Abstract
Despite the identification of severe acute respiratory syndrome-related coronavirus (SARSr-CoV) in Rhinolophus Chinese horseshoe bats (SARSr-Rh-BatCoV) in China, the evolutionary and possible recombination origin of SARSr-CoV remains undetermined. We carried out the first study to investigate the migration pattern and SARSr-Rh-BatCoV genome epidemiology in Chinese horseshoe bats during a 4-year period. Of 1,401 Chinese horseshoe bats from Hong Kong and Guangdong, China, that were sampled, SARSr-Rh-BatCoV was detected in alimentary specimens from 130 (9.3%) bats, with peak activity during spring. A tagging exercise of 511 bats showed migration distances from 1.86 to 17 km. Bats carrying SARSr-Rh-BatCoV appeared healthy, with viral clearance occurring between 2 weeks and 4 months. However, lower body weights were observed in bats positive for SARSr-Rh-BatCoV, but not Rh-BatCoV HKU2. Complete genome sequencing of 10 SARSr-Rh-BatCoV strains showed frequent recombination between different strains. Moreover, recombination was detected between SARSr-Rh-BatCoV Rp3 from Guangxi, China, and Rf1 from Hubei, China, in the possible generation of civet SARSr-CoV SZ3, with a breakpoint at the nsp16/spike region. Molecular clock analysis showed that SARSr-CoVs were newly emerged viruses with the time of the most recent common ancestor (tMRCA) at 1972, which diverged between civet and bat strains in 1995. The present data suggest that SARSr-Rh-BatCoV causes acute, self-limiting infection in horseshoe bats, which serve as a reservoir for recombination between strains from different geographical locations within reachable foraging range. Civet SARSr-CoV is likely a recombinant virus arising from SARSr-CoV strains closely related to SARSr-Rh-BatCoV Rp3 and Rf1. Such frequent recombination, coupled with rapid evolution especially in ORF7b/ORF8 region, in these animals may have accounted for the cross-species transmission and emergence of SARS.
Coronaviruses can infect a wide variety of animals, causing respiratory, enteric, hepatic, and neurological diseases with different degrees of severity. On the basis of genotypic and serological characteristics, coronaviruses were classified into three distinct groups (2, 20, 54). Among coronaviruses that infect humans, human coronavirus 229E (HCoV-229E) and human coronavirus NL63 (HCoV-NL63) belong to group 1 coronaviruses and human coronavirus OC43 (HCoV-OC43), and human coronavirus HKU1 (HCoV-HKU1) belong to group 2 coronaviruses, whereas severe acute respiratory syndrome-related coronavirus (SARSr-CoV) has been classified as a group 2b coronavirus, distantly related to group 2a, and the recently discovered group 2c and 2d coronaviruses (6, 8, 10, 18, 31, 38, 43, 46, 49, 50). Recently, the Coronavirus Study Group of the International Committee for Taxonomy of Viruses has proposed renaming the traditional group 1, 2, and 3 coronaviruses Alphacoronavirus, Betacoronavirus, and Gammacoronavirus, respectively (http://talk.ictvonline.org/media/p/1230.aspx).
Among all coronaviruses, SARSr-CoV has caused the most severe disease in humans, with over 700 fatalities since the SARS epidemic in 2003. Although the identification of SARSr-CoV in Himalayan palm civets and raccoon dogs in live animal markets in southern China suggested that wild animals could be the origin of SARS (11), the presence of the virus in only market or farmed civets, but not civets in the wild, and the rapid evolution of SARSr-CoV genomes in market civets suggested that these caged animals were only intermediate hosts (24, 39, 42, 52). Since bats are commonly found and served in wild animal markets and restaurants in Guangdong, China (47), we have previously carried out a study of bats from the region and identified a SARSr-CoV in Rhinolophus Chinese horseshoe bats (SARSr-Rh-BatCoV) (21). Similar viruses have also been found in three other species of horseshoe bats in mainland China (25), supporting the hypothesis that horseshoe bats are a reservoir of SARSr-CoV. Recently, viruses closely related to SARSr-Rh-BatCoV in China were also reported in Chaerophon bats from Africa, although only partial RNA-dependent RNA polymerase (RdRp) sequences were available (41). In addition, more than 10 previously unrecognized coronaviruses of huge diversity have since been identified in bats from China and other countries (1, 3, 5, 9, 22, 27, 32, 33, 40, 46, 51), suggesting that bats play an important role in the ecology and evolution of coronaviruses.
As a result of the unique mechanism of viral replication, coronaviruses have a high frequency of recombination (20). Such a high recombination rate, coupled with the infidelity of the polymerases of RNA viruses, may allow them to adapt to new hosts and ecological niches (12, 48). Recombination in coronaviruses was first recognized between different strains of murine hepatitis virus (MHV) and subsequently in other coronaviruses, such as infectious bronchitis virus, between MHV and bovine coronavirus, and between feline coronavirus type I and canine coronavirus generating feline coronavirus type II (12, 16, 17, 23). Recently, by complete genome analysis of 22 strains of HCoV-HKU1, we have also documented for the first time that natural recombination events in a human coronavirus can give rise to three different genotypes (48).
Although previous studies have attempted to study the possible evolutionary and recombination origin of SARSr-CoV, no definite conclusion can be made on whether the viruses from bats are the direct ancestor of SARSr-CoV in civets and humans, given the paucity of available strains and genome sequences. To better define the epidemiology and evolution of SARSr-Rh-BatCoV in China and their role as a recombination origin of SARSr-CoV in civets, we carried out a 4-year study on coronaviruses in Chinese horseshoe bats in Hong Kong and Guangdong Province of southern China. Bat tagging was also performed to study the migration pattern of bats and viral persistence. The complete genomes of 10 strains of SARSr-Rh-BatCoV obtained at different time were sequenced and compared to previously sequenced genomes. With the availability of this larger set of genome sequences for more accurate analysis, recombination and molecular clock analyses were performed to elucidate the evolutionary origin and time of interspecies transmission of SARSr-CoV.
MATERIALS AND METHODS
Sample collection and bat tagging.
Sample collection was approved by and performed in collaboration with the Department of Agriculture, Fisheries and Conservation (AFCD) of the Hong Kong Special Administrative Region (HKSAR) and Guangzhou Center for Disease Control and Prevention, Guangzhou, China. Chinese horseshoe bats (Rhinolophus sinicus) were captured from various locations in Hong Kong and in Guangdong Province of southern China over a 4-year period (April 2004 to March 2008). Respiratory and alimentary specimens of the bars were collected using procedures described previously (21, 53). All specimens were placed in viral transport medium before transportation to the laboratory for RNA extraction. To assess the migration range and chronicity of coronavirus infections, 511 bats from Hong Kong were also tagged during sample collection before release. Tagged bats, when identified in subsequent site visits, were recaptured and recorded for sample collection before release.
RNA extraction.
Viral RNA was extracted from the respiratory and alimentary specimens using QIAamp viral RNA minikit (QIAgen, Hilden, Germany). The RNA was eluted in 50 μl of AVE buffer and was used as the template for reverse transcription-PCR (RT-PCR).
RT-PCR for coronaviruses and DNA sequencing.
Coronavirus screening was performed by amplifying a 440-bp fragment of the RdRp gene of coronaviruses using conserved primers (5′-GGTTGGGACTATCCTAAGTGTGA-3′ and 5′-CCATCATCAGATAGAATCATCATA-3′) designed by multiple alignments of the nucleotide sequences of available RdRp genes of known coronaviruses (49). Reverse transcription was performed using the SuperScript III kit (Invitrogen, San Diego, CA). The PCR mixture (25 μl) contained cDNA, PCR buffer (10 mM Tris-HCl [pH 8.3], 50 mM KCl, 3 mM MgCl2, and 0.01% gelatin), 200 μM (each) deoxynucleoside triphosphates (dNTPs), and 1.0 U Taq polymerase (Applied Biosystems, Foster City, CA). The mixtures were amplified in 40 cycles of PCR, with 1 cycle consisting of 1 min at 94°C, 1 min at 48°C, and 1 min at 72°C, and a final extension step of 10 min at 72°C in an automated thermal cycler (Applied Biosystems, Foster City, CA). Standard precautions were taken to avoid PCR contamination, and no false-positive result was observed for the negative controls.
