Abstract
Prophylactic or therapeutic immunomodulation is an antigen-independent strategy that induces nonspecific immune system activation, thereby enhancing host defense to disease. In this study, we investigated the effect of prophylactic immunomodulation on the outcome of influenza virus infection using three bacterially derived immune-enhancing agents known for promoting distinct immunological profiles. BALB/c mice were treated nasally with either cholera toxin (CT), a mutant form of the CT-related Escherichia coli heat-labile enterotoxin designated LT(R192G), or CpG oligodeoxynucleotide. Mice were subsequently challenged with a lethal dose of influenza A/PR/8/34 virus 24 h after the last immunomodulation treatment and either monitored for survival or sacrificed postchallenge for viral and immunological analysis. Treatment with the three immunomodulators prevented or delayed mortality and weight loss, but only CT and LT(R192G) significantly reduced initial lung viral loads as measured by plaque assay. Analysis performed 4 days postinfection indicated that prophylactic treatments with CT, LT(R192G), or CpG resulted in significantly increased numbers of CD4 T cells, B cells, and dendritic cells and altered costimulatory marker expression in the airways of infected mice, coinciding with reduced expression of pulmonary chemokines and the appearance of inducible bronchus-associated lymphoid tissue-like structures in the lungs. Collectively, these results suggest that, despite different immunomodulatory mechanisms, CT, LT(R192G), and CpG induce an initial inflammatory process and enhance the immune response to primary influenza virus challenge while preventing potentially damaging chemokine expression. These studies provide insight into the immunological parameters and immune modulation strategies that have the potential to enhance the nonspecific host response to influenza virus infection.
Influenza viruses cause acute, contagious respiratory disease. Despite the availability of vaccines and antiviral therapies, influenza virus infections cause considerable morbidity and mortality each year. It is estimated that during seasonal epidemics 10% of the world population is infected, resulting in 2 to 3 million severe cases and up to 500,000 deaths (1). The failure of conventional methods to prevent illness and death from influenza is attributed to the continuous antigenic variability of the virus due to mutations (antigen drift) and reassortments (antigenic shift). The inadequacy of current anti-influenza virus treatments is particularly concerning in the case of influenza pandemics with new viral strains for which effective vaccines would not be initially available. Thus, an antigen-independent prophylactic treatment that could nonspecifically enhance immune responses to negate or inhibit the progression of influenza virus infection would provide invaluable benefits.
Several recent studies have explored the use of immunomodulation strategies as prophylaxis or therapeutic treatments to modify the immune response to influenza virus infection, thereby preventing or decreasing viral burden, disease symptoms, and mortality. These strategies have one of two distinct immunologic goals: either to increase immune system activation and/or Th1 responses specific against influenza virus, or alternatively, decrease inflammation and immunopathology. The first strategy has been demonstrated in animal models by administering host proteins/glycoproteins that function in immune defense, such as the pattern recognition receptor (PRR) mindin (28), milk-derived glycoproteins (61), and virally delivered interferon (IFN) cytokines (27). Immunomodifiers of microbial origin have also been used to enhance host response to infection, including the binding subunit of cholera toxin (CT-B) (49), Th1-promoting Toll-like receptor (TLR) agonists CpG oligodeoxynucleotides (ODN) (15, 82), poly(I:C) (81), 3 M-011 (23), and synthetic lipid A analogs (11). Immunomodulators used in the second strategy, with the aim to prevent detrimental inflammation, have been associated with improved infection outcomes and include enterotoxin mutant LT(S63K) (80) and anti-inflammatory COX-2 inhibitors (84). However, immunomodulation does not always result in beneficial responses to infection. Administration of Δ9-THC, an immunosuppressive compound, decreased cellular infiltration and increased viral load when given prior to and during influenza virus infection (7). Similarly, sphingosine 1-phosphate (S1P) analog, an immunotherapeutic agent, was found to suppress induction of T-cell responses to influenza virus (46). Lastly, fish oil-fed mice demonstrated reduced lung inflammation, cellular infiltration, and cytokine secretion but increased mortality during influenza virus infection (60).
