1.63 ± 0.33 (−3) | 2.63 ± 0.16b,c (+57) |
| Surface area of substratum occupied by cells (%) | 32.0 ± 9.9 | 27.4 ± 13.8 (−14) | 27.0 ± 2.7 (−16) |
| Avg no. of microcoloniesd | 21.0 ± 5.2 | 23.7 ± 7.9 (+12) | 14.7 ± 0.5 (−30) |
| Avg microcolony area (μm2) | 168 ± 50 | 102 ± 37 (−39) | 118 ± 9 (−30) |
As determined using COMSTAT analysis of CLSM images. Data are expressed as means ± standard deviations of three biological replicates. The percent change compared with the control is shown in parentheses. The biometric data were statistically analyzed using the Kruskal-Wallis test and Mann-Whitney U Wilcoxon rank sum test with a Bonferroni correction for type 1 error.
Significantly different from control (P < 0.05).
Significantly different from metronidazole result (P < 0.05).
Microcolonies were defined as clusters of cells with >500 pixel counts.
In this study we have demonstrated that oxantel is a promising therapeutic for the control of the pathogen P. gingivalis by disrupting biofilm development and stimulating release of cells from biofilm microcolonies.
Acknowledgments
This work was supported by the Australian National Health and Medical Research Council and an International Association for Dental Research/Glaxo Smith Kline Innovation in Oral Care Award. C.S.A. acknowledges the support of the IPRS, MIRS, and CRC scholarships from The University of Melbourne and The CRC for Oral Health Science.
Footnotes
Published ahead of print on 28 December 2009.
REFERENCES
- 1.Baughn, A. D., and M. H. Malamy. 2003. The essential role of fumarate reductase in haem-dependent growth stimulation of Bacteroides fragilis. Microbiology 149:1551-1558. [DOI] [PubMed] [Google Scholar]
- 2.Bhattacharyya, S., G. Zhongming, P. Krishnamurthy, T. Chambers, S. Hadnis, J. Fox, and B. Dunn. 2001. Helicobacter phylori requires fumarate reductase for acid resistance. Gastroenterology 120:A655. [Google Scholar]
- 3.Capestany, C. A., M. Kuboniwa, I. Y. Jung, Y. Park, G. D. Tribble, and R. J. Lamont. 2006. Role of the Porphyromonas gingivalis InlJ protein in homotypic and heterotypic biofilm development. Infect. Immun. 74:3002-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, W., K. Honma, A. Sharma, and H. K. Kuramitsu. 2006. A universal stress protein of Porphyromonas gingivalis is involved in stress responses and biofilm formation. FEMS Microbiol. Lett. 264:15-21. [DOI] [PubMed] [Google Scholar]
- 5.Choi, W. Y., O. R. Lee, W. K. Lee, W. K. Kim, C. S. Chung, and B. O. Ough. 1979. A clinical trial of oxantel and pyrantel against intestinal nematodes infections. Kisaengchunghak Chapchi 17:60-66. [DOI] [PubMed] [Google Scholar]
- 6.Dashper, S. G., C. S. Ang, P. D. Veith, H. L. Mitchell, A. W. Lo, C. A. Seers, K. A. Walsh, N. Slakeski, D. Chen, J. P. Lissel, C. A. Butler, N. M. O'Brien-Simpson, I. G. Barr, and E. C. Reynolds. 2009. Response of Porphyromonas gingivalis to heme limitation in continuous culture. J. Bacteriol. 191:1044-1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Garcia, E. G. 1976. Treatment for trichuriasis with oxantel. Am. J. Trop. Med. Hyg. 25:914-915. [DOI] [PubMed] [Google Scholar]
- 8.Ge, Z., Y. Feng, C. A. Dangler, S. Xu, N. S. Taylor, and J. G. Fox. 2000. Fumarate reductase is essential for Helicobacter pylori colonization of the mouse stomach. Microb. Pathog. 29:279-287. [DOI] [PubMed] [Google Scholar]
- 9.Heydorn, A., A. T. Nielsen, M. Hentzer, C. Sternberg, M. Givskov, B. K. Ersboll, and S. Molin. 2000. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology 146:2395-2407. [DOI] [PubMed] [Google Scholar]
- 10.Horton, J. 2003. The efficacy of anthelmintics: past, present, and future, p. 143-155. In D. W. T. Crompton, A. Montresor, M. C. Nesheim, and L. Savioli (ed.), Controlling disease due to helminth infections. WHO, Geneva, Switzerland.
- 11.Klein, R. A., D. J. Linstead, and M. V. Wheeler. 1975. Carbon dioxide fixation in trypanosomatids. Parasitology 71:93-107. [DOI] [PubMed] [Google Scholar]
- 12.Kroeger, A., V. Geisler, E. Lemma, F. Theis, and R. Lenger. 1992. Bacterial fumarate respiration. Arch. Microbiol. 158:311-314. [Google Scholar]
- 13.Lamont, R. J., and H. F. Jenkinson. 1998. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol. Mol. Biol. Rev. 62:1244-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malkoski, M., S. G. Dashper, N. M. O'Brien-Simpson, G. H. Talbo, M. Macris, K. J. Cross, and E. C. Reynolds. 2001. Kappacin, a novel antibacterial peptide from bovine milk. Antimicrob. Agents Chemother. 45:2309-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mendz, G. L., S. L. Hazell, and S. Srinivasan. 1995. Fumarate reductase: a target for therapeutic intervention against Helicobacter pylori. Arch. Biochem. Biophys. 321:153-159. [DOI] [PubMed] [Google Scholar]
- 16.Mendz, G. L., D. J. Meek, and S. L. Hazell. 1998. Characterization of fumarate transport in Helicobacter pylori. J. Membr. Biol. 165:65-76. [DOI] [PubMed] [Google Scholar]
- 17.Mileni, M., F. MacMillan, C. Tziatzios, K. Zwicker, A. H. Haas, W. Mantele, J. Simon, and C. R. Lancaster. 2006. Heterologous production in Wolinella succinogenes and characterization of the quinol:fumarate reductase enzymes from Helicobacter pylori and Campylobacter jejuni. Biochem. J. 395:191-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nielsen, L. S., H. Bundgaard, and E. Falch. 1992. Prodrugs of thiabendazole with increased water-solubility. Acta Pharm. Nord. 4:43-49. [PubMed] [Google Scholar]
- 19.Oliver, R. C., L. J. Brown, and H. Loe. 1998. Periodontal diseases in the United States population. J. Periodontol. 69:269-278. [DOI] [PubMed] [Google Scholar]
- 20.O'Toole, G. A., and R. Kolter. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol. Microbiol. 28:449-461. [DOI] [PubMed] [Google Scholar]
- 21.Prichard, R. K. 1970. Mode of action of the anthelminthic thiabendazole in Haemonchus contortus. Nature 228:684-685. [DOI] [PubMed] [Google Scholar]
- 22.Smith, M. A., G. L. Mendz, M. A. Jorgensen, and S. L. Hazell. 1999. Fumarate metabolism and the microaerophily of Campylobacter species. Int. J. Biochem. Cell Biol. 31:961-975. [DOI] [PubMed] [Google Scholar]
- 23.Turrens, J. F. 1989. The role of succinate in the respiratory chain of Trypanosoma brucei procyclic trypomastigotes. Biochem. J. 259:363-368. [DOI] [PMC free article] [PubMed] [Google Scholar]