The PCR products were gel purified using the QIAquick gel extraction kit (QIAgen, Hilden, Germany). Both strands of the PCR products were sequenced twice with an ABI Prism 3700 DNA analyzer (Applied Biosystems, Foster City, CA) using the two PCR primers. The sequences of the PCR products were compared with known sequences of the RdRp genes of coronaviruses in the GenBank database.
All cDNAs positive for SARSr-Rh-BatCoV were subjected to Rh-BatCoV HKU2 screening using Rh-BatCoV HKU2-specific primers (5′-GGAGTATGCAGCGTTGGGTTA-3′ and 5′-GACACATAGCGCTCAAGCAAA-3′), and all cDNAs positive for Rh-BatCoV HKU2 were subjected to SARSr-Rh-BatCoV screening using SARSr-Rh-BatCoV-specific primers (5′-CAAGTGGGGTAAGGCTAGACTTT-3′ and 5′-AACATATTATGCCAGCCACCATA-3′) using the PCR conditions described above.
Statistical analysis.
Comparison of the body weights of bats in different groups was performed using Student's t test and covariate analysis (SPSS version 11.5). A P of <0.05 was regarded as statistically significant.
Complete genome sequencing of SARSr-Rh-BatCoV.
Ten complete genomes of SARSr-Rh-BatCoV detected in the present study were amplified and sequenced using the RNA extracted from an alimentary specimen as the template. The RNA was converted to cDNA by a combined random priming and oligo(dT) priming strategy. The cDNA was amplified by degenerate primers as described previously (21). A total of 57 sets of primers, available on request, were used for PCR. The 5′ end of the viral genome was confirmed by rapid amplification of cDNA ends (RACE) using the 5′/3′ RACE kit (Roche, Germany). Sequences were assembled and manually edited to produce the final sequences.
Genome analysis.
The nucleotide sequences of the genomes and the deduced amino acid sequences of the open reading frames (ORFs) were compared to those of other coronaviruses using the CoVDB coronavirus database (14). Phylogenetic tree construction was performed using the neighbor-joining method with ClustalX 1.83.
Bootscan analysis.
Sliding window analysis was used to detect possible recombination, using nucleotide alignment of the available genome sequences of different SARSr-Rh-BatCoV strains and civet SARSr-CoV SZ3 generated by ClustalX version 1.83 and edited manually. Bootscan analysis was performed using Simplot version 3.5.1 (26) (F84 model; window size, 1,500 bp; step size, 300 bp) with selected strains, including SARSr-Rh-BatCoV Rf1 and civet SARSr-CoV SZ3, as the query sequence.
Estimation of synonymous and nonsynonymous substitution rates.
The number of synonymous substitutions per synonymous site, Ks, and the number of nonsynonymous substitutions per nonsynonymous site, Ka, for each coding region were calculated for all available SARSr-Rh-BatCoV, civet SARSr-CoV, and human SARSr-CoV genomes using the Nei-Gojobori method (Jukes-Cantor) in MEGA 3.1 (19). Identical genes were excluded from analysis.
Estimation of divergence dates.
The time of the most recent common ancestor (tMRCA) and the time of divergence were estimated on the basis of an alignment of ORF1 sequences, using the uncorrelated exponentially distributed relaxed clock model (UCED) in BEAST version 1.4 (7). Under this model, the rates were allowed to vary at each branch drawn independently from an exponential distribution. The sampling dates of all strains were collected from the literature or from the present study and were used as calibration points. Depending on the data set, Markov chain Monte Carlo (MCMC) sample chains were run for 1 × 108 states, sampling every 1,000 generations under the GTR nucleotide substitution model, determined by MODELTEST and allowing γ-rate heterogeneity for all data sets. A constant population coalescent prior was assumed for all data sets. The median and the highest posterior density regions at 95% (HPD) were calculated for each of these parameters from two identical but independent MCMC chains using TRACER 1.3 (http://beast.bio.ed.ac.uk). The tree was annotated by TreeAnnotator, a program of BEAST and displayed by FigTree (http://tree.bio.ed.ac.uk/software/figtree/).
Nucleotide sequence accession numbers.
The nucleotide sequences of the 10 genomes of SARSr-Rh-BatCoV have been lodged within the GenBank sequence database under accession no. GQ153539 to GQ153548.
RESULTS
Epidemiology of SARSr-Rh-BatCoV in Chinese horseshoe bats.
A total of 1,398 respiratory specimens and 1,648 alimentary specimens from 1,337 and 64 Chinese horseshoe bats were obtained from Hong Kong and in Guangdong Province in southern China, respectively, over the 4-year study period. RT-PCR of a 440-bp fragment in the RdRp genes of coronaviruses was positive for SARSr-Rh-BatCoV in respiratory specimens from 2 of the 1,337 Chinese horseshoe bats from Hong Kong and in alimentary specimens from 126 (9.4%) of the 1,337 Chinese horseshoe bats from Hong Kong and 4 (6.3%) of the 64 Chinese horseshoe bats from Guangdong, China, with ≥99% nucleotide identities to SARSr-Rh-BatCoV (GenBank accession no. DQ022305) (21). Another previously described group 1 coronavirus, Rhinolophus bat coronavirus HKU2 (Rh-BatCoV HKU2), coinfecting Chinese horseshoe bats, was identified in alimentary specimens from 62 (4.6%) bats from Hong Kong and from 7 (10.9%) bats from Guangdong, China, with ≥99% nucleotide identities to Rh-BatCoV HKU2 (GenBank accession no. DQ249235) (22). Seventeen bats from Hong Kong and three bats from Guangdong, China, were coinfected by SARSr-Rh-BatCoV and Rh-BatCoV HKU2. The 126 bats from Hong Kong positive for SARSr-Rh-BatCoV were from 15 of the 27 sampling locations in Hong Kong, with bats from seven locations harboring both viruses (Fig. 1). Peak activity of both SARSr-Rh-BatCoV and Rh-BatCoV HKU2 was observed in the spring (see Fig. S1 in the supplemental material). However, the prevalence of SARSr-Rh-BatCoV was higher than that of Rh-BatCoV HKU2 during the spring of 2005 and 2007, while the prevalence of Rh-BatCoV HKU2 was higher than that of SARSr-Rh-BatCoV in the spring of 2006.
FIG. 1.
Map showing the locations of bat sampling and tagging in Hong Kong. Squares represent the locations where bats were positive for SARSr-Rh-BatCoV, dark circles represent locations where bats were positive for Rh-BatCoV HKU2, and triangles represent locations where the bats were positive for both Rh-BatCoV HKU2 and SARSr-Rh-BatCoV. The percentages indicate the proportion of bats positive for SARSr-Rh-BatCoV, Rh-BatCoV HKU2, or SARSr-Rh-BatCoV/Rh-BatCoV HKU2 at each location. Blank circles represent locations negative for SARSr-Rh-BatCoV and Rh-BatCoV HKU2. The red circle represents the location of Shenzhen Dongman market (SZDM) in China where civet SARSr-CoV was first identified. The arrows indicate the direction of migration of Chinese horseshoe bats as demonstrated in the tagging exercise.