These studies highlight the need for experiments that clarify the consequences of various immunomodulation strategies on influenza virus infection and the particular requirements for generating a protective response. Furthermore, very little attention has been given to the mechanisms by which different immunomodulators with unique effector functions modulate the host response when evaluated in the same infection model. To address these questions and increase our understanding of the consequences brought about by prophylactic immunomodulation in pulmonary disease, we chose to compare the effects of pulmonary delivery of three well-characterized vaccine adjuvants on the outcome of influenza virus infection in a murine model. The immunomodulators used in this study are CpG, a nontoxic protein designated LT(R192G) that was derived from the cholera-related heat-labile enterotoxin produced by Escherichia coli, and CT. These bacterially derived agents, known to promote distinct effector functions, are excellent immunomodulators, as they induce strong immune activation and have been previously evaluated as components of influenza vaccines (29, 42, 49, 53, 56, 58). CpG ODNs are synthetic unmethylated oligodeoxynucleotides containing CpG motifs that trigger a TLR9-dependent MyD88 signaling pathway. CpG treatment results in potent Th1 cytokine expression (IFNs and interleukin-12 [IL-12]), activation of dendritic cells (DCs), NK cells, and B cells, and induction of Th1 cells and a Th1 antibody profile (30, 35, 83). CpG has been extensively studied in animal models of systemic and pulmonary infectious diseases caused by influenza virus (15, 82) and other bacterial, fungal, and parasitic pathogens (3, 9, 15, 17, 25, 34, 51, 77).
Bacterially derived ADP-ribosylating enterotoxins, including CT from Vibrio cholerae and LT from E. coli, are robust systemic and mucosal adjuvants. Both in vitro and in vivo studies have demonstrated that CT induces secretion of Th2 cytokines (IL-4, IL-5, IL-6, and IL-10) by immune system cells, maturation of DCs, generation of Th2 and T-regulatory cells, and active suppression of Th1 responses (2, 32, 38, 39, 47, 49, 53, 56). Studies in vivo have also shown that intranasal delivery of CT-B, the binding subunit of the enterotoxin, combined with minimal levels of CT holotoxin, induces protective effects in influenza virus-infected mice (49). In contrast to CpG and CT, LT and LT(R192G) induce a more balanced cytokine and antibody subclass profile indicative of a mixed Th1/Th2 immune response (16, 45, 73). LT(R192G) has yet to be evaluated as a prophylactic immunomodulator, but another LT mutant, LT(S63K), has demonstrated some protective effects against influenza virus, respiratory syncytial virus (RSV), and Cryptococcus neoformans infections (80). Although safety concerns limit the use of native enterotoxins for intranasal or intrapulmonary use in humans (54, 76), animal model studies are warranted because they enhance our understanding of the initial responses that can ultimately lead to protection of the host against infection. In addition, the use of these enterotoxins in laboratory research has the potential to be translated into clinical application by using mutated low-toxinogenic derivatives that retain their immunomodulatory properties.
In this study we used a comprehensive approach to evaluate the effects of intrapulmonary delivery of three strong immunomodulators prior to influenza virus infection in a murine model. We hypothesized that the unique immunologic effects induced by prophylactic treatment with CT, LT(R192G), or CpG would differentially affect survival, viral loads, and immune responses of BALB/c mice to influenza A/PR/8/34 (H1N1) virus infection. The relevance of this study to influenza virus disease pathogenesis and infectious disease immunomodulation strategies is discussed.
MATERIALS AND METHODS
Immunomodulators and virus.