A total of 511 Chinese horseshoe bats from 11 sites were tagged, with 152 (29.7%) recapturing episodes from six sites during subsequent visits (Fig. 2). A total of 113 tagged bats were recaptured, with 84 bats recaptured once, 21 recaptured twice, 6 recaptured three times, and 2 recaptured four times after tagging. The time interval between tagging and recapture of the same bat ranged from 2 weeks to 21 months. Migration between water tunnels at short distances was most common (Fig. 1). The longest distance of migration was approximately 17 km within 3 months from tagging to recapture (October 2006 to January 2007), while the shortest distance between two habitats was 1.86 km. Sixteen bats were positive for SARSr-Rh-BatCoV, and 23 were positive for Rh-BatCoV HKU2 at the time of tagging, with one bat being positive for both viruses. Among these 38 bats, 10 bats were recaptured, but all were subsequently negative for coronaviruses within a period of 4 to 16 months (Fig. 2). Twenty-three and nine bats initially negative for coronaviruses were subsequently positive for SARSr-Rh-BatCoV and Rh-BatCoV HKU2, respectively, among which seven bats were positive for both viruses. However, only one of these bats was positive for coronavirus at more than one episode, which carried SARSr-Rh-BatCoV at first and both SARSr-Rh-BatCoV and Rh-BatCoV HKU2 during the next visit at the same site 2 weeks later (Fig. 2). With the longest shedding period of 2 weeks and the shortest documented clearance time of 4 months in bats positive for SARSr-Rh-BatCoV, it is estimated that viral clearance occurred between 2 weeks and 4 months.
FIG. 2.
Schematic diagram showing the number of Rhinolophus sinicus (Chinese horseshoe) bats tagged and recaptured, and the presence of coronaviruses among these tagged bats. The numbers in boldface type indicate the number of bats successfully recaptured. The numbers in roman type (not boldface type) following dashed lines are the numbers of bats not recaptured in subsequent visits. The numbers in parentheses are the number of recaptured bats positive for coronaviruses. CoV +ve, coronavirus positive; CoV -ve, coronavirus negative.
No disease association was observed in bats positive for SARSr-Rh-BatCoV or Rh-BatCoV HKU2. However, lower body weights were observed in bats positive for SARSr-Rh-BatCoV (body weight [mean ± standard deviation {SD}], 10.9 g ± 1.4 g) than those negative for coronaviruses (body weight [mean ± SD], 11.6 ± 2.2 g) (P < 0.0001 by Student's t test). A similar phenomenon was not observed when bats positive for Rh-BatCoV HKU2 (body weight [mean ± SD], 11.5 ± 1.5 g) were used for comparison (P = 0.783 by Student's t test). To control for the confounding effect of age and possible lower body weights after hibernation during which SARSr-Rh-BatCoV showed the highest detection rate, covariate analysis was performed using only data from the peak season (during March) with forearm lengths (which correlate with age) as a possible cofactor. The results showed that the SARSr-Rh-BatCoV carriage state is an independent factor in association with lower body weight (P = 0.002). Similarly, no significant difference in body weight was observed when similar analysis was performed on bats positive for Rh-BatCoV HKU2 despite its similar seasonality, suggesting that this phenomenon is specific to SARSr-Rh-BatCoV.
Complete genome comparison of SARSr-Rh-BatCoV genomes.
In addition to the eight previously described genomes of SARSr-Rh-BatCoVs, complete genome sequence data of 10 additional strains of SARSr-Rh-BatCoV were obtained by assembly of the sequences of the RT-PCR products obtained directly from 10 individual specimens collected at different times. Eight strains were detected in bats from Hong Kong, while two strains were from bats from Guangdong, China (see Table S1 in the supplemental material). Their genome sizes were 29,677 to 29,716 nucleotides, with a G+C content of 41%, comparable to the previously reported genomes. The eight Hong Kong strains were more closely related to each other with an overall nucleotide identity of 99.9%, while the two strains from Guangdong, China, had 98.5% nucleotide identity to the Hong Kong strains. Except for strain HKU3-8 from Guangdong, China, all SARSr-Rh-BatCoV strains share the same genome organization, containing the putative transcription regulatory sequence (TRS) motif, 5′-ACGAAC-3′, at the 3′ end of the 5′ leader sequence and preceding each ORF except ORF7b.
Similar to previous findings, analysis of the full-length sequences of all currently available SARSr-Rh-BatCoV genomes showed that the major differences between SARSr-Rh-BatCoV genomes and civet/human SARSr-CoV genomes were observed in spike (mainly S1 domain), ORF3, and ORF8 regions, which were also the most variable regions among human SARSr-CoV genomes (21, 25, 34). All genomes possessed 87% nucleotide identities to civet and human SARSr-CoV, except for SARSr-Rh-BatCoV strain Rp3, which possessed 91% and 92% nucleotide identities to civet and human strains, respectively. The higher overall sequence similarity of strain Rp3 to civet and human strains is mainly due to the higher sequence homology within the ORF1 region. At nsp2, nsp3, nsp12, and nsp14 regions, strain Rp3 possessed the highest amino acid identities among all SARSr-Rh-BatCoV strains to the corresponding regions in civet SARSr-CoV SZ3 (see Table S2 in the supplemental material). Interestingly, the sequences of our SARSr-Rh-BatCoV strains from Hong Kong and Guangdong, China, possessed higher (98%) amino acid identities in the nps1 region to civet SARSr-CoV than other strains from China (92 to 93%). On the other hand, at ORF3a and ORF8, the sequences of strains Rf1 and 273/04 possessed the highest amino acid identities to those of civet SARSr-CoV (see Table S2 in the supplemental material).
ORF8 represents the most variable region within the SARSr-CoV genomes. In contrast to human SARSr-CoV which contains a 29-bp deletion at ORF8 region which resulted in two overlapping ORFs, ORF8a and ORF8b, all SARSr-Rh-BatCoV genomes except that of strain HKU3-8 contain a single long ORF8, similar to civet SARSr-CoV. This 29-bp deletion present only in human strains has been shown to disrupt the functional expression of the ORF8 region (30). Strain HKU3-8 has a short deletion at the ORF8 region that breaks this ORF into three small ones (see Fig. S2 in the supplemental material). This 26-bp deletion was only 14 bp downstream of the 29-bp deletion in human SARSr-CoV, suggesting that this region is a frequent site for deletions. As a result of the frequent deletions observed within this region, the ORF8 region of SARSr-Rh-BatCoV possessed very low (35 to 37%) amino acid identities to that of civet SARSr-CoV, except for two strains, Rf1 and 273/04, which possessed 80% amino acid identities (see Table S2 in the supplemental material).
Phylogenetic analysis.
Phylogenetic trees were constructed using the nucleotide sequences of the nonstructural protein 3 (nsp3), RdRp, spike (S), ORF3a, envelope protein (E), membrane protein (M), ORF8, and nucleocapsid protein (N) genes of SARSr-CoV (see Fig. S3 in the supplemental material). In general, SARSr-Rh-BatCoV strains from the same geographical area are more closely related to each other. Among all SARSr-Rh-BatCoV genomes, strain Rp3 from Guangxi Province, China, is most closely related to human and civet strains in the ORF1 region, as exemplified by their close clustering in the nsp3 and RdRp trees. However, from the S gene onwards, strain Rp3 was more closely related to other SARSr-Rh-BatCoVs than to human and civet strains. Moreover, from ORF3a to ORF8, clustering of human and civet SARSr-CoVs with strains Rf1 and 273/04 from Hubei Province, China, was observed. This suggested that civet SARSr-CoV may have arisen from recombination between strains from different geographical locations that were related to present strains from Guangxi and Hubei provinces in China.
Recombination analysis.
To detect recombination between genomes of different strains of SARSr-Rh-BatCoV or civet SARSr-CoV, sliding window analysis was conducted. Results showed frequent recombination events among the bat viruses in China. When civet SARSr-CoV SZ3 was used as the query sequence with SARSr-Rh-BatCoV strains Rm1, Rf1, and Rp3 as the potential parents, a recombination breakpoint at the nsp16/S intergenic region was identified (Fig. 3). Upstream of this breakpoint before position 21300, high bootstrap support for clustering of civet SARSr-CoV SZ3 with SARSr-Rh-BatCoV strain Rp3 was observed. However, an abrupt change in clustering occurred after position 21300, with high bootstrap support for clustering of civet SARSr-CoV SZ3 with SARSr-Rh-BatCoV strain Rf1. This is in line with results from phylogenetic analysis, where the ORF1 sequences of civet and human SARSr-CoV strains clustered with SARSr-Rh-BatCoV Rp3, but the sequences from ORF3a to ORF8 clustered with SARSr-Rh-BatCoV strains Rf1 and 273/04.
FIG. 3.
Bootscan analysis using the genome sequence of civet SARSr-CoV strain SZ3 as the query sequence. Bootscanning was conducted with Simplot version 3.5.1 (F84 model; window size, 1,500 bp; step size, 300 bp). SARSr-Rh-BatCoV strain Rf1, SARSr-Rh-BatCoV strain Rp3, and SARSr-Rh-BatCoV strain Rm1 were examined by bootscan analysis.
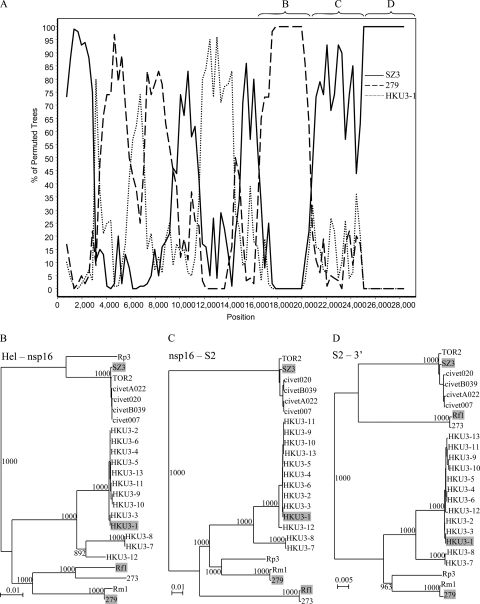
Apart from this recombination event, other putative recombination events were also observed when SARSr-Rh-BatCoV strains were used as the query sequence. When SARSr-Rh-BatCoV strain Rf1 was used as the query sequence with SARSr-Rh-BatCoV HKU3-1, 279/04, and civet SARSr-CoV SZ3 as the potential parents, putative recombination events were observed throughout the genome, as shown by frequent shuffle of clustering with the three putative parent strains (Fig. 4A). The most notable site occurred at around position 20700, corresponding to nsp16. From positions 16400 to 20700, high bootstrap support for clustering with strain 279/04 was observed. From position 20700 onwards and especially toward the 3′end of the genome, high bootstrap support for clustering with civet SARSr-CoV SZ3 was observed. These bootscan results were also supported by the shifting of positions upon phylogenetic analysis (Fig. 4B). From positions 16400 to 20700 (corresponding to helicase to nsp16), SARSr-Rh-BatCoV Rf1 was clustered with strain 279/04 with a bootstrap value of 1,000 away from civet SARSr-CoV. From positions 20700 to 25000 (corresponding to nsp16 to S2), it exhibited a distant relationship with both other SARSr-Rh-BatCoVs and civet SARSr-CoV. However, from position 25000 (S2) onwards, it was more closely related to civet and human SARSr-CoV strains than to other SARSr-Rh-BatCoV. Similar results were also observed when similar analysis was performed using strains 273/04, 279/04, and Rm1 as the query sequence with corresponding strains as the potential parents.
FIG. 4.
(A) Bootscan analysis using the genome sequence of SARSr-Rh-BatCoV strain Rf1 as the query sequence (A) and phylogenetic analysis of its partial sequences to the corresponding regions in other SARSr-CoVs (indicated by the letters B to D above the graph). Bootscanning was conducted with Simplot version 3.5.1 (F84 model; window size, 1,500 bp; step size, 300 bp) on a gapless nucleotide alignment, generated with ClustalX. SARSr-Rh-BatCoV strain 279/04 (279), civet SARSr-CoV strain SZ3, and SARSr-Rh-BatCoV strain HKU3-1 were examined by bootscan analysis. (B to D) Phylogenetic trees were constructed for the regions corresponding to positions 16400 to 20700 (B), 20700 to 25000 (C), and 25000 to 3′ end (D) by the neighbor-joining method using Kimura's two-parameter correction, and bootstrap values were calculated from 1,000 trees. Shaded strains represent strains included in bootscan analysis. Hel, helicase. Bars, 0.01 nucleotide substitution (B and C) or 0.005 nucleotide substitution (D).
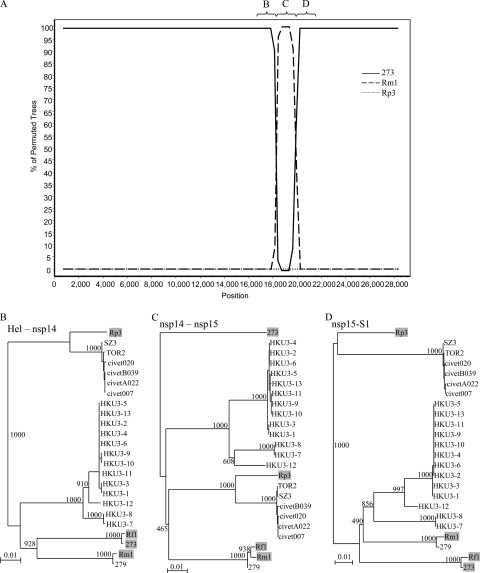
On the other hand, when SARSr-Rh-BatCoV strain Rf1 was used as the query sequence with strains 273/04, Rm1, and Rp3 as the potential parents, a single recombination breakpoint from position 18300 to 19900 corresponding to nsp14/15 (Fig. 5A) was observed. Before position 18300 and after position 19900, high bootstrap support for clustering between strains Rf1 and 273/04 was observed, whereas between these two positions, an abrupt shift in phylogenetic signals occurred, with high bootstrap support for clustering with strain Rm1. In fact, from phylogenetic analysis of other regions of the whole genome, strain Rf1 is closely related to 273/04, and SARSr-Rh-BatCoV Rm1 is closely related to SARSr-Rh-BatCoV 279/04, except in this breakpoint region, where discordance of phylogenetic positions was observed (Fig. 5B). These results suggest that SARSr-Rh-BatCoV strains from different bat species may undergo frequent recombination.
FIG. 5.
(A) Bootscan analysis using the genome sequence of SARSr-Rh-BatCoV strain Rf1 as the query sequence (A) and phylogenetic analysis of its partial sequences to the corresponding regions in other SARSr-CoVs (indicated by the letters B to D above the graph). Bootscanning was conducted with Simplot version 3.5.1 (F84 model; window size, 1500 bp; step size, 300 bp) on a gapless nucleotide alignment, generated with ClustalX. SARSr-Rh-BatCoV strain Rm1, SARSr-Rh-BatCoV strain 273/04 (273), and SARSr-Rh-BatCoV strain Rp3 were examined by bootscan analysis. (B to D) Phylogenetic trees were constructed for the regions before position 18300 (B), positions 18300 to 19900 (C), and after position 19900 (D) by the neighbor-joining method using Kimura's two-parameter correction, and bootstrap values were calculated from 1,000 trees. Shaded strains represent strains included in bootscan analysis. Bar, 0.01 nucleotide substitution.
Estimation of synonymous and nonsynonymous substitution rates.
Using all available SARSr-Rh-BatCoV genome sequences for analysis, except for strain HKU3-8 not used for ORF8 analysis, the Ka/Ks ratios for the various coding regions, compared to those of civet SARSr-CoV and human SARSr-CoV, are shown in Table 1. Notably, the Ka/Ks ratio for the S of SARSr-Rh-BatCoV is only 0.054, compared to that of civet SARSr-CoV (1.5) and human SARSr-CoV (1.0), suggesting that the spike gene of SARSr-Rh-BatCoV is unlikely under positive selection. Moreover, the Ka/Ks ratio for ORF3a, E, and M of SARSr-Rh-BatCoV were also markedly lower than that for civet and/or human SARSr-CoV. On the other hand, the highest Ka/Ks ratios were observed at ORF7b (0.546) and ORF8 (0.554), suggesting that this region is under strong positive selection.
TABLE 1.
Nonsynonymous and synonymous substitutions in the coding regions of SARSr-Rh-BatCoV, civet SARSr-CoV, and human SARSr-CoV genomesa
| Gene | SARSr-Rh-BatCoV |
Civet SARSr-CoV |
Human SARSr-CoV |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Ka | Ks | Ka/Ks ratio | Ka | Ks | Ka/Ks ratio | Ka | Ks | Ka/Ks ratio | |
| ORF1 | 0.012 | 0.259 | 0.046 | 0.001 | 0.002 | 0.500 | 0.000 | 0.001 | 0.000 |
| ORF3a | 0.029 | 0.181 | 0.160 | 0.004 | 0.002 | 2.000 | 0.005 | 0.004 | 1.250 |
| 0.005 | 0.008 | 0.625 | |||||||
| S | 0.023 | 0.428 | 0.054 | 0.003 | 0.002 | 1.500 | 0.002 | 0.002 | 1.000 |
| E | 0.007 | 0.046 | 0.152 | 0.008 | 0.004 | 2.000 | |||
| M | 0.006 | 0.110 | 0.055 | 0.001 | 0.007 | 0.143 | 0.005 | 0.003 | 1.667 |
| ORF6 | 0.011 | 0.073 | 0.151 | 0.007 | 0 | 0.005 | 0.011 | 0.455 | |
| ORF7a | 0.013 | 0.177 | 0.073 | 0.001 | 0.019 | 0.053 | 0.003 | 0.012 | 0.250 |
| ORF7b | 0.100 | 0.183 | 0.546 | 0.010 | 0.000 | ||||
| ORF8 | 0.215 | 0.388 | 0.554 | 0.007 | |||||
| ORF8a | 0.013 | 0.019 | 0.684 | ||||||
| ORF8b | 0.003 | 0.000 | |||||||
| N | 0.011 | 0.106 | 0.104 | 0.001 | 0.007 | 0.143 | 0.002 | 0.003 | 0.667 |
The number of synonymous substitutions per synonymous site (Ks), the number of nonsynonymous substitutions per nonsynonymous site (Ka), and the Ka/Ks ratio for each coding region were calculated for all available SARSr-Rh-BatCoV, civet SARSr-CoV, and human SARSr-CoV genomes.
Estimation of divergence dates.
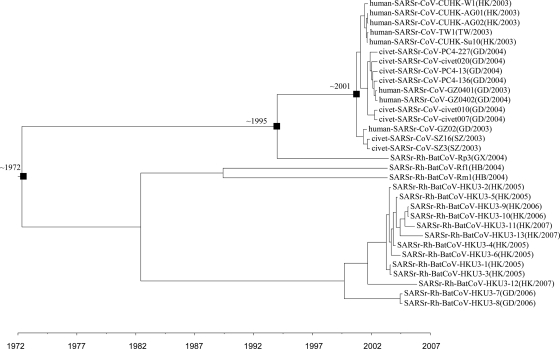
Using the uncorrelated relaxed clock model on ORF1ab, the date of the most recent common ancestor (MRCA) of all SARSr-CoVs was estimated to be 1972.39 (HPDs, 1935.28 to 1990.63), approximately 31 years before the SARS epidemic (Fig. 6). The date of divergence between human or civet SARSr-CoV and the closest SARSr-Rh-BatCoV was estimated to be 1995.10 (HPDs, 1986.53 to 2000.13), approximately 8 years before the SARS epidemic. Moreover, the MRCA date of human and civet SARSr-CoV was estimated to be 2001.36 (HPDs, 1999.16 to 2002.14). The estimated mean substitution rate of the ORF1ab data set under the UCED model was 2.82 ×10−3 substitution per site per year. This estimate is comparable to a previous estimation using fewer SARSr-Rh-BatCoV genome sequences (2.79 × 10−3 substitution per site per year) and the estimate in other RNA viruses (13, 15).
FIG. 6.
Estimation of the time of interspecies transmission of SARSr-CoV. Squares denote the MRCA of all SARSr-CoV (1972), the MRCA of human/civet SARSr-CoV and the closest SARSr-Rh-BatCoV (1995), and the MRCA of human and civet SARSr-CoV (2001), respectively.
DISCUSSION
This is the first report on the migration pattern of horseshoe bats in China and its relation to the epidemiology of coronaviruses. In this study, SARSr-Rh-BatCoV was found among 9.4% and 6.3% of alimentary specimens from Chinese horseshoe bats from Hong Kong and Guangdong, China, respectively, with some bats coinfected with a group 1 coronavirus, Rh-BatCoV HKU2. Both viruses showed peak activity during spring, with an apparent alternate biennial activity. Mating and feeding activity soon after hibernation in spring may have facilitated the spread of the virus within the same roost and from roost to roost. Although no disease association could be observed, lower body weights were observed for bats positive for SARSr-Rh-BatCoV (but not for bats positive for Rh-BatCoV HKU2) than those negative for coronaviruses. The results of a tagging exercise showed that long-distance migration of Chinese horseshoe bats is uncommon, with the longest distance being 17 km from a northern location in fall to an eastern location in winter, compatible with data from other Rhinolophus species which may migrate up to 30 km for hibernation (28, 29). Nevertheless, such migration distances are sufficient for migration between Hong Kong and many areas in Shenzhen, China, including the wild animal markets where the first civet SARSr-CoV was identified (Fig. 1). Such foraging ranges could have allowed for mixing of different SARSr-Rh-BatCoV strains of different geographical origins. Except for one bat which carried SARSr-Rh-BatCoV for at least 2 weeks, all bats positive for coronavirus were cleared of the same virus during recapture. Moreover, tagged individuals positive for SARSr-Rh-BatCoV were healthy during subsequent recapture, evidencing survival after the viral infection as reported for European bat lyssavirus in meridional serotine bats from Spain (44). The present findings suggest that SARSr-Rh-BatCoV causes an acute, self-limiting infection associated with weight loss in Chinese horseshoe bats, with viral clearance occurring between 2 weeks to 4 months. This is compatible with our previous finding that the presence of neutralizing antibody in their sera correlated with a lower viral load in alimentary specimens (21).
The present study revealed that recombination events are common between different SARSr-Rh-BatCoV strains from different species of bats and geographical locations, which may account for the emergence of a civet SARSr-CoV capable of cross-species transmission from bats to civets and from civets to humans. Genome sequence comparison of SARSr-Rh-BatCoVs from horseshoe bats and human/civet SARSr-CoV showed that they shared only 87 to 92% nucleotide identity. Therefore, genetic events, such as mutation and/or recombination, would have occurred during the evolution of these SARSr-CoVs before the possible emergence of direct progenitors of SARSr-CoV capable of infecting palm civets and subsequently humans. Reconstruction of chimeric recombinant SARSr-CoV using the S sequences of various SARSr-Rh-BatCoV found in horseshoe bats will reveal if any particular part of the S sequence is important for infection of civets and/or humans (37). In the present study, frequent recombination events were identified among SARSr-CoVs. Moreover, civet SARSr-CoV strain SZ3 was shown to be a potential recombinant of SARSr-Rh-BatCoV strain Rp3 from Guangxi Province, China, and SARSr-Rh-BatCoV strain Rf1 from Hubei Province, China, by both phylogenetic and bootscan analyses, with the recombination breakpoint identified at the nsp16/S intergenic region. This suggests that civet SARSr-CoV may have either evolved from an ancestor that is a direct recombinant between strains Rp3 and Rf1 or is a direct recombinant of lineages closely related to Rp3 and Rf1 that are yet to be identified. This finding is in line with a previous study showing that SARSr-Rh-BatCoV Rp3 was a potential recombinant of SARSr-Rh-BatCoV and an unidentified lineage closely related to civet SARSr-CoV (13). However, civet SARSr-CoV was not used as the query sequence for analysis in this study, and no conclusion was drawn on the origin of civet strains. In addition, we also detected other potential recombination events when different SARSr-Rh-BatCoV strains were used as the query sequence for analysis, with the most notable recombination sites found to be located at nsp16 and nsp14/15. We have previously described the first evidence of natural recombination in a human coronavirus, HCoV-HKU1, that led to the generation of different genotypes, which also represents the first report to describe a distribution of the recombination spots in the entire genome of field isolates of a coronavirus (46). Interestingly, in this report, the most significant recombination event was also observed at nsp16. Therefore, the 3′ region of ORF1 and the ORF1/S junction are likely frequent sites of natural recombination in coronaviruses. Alternatively, the apparent hot spot of recombination may be due to the nonviability of the recombinant viruses that cross over at other points. In fact, recombination at the junction of the nonstructural and structural genes essentially defines the evolution of the nidoviruses. In wildlife food markets and restaurants in southern China, a huge variety of animals of different geographical origins are often caged in a crowded environment, which may allow cross-species viral transmission and recombination events (47). Further surveillance studies of different horseshoe bat species from different provinces of China and genome analysis of their SARSr-Rh-BatCoV strains may reveal further evidence for the recombination origin of SARSr-CoV.
With the availability of a larger set of SARSr-Rh-BatCoV genome sequences for analysis, the present study also attempts to more accurately estimate the time of emergence of SARSr-CoV in civets, which was shown to be only 8 years before the SARS epidemic in 2003. Despite the identification of SARSr-Rh-BatCoV in various horseshoe bat species from China, there has not been sufficient evidence to determine if bats are the host for the direct ancestor of civet and human SARSr-CoV. On the basis of the considerable phylogenetic distance between bat and civet/human strains, one study concluded that the current bat strains are unlikely to be the direct ancestor (34). A subsequent study using molecular dating analysis showed that the estimated date of interspecies transmission event from bats to an amplifying host, such as the civet, was 17 years (HPD, 2 to 39 years) before the SARS epidemic (45). It was therefore concluded that there may be a yet unidentified intermediate host between bats and civets or unidentified SARSr-Rh-BatCoV strains that are even more closely related to civet SARSr-CoV. Another study estimated the date of interspecies jumping to be much more recent, approximately 4.08 years (HPD, 1.45 to 8.84 years) before the SARS epidemic (13). Results from the present study are more in line with the latter estimate, with the date of divergence between human/civet and bat strains estimated to be 8 years (HPDs, 2.9 to 16.5 years) before the SARS epidemic. The discrepancies between the different estimated results may be due to the choice of different gene sequences and different number of strains for analysis. The study that concluded a much older date of divergence used only the helicase domain sequence for their analysis, whereas the other study, similar to this study but with fewer strains, used the complete ORF1 sequence for analysis. The availability of sequences of more strains collected over a longer period of time may further improve the accuracy of such estimation. On the other hand, all three studies supported that SARSr-CoV are likely a newly emerged subgroup of Betacoronavirus, with the median date of their MRCA estimated to be from 1961 to 1982 (13, 45). The emergence of diverse virus strains in the different Rhinolophus species within a few decades suggested that this novel group of coronaviruses is rapidly evolving and may easily cross the species barrier.
Comparison of all available complete genome sequences of SARSr-Rh-BatCoVs showed that their genome sequences were closely related with the same genome organization, except for strain HKU3-8 from Guangdong, China, with three small ORFs at ORF8 region instead of a single ORF. In particular, strains from the same geographical location were highly similar. Upon phylogenetic analysis of the individual ORFs, the strains from Hong Kong and Guangdong, Guangxi, and Hubei, China, formed separate clusters for most of the time, although the strains from Hong Kong and Guangdong, China, are more closely related, probably due to the close geographical proximity. However, given the frequent recombination and short genetic distance among the different bat strains, no distinct subgroups can be classified. Similar to previous studies, the most variable regions in the SARSr-Rh-BatCoV genomes were located in S, ORF3, and ORF8 (21, 25). In particular, the frequent deletions in ORF8 region in SARSr-Rh-BatCoV, together with the previously reported 29-bp deletion in human SARSr-CoV, suggested that this is a frequent site for deletions in SARSr-CoV. Moreover, the relatively high Ka/Ks ratios observed at ORF7b and ORF8 further supported that it is a region subject to rapid evolution under strong positive selection. The human SARSr-CoV 7b protein is an integral membrane protein localized in the Golgi apparatus and contributes to virus-induced apoptosis (35, 36). The human SARSr-CoV ORF8a enhances viral replication and induces apoptosis through a mitochondrion-dependent pathway, whereas the longer ORF8 protein of civet and early human isolates is a cleaved protein stable in the endoplasmic reticulum (4). Further studies are required to understand the biological significance of the high mutation rate in this part of the SARSr-CoV genomes.
Supplementary Material
Acknowledgments
We thank Alan Chi-Kong Wong, Siu-Fai Leung, Chik-Chuen Lay, Ping-Man So, and K. F. Chan (Hong Kong Special Administrative Region [HKSAR] Department of Agriculture, Fisheries and Conservation [AFCD]) and Hong Kong Police Force for facilitation and support; Cynthia S. M. Chan and Joseph W. K. So from AFCD; and King-Shun Lo (Laboratory Animal Unit) and Cassius Chan for their excellent technical assistance and collection of animal specimens. We are grateful to the generous support of Carol Yu, Richard Yu, Hui Hoy, and Hui Ming in the genomic sequencing platform.
This work was partly supported by the Research Grant Council Grant (HKU 7687/07M); University Development Fund and Outstanding Young Researcher Award, The University of Hong Kong; The Tung Wah Group of Hospitals Fund for Research in Infectious Diseases; the HKSAR Research Fund for the Control of Infectious Diseases (04050232) of the Health, Welfare and Food Bureau; the Providence Foundation Limited in memory of the late Lui Hac Minh; and Consultancy Service for Enhancing Laboratory Surveillance of Emerging Infectious Disease for the HKSAR Department of Health.
Footnotes
Published ahead of print on 13 January 2010.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Brandão, P. E., K. Scheffer, L. Y. Villarreal, S. Achkar, N. Oliveira Rde, O. Fahl Wde, J. G. Castilho, I. Kotait, and L. J. Richtzenhain. 2008. A coronavirus detected in the vampire bat Desmodus rotundus. Braz. J. Infect. Dis. 12:466-468. [DOI] [PubMed] [Google Scholar]
- 2.Brian, D. A., and R. S. Baric. 2005. Coronavirus genome structure and replication. Curr. Top. Microbiol. Immunol. 287:1-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrington, C. V., J. E. Foster, H. C. Zhu, J. X. Zhang, G. J. Smith, N. Thompson, A. J. Auguste, V. Ramkissoon, A. A. Adesiyun, and Y. Guan. 2008. Detection and phylogenetic analysis of group 1 coronaviruses in South American bats. Emerg. Infect. Dis. 14:1890-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, C. Y., Y. H. Ping, H. C. Lee, K. H. Chen, Y. M. Lee, Y. J. Chan, T. C. Lien, T. S. Jap, C. H. Lin, L. S. Kao, and Y. M. Chen. 2007. Open reading frame 8a of the human severe acute respiratory syndrome coronavirus not only promotes viral replication but also induces apoptosis. J. Infect. Dis. 196:405-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dominguez, S. R., T. J. O'Shea, L. M. Oko, and K. V. Holmes. 2007. Detection of group 1 coronaviruses in bats in North America. Emerg. Infect. Dis. 13:1295-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drosten, C., S. Günther, W. Preiser, S. van der Werf, H. R. Brodt, S. Becker, H. Rabenau, M. Panning, L. Kolesnikova, R. A. Fouchier, A. Berger, A. M. Burguière, J. Cinatl, M. Eickmann, N. Escriou, K. Grywna, S. Kramme, J. C. Manuguerra, S. Müller, V. Rickerts, M. Stürmer, S. Vieth, H. D. Klenk, A. D. Osterhaus, H. Schmitz, and H. W. Doerr. 2003. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 348:1967-1976. [DOI] [PubMed] [Google Scholar]
- 7.Drummond, A. J., and A. Rambaut. 2007. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 7:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fouchier, R. A., N. G. Hartwig, T. M. Bestebroer, B. Niemeyer, J. C. de Jong, J. H. Simon, and A. D. Osterhaus. 2004. A previously undescribed coronavirus associated with respiratory disease in humans. Proc. Natl. Acad. Sci. U. S. A. 101:6212-6216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gloza-Rausch, F., A. Ipsen, A. Seebens, M. Gottsche, M. Panning, J. F. Drexler, N. Petersen, A. Annan, K. Grywna, M. Muller, S. Pfefferle, and C. Drosten. 2008. Detection and prevalence patterns of group I coronaviruses in bats, northern Germany. Emerg. Infect. Dis. 14:626-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorbalenya, A. E., E. J. Snijder, and W. J. Spaan. 2004. Severe acute respiratory syndrome coronavirus phylogeny: toward consensus. J. Virol. 78:7863-7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan, Y., B. J. Zheng, Y. Q. He, X. L. Liu, Z. X. Zhuang, C. L. Cheung, S. W. Luo, P. H. Li, L. J. Zhang, Y. J. Guan, K. M. Butt, K. L. Wong, K. W. Chan, W. Lim, K. F. Shortridge, K. Y. Yuen, J. S. Peiris, and L. L. Poon. 2003. Isolation and characterization of viruses related to the SARS coronavirus from animals in southern China. Science 302:276-278. [DOI] [PubMed] [Google Scholar]
- 12.Herrewegh, A. A., I. Smeenk, M. C. Horzinek, P. J. Rottier, and R. J. de Groot. 1998. Feline coronavirus type II strains 79-1683 and 79-1146 originate from a double recombination between feline coronavirus type I and canine coronavirus. J. Virol. 72:4508-4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hon, C. C., T. Y. Lam, Z. L. Shi, A. J. Drummond, C. W. Yip, F. Zeng, P. Y. Lam, and F. C. Leung. 2008. Evidence of the recombinant origin of a bat severe acute respiratory syndrome (SARS)-like coronavirus and its implications on the direct ancestor of SARS coronavirus. J. Virol. 82:1819-1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang, Y., S. K. Lau, P. C. Woo, and K. Y. Yuen. 2008. CoVDB: a comprehensive database for comparative analysis of coronavirus genes and genomes. Nucleic Acids Res. 36:D504-D511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jenkins, G. M., A. Rambaut, O. G. Pybus, and E. C. Holmes. 2002. Rates of molecular evolution in RNA viruses: a quantitative phylogenetic analysis. J. Mol. Evol. 54:156-165. [DOI] [PubMed] [Google Scholar]
- 16.Keck, J. G., G. K. Matsushima, S. Makino, J. O. Fleming, D. M. Vannier, S. A. Stohlman, and M. M. Lai. 1988. In vivo RNA-RNA recombination of coronavirus in mouse brain. J. Virol. 62:1810-1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kottier, S. A., D. Cavanagh, and P. Britton. 1995. Experimental evidence of recombination in coronavirus infectious bronchitis virus. Virology 213:569-580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ksiazek, T. G., D. Erdman, C. S. Goldsmith, S. R. Zaki, T. Peret, S. Emery, S. Tong, C. Urbani, J. A. Comer, W. Lim, P. E. Rollin, S. F. Dowell, A. E. Ling, C. D. Humphrey, W. J. Shieh, J. Guarner, C. D. Paddock, P. Rota, B. Fields, J. DeRisi, J. Y. Yang, N. Cox, J. M. Hughes, J. W. LeDuc, W. J. Bellini, L. J. Anderson, and SARS Working Group. 2003. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 348:1953-1966. [DOI] [PubMed] [Google Scholar]
- 19.Kumar, S., K. Tamura, and M. Nei. 2004. MEGA3: integrated software for Molecular Evolutionary Genetics Analysis and sequence alignment. Brief. Bioinform. 5:150-163. [DOI] [PubMed] [Google Scholar]
- 20.Lai, M. M., and D. Cavanagh. 1997. The molecular biology of coronaviruses. Adv. Virus Res. 48:1-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lau, S. K., P. C. Woo, K. S. Li, Y. Huang, H. W. Tsoi, B. H. Wong, S. S. Wong, S. Y. Leung, K. H. Chan, and K. Y. Yuen. 2005. Severe acute respiratory syndrome coronavirus-like virus in Chinese horseshoe bats. Proc. Natl. Acad. Sci. U. S. A. 102:14040-14045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lau, S. K., P. C. Woo, K. S. Li, Y. Huang, M. Wang, C. S. Lam, H. Xu, R. Guo, K. H. Chan, B. J. Zheng, and K. Y. Yuen. 2007. Complete genome sequence of bat coronavirus HKU2 from Chinese horseshoe bats revealed a much smaller spike gene with a different evolutionary lineage from the rest of the genome. Virology 367:428-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lavi, E., J. A. Haluskey, and P. S. Masters. 1998. The pathogenesis of MHV nucleocapsid gene chimeric viruses. Adv. Exp. Med. Biol. 440:537-541. [DOI] [PubMed] [Google Scholar]
- 24.Li, W., C. Zhang, J. Sui, J. H. Kuhn, M. J. Moore, S. Luo, S. K. Wong, I. C. Huang, K. Xu, N. Vasilieva, A. Murakami, Y. He, W. A. Marasco, Y. Guan, H. Choe, and M. Farzan. 2005. Receptor and viral determinants of SARS-coronavirus adaptation to human ACE2. EMBO J. 24:1634-1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li, W., Z. Shi, M. Yu, W. Ren, C. Smith, J. H. Epstein, H. Wang, G. Crameri, Z. Hu, H. Zhang, J. Zhang, J. McEachern, H. Field, P. Daszak, B. T. Eaton, S. Zhang, and L. F. Wang. 2005. Bats are natural reservoirs of SARS-like coronaviruses. Science 310:676-679. [DOI] [PubMed] [Google Scholar]
- 26.Lole, K. S., R. C. Bollinger, R. S. Paranjape, D. Gadkari, S. S. Kulkarni, N. G. Novak, R. Ingersoll, H. W. Sheppard, and S. C. Ray. 1999. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 73:152-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muller, M. A., J. T. Paweska, P. A. Leman, C. Drosten, K. Grywna, A. Kemp, L. Braack, K. Sonnenberg, M. Niedrig, and R. Swanepoel. 2007. Coronavirus antibodies in African bat species. Emerg. Infect. Dis. 13:1367-1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neuweiler, G. 2000. The biology of bats, p. 266. Oxford University Press, New York, NY.
- 29.Nowak, R. M., and J. L. Paradiso. 1983. Walker's mammals of the world, p. 230. The Johns Hopkins University Press, Baltimore, MD.
- 30.Oostra, M., C. A. de Haan, and P. J. Rottier. 2007. The 29-nucleotide deletion present in human but not in animal severe acute respiratory syndrome coronaviruses disrupts the functional expression of open reading frame 8. J. Virol. 81:13876-13888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peiris, J. S., S. T. Lai, L. L. Poon, Y. Guan, L. Y. Yam, W. Lim, J. Nicholls, W. K. Yee, W. W. Yan, M. T. Cheung, V. C. Cheng, K. H. Chan, D. N. Tsang, R. W. Yung, T. K. Ng, and K. Y. Yuen. 2003. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet 361:1319-1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pfefferle, S., S. Oppong, J. F. Drexler, F. Gloza-Rausch, A. Ipsen, A. Seebens, M. A. Muller, A. Annan, P. Vallo, Y. Adu-Sarkodie, T. F. Kruppa, and C. Drosten. 2009. Distant relatives of severe acute respiratory syndrome coronavirus and close relatives of human coronavirus 229E in bats, Ghana. Emerg. Infect. Dis. 15:1377-1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poon, L. L., D. K. Chu, K. H. Chan, O. K. Wong, T. M. Ellis, Y. H. Leung, S. K. Lau, P. C. Woo, K. Y. Suen, K. Y. Yuen, Y. Guan, and J. S. Peiris. 2005. Identification of a novel coronavirus in bats. J. Virol. 79:2001-2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ren, W., W. Li, M. Yu, P. Hao, Y. Zhang, P. Zhou, S. Zhang, G. Zhao, Y. Zhong, S. Wang, L. F. Wang, and Z. Shi. 2006. Full-length genome sequences of two SARS-like coronaviruses in horseshoe bats and genetic variation analysis. J. Gen. Virol. 87:3355-3359. [DOI] [PubMed] [Google Scholar]
- 35.Schaecher, S. R., E. Touchette, J. Schriewer, R. M. Buller, and A. Pekosz. 2007. Severe acute respiratory syndrome coronavirus gene 7 products contribute to virus-induced apoptosis. J. Virol. 81:11054-11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schaecher, S. R., M. S. Diamond, and A. Pekosz. 2008. The transmembrane domain of the severe acute respiratory syndrome coronavirus ORF7b protein is necessary and sufficient for its retention in the Golgi complex. J. Virol. 82:9477-9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheahan, T., B. Rockx, E. Donaldson, D. Corti, and R. Baric. 2008. Pathways of cross-species transmission of synthetically reconstructed zoonotic severe acute respiratory syndrome coronavirus. J. Virol. 82:8721-8732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snijder, E. J., P. J. Bredenbeek, J. C. Dobbe, V. Thiel, J. Ziebuhr, L. L. Poon, Y. Guan, M. Rozanov, W. J. Spaan, and A. E. Gorbalenya. 2003. Unique and conserved features of genome and proteome of SARS-coronavirus, an early split-off from the coronavirus group 2 lineage. J. Mol. Biol. 331:991-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Song, H. D., C. C. Tu, G. W. Zhang, S. Y. Wang, K. Zheng, L. C. Lei, Q. X. Chen, Y. W. Gao, H. Q. Zhou, H. Xiang, H. J. Zheng, S. W. Chern, F. Cheng, C. M. Pan, H. Xuan, S. J. Chen, H. M. Luo, D. H. Zhou, Y. F. Liu, J. F. He, P. Z. Qin, L. H. Li, Y. Q. Ren, W. J. Liang, Y. D. Yu, L. Anderson, M. Wang, R. H. Xu, X. W. Wu, H. Y. Zheng, J. D. Chen, G. Liang, Y. Gao, M. Liao, L. Fang, L. Y. Jiang, H. Li, F. Chen, B. Di, L. J. He, J. Y. Lin, S. Tong, X. Kong, L. Du, P. Hao, H. Tang, A. Bernini, X. J. Yu, O. Spiga, Z. M. Guo, H. Y. Pan, W. Z. He, J. C. Manuguerra, A. Fontanet, A. Danchin, N. Niccolai, Y. X. Li, C. I. Wu, and G. P. Zhao. 2005. Cross-host evolution of severe acute respiratory syndrome coronavirus in palm civet and human. Proc. Natl. Acad. Sci. U. S. A. 102:2430-2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tang, X. C., J. X. Zhang, S. Y. Zhang, P. Wang, X. H. Fan, L. F. Li, G. Li, B. Q. Dong, W. Liu, C. L. Cheung, K. M. Xu, W. J. Song, D. Vijaykrishna, L. L. Poon, J. S. Peiris, G. J. Smith, H. Chen, and Y. Guan. 2006. Prevalence and genetic diversity of coronaviruses in bats from China. J. Virol. 80:7481-7490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tong, S., C. Conrardy, S. Ruone, I. V. Kuzmin, X. Guo, Y. Tao, M. Niezgoda, L. Haynes, B. Agwand, R. F. Breiman, L. J. Anderson, and C. E. Rupprecht. 2009. Detection of novel SARS-like and other coronaviruses in bats from Kenya. Emerg. Infect. Dis. 15:482-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tu, C., G. Crameri, X. Kong, J. Chen, Y. Sun, M. Yu, H. Xiang, X. Xia, S. Liu, T. Ren, Y. Yu, B. T. Eaton, H. Xuan, and L. F. Wang. 2004. Antibodies to SARS coronavirus in civets. Emerg. Infect. Dis. 10:2244-2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Hoek, L., K. Pyrc, M. F. Jebbink, W. Vermeulen-Oost, R. J. Berkhout, K. C. Wolthers, P. M. Wertheim-van Dillen, J. Kaandorp, J. Spaargaren, and B. Berkhout. 2004. Identification of a new human coronavirus. Nat. Med. 10:368-373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vázquez-Morón, S., J. Juste, C. Ibáñez, E. Ruiz-Villamor, A. Avellón, M. Vera, and J. E. Echevarría. 2008. Endemic circulation of European bat lyssavirus type 1 in serotine bats, Spain. Emerg. Infect. Dis. 14:1263-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vijaykrishna, D., G. J. Smith, J. X. Zhang, J. S. Peiris, H. Chen, and Y. Guan. 2007. Evolutionary insights into the ecology of coronaviruses. J. Virol. 81:4012-4020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woo, P. C., M. Wang, S. K. Lau, H. Xu, R. W. Poon, R. Guo, B. H. Wong, K. Gao, H. W. Tsoi, Y. Huang, K. S. Li, C. S. Lam, K. H. Chan, B. J. Zheng, and K. Y. Yuen. 2007. Comparative analysis of 12 genomes of three novel group 2c and group 2d coronaviruses reveals unique group and subgroup features. J. Virol. 81:1574-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woo, P. C., S. K. Lau, and K. Y. Yuen. 2006. Infectious diseases emerging from Chinese wet-markets: zoonotic origins of severe respiratory viral infections. Curr. Opin. Infect. Dis. 19:401-407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Woo, P. C., S. K. Lau, C. C. Yip, Y. Huang, H. W. Tsoi, K. H. Chan, and K. Y. Yuen. 2006. Comparative analysis of 22 coronavirus HKU1 genomes reveals a novel genotype and evidence of natural recombination in coronavirus HKU1. J. Virol. 80:7136-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Woo, P. C., S. K. Lau, C. M. Chu, K. H. Chan, H. W. Tsoi, Y. Huang, B. H. Wong, R. W. Poon, J. J. Cai, W. K. Luk, L. L. Poon, S. S. Wong, Y. Guan, J. S. Peiris, and K. Y. Yuen. 2005. Characterization and complete genome sequence of a novel coronavirus, coronavirus HKU1, from patients with pneumonia. J. Virol. 79:884-895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Woo, P. C., S. K. Lau, H. W. Tsoi, Y. Huang, R. W. Poon, C. M. Chu, R. A. Lee, W. K. Luk, G. K. Wong, B. H. Wong, V. C. Cheng, B. S. Tang, A. K. Wu, R. W. Yung, H. Chen, Y. Guan, K. H. Chan, and K. Y. Yuen. 2005. Clinical and molecular epidemiological features of coronavirus HKU1-associated community-acquired pneumonia. J. Infect. Dis. 192:1898-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Woo, P. C., S. K. Lau, K. S. Li, R. W. Poon, B. H. Wong, H. W. Tsoi, B. C. Yip, Y. Huang, K. H. Chan, and K. Y. Yuen. 2006. Molecular diversity of coronaviruses in bats. Virology 351:180-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yang, Z. Y., H. C. Werner, W. P. Kong, K. Leung, E. Traggiai, A. Lanzavecchia, and G. J. Nabel. 2005. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl. Acad. Sci. U. S. A. 102:797-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yob, J. M., H. Field, A. M. Rashdi, C. Morrissy, B. van der Heide, P. Rota, A. bin Adzhar, J. White, P. Daniels, A. Jamaluddin, and T. Ksiazek. 2001. Nipah virus infection in bats (order Chiroptera) in peninsular Malaysia. Emerg. Infect. Dis. 7:439-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ziebuhr, J. 2004. Molecular biology of severe acute respiratory syndrome coronavirus. Curr. Opin. Microbiol. 7:412-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.