Mouse-passaged influenza A/PR/8/34 (H1N1) virus was propagated in the allantoic cavity of 10-day-old specific-pathogen-free embryonated chicken eggs. Infected allantoic fluid was pooled, clarified by centrifugation, and kept frozen at −80°C until use in challenge studies. The immunomodulators LT(R192G) and CT were prepared by using galactose affinity chromatography in our laboratory as described previously (4, 8, 10). Briefly, toxins were purified from cultures grown overnight in a 10-liter fermentor. Cells were harvested by centrifugation and lysed in a microfluidizer M-110L (Microfluidics, Newton, MA). The cell lysate was dialyzed overnight in TEAN (0.2 M NaCl, 0.05 M Tris, 0.001 M EDTA, 0.003 M NaN3, pH 7.5), clarified by centrifugation, and subjected to chromatography on separate immobilized d-galactose columns (Pierce, Rockford, IL). Toxins were eluted with 0.3 M galactose in TEAN and passed through an endotoxin removal column (Pierce, Rockford, IL). The composition and purity of each protein was checked by SDS-PAGE and a Limulus amebocyte lysate assay (Lonza Inc., Walkersville, MD). CpG ODN 1826 (CpG) obtained from Coley Pharmaceuticals (Wellesley, MA) has a mouse-specific, nuclease-resistant phosphorothioate backbone with the sequence 5′-TCCATGACGTTCCTGACGTT-3′.
Treatment, infection, and tissue harvest.
Six- to eight-week-old female BALB/c mice were purchased from Charles River and housed in filter-top cages. All mouse studies were approved by the Tulane University Institutional Animal Care and Use Committee. Animals were treated once or twice with immunomodulators by nasal administration. For these studies, mice were anesthetized with ketamine-xylazine and suspended on a wire by their front teeth while 30 μl of a saline solution containing 5 μg of CT, LT(R192G), or CPG ODN was instilled into their nares. Control animals were treated with the same volume of saline or left untreated. Ten minutes after the treatment, animals were returned to their cages and observed until recovered from anesthesia. Twenty-four hours after receiving the last immunomodulator treatment, mice were either sacrificed and tissues collected for analysis or they were infected by nasal application of 30 μl containing 200 PFU/ml of influenza A/PR/8/34 virus. For survival studies, groups of 10 mice were treated with the immunomodulators, infected with influenza virus, and observed daily for signs of illness or mortality, and individual body weights were recorded as indicated. Moribund mice were humanely euthanized. Surviving mice were euthanized on day 21 postchallenge and their lungs were processed for analysis of viral burden and histology. For short-term studies, groups of five mice were treated and infected as described above and then euthanized for tissue collection 4 or 6 days after infection. The left lung lobe was removed to use in plaque assay or histological analyses while the rest of the lung was lavaged to recover lung airway cells. Briefly, the trachea was surgically exposed and bronchoalveolar lavage (BAL) was performed through a catheter delivering 0.8 ml of saline supplemented with a protease inhibitor cocktail (Roche, Mannheim, Germany). The BAL supernatant was aliquoted and frozen for cytokine analysis, while collected BAL cells were pelleted, counted, and used for cellular analysis by microscopy and flow cytometry.
Cytokine analysis.
Frozen BAL fluid samples were thawed and immediately analyzed using either a 23-plex mouse cytokine assay (Bio-Rad, Hercules, CA) with a Bioplex 200 array reader (Bio-Rad) or IFN-α and IFN-β enzyme-linked immunosorbent assay (ELISA) kits (PBL Biomedical Laboratories, New Brunswick, NJ) following the manufacturers' instructions.
Cytospin and flow cytometry.
A total of 5 × 104 BAL cells were loaded into cytospin funnels (ThermoShandon, Pittsburg, PA), spun onto slides, and stained with Hema3 (Fisher Scientific, Kalamazoo, MI). A coverslip was added with Permount (Fisher Sci, Fairlawn, NJ), and differential cell counts were made microscopically by counting 100 cells per slide. The total numbers of BAL cells was calculated by multiplying the total number of recovered cells by the percentage of each differential cell type. Remaining BAL cell samples were resuspended at 1 × 106 cells/100 μl in flow cytometry buffer (phosphate-buffered saline [PBS] containing 1% bovine serum albumin [BSA], 0.1% NaN3), incubated with Fc Block (BD Biosciences, San Jose, CA), and stained with the following fluorescently labeled antibodies: anti-mouse CD3-Pacific Blue, F4/80-Pacific Blue, CD80-fluorescein isothiocyanate [FITC], CD86-phycoerythrin [PE], CD11b-PE, NK1.1 PE-Cy5, CD19-PE-Cy5.5, CD4-allophycocyanin [APC], CD11c-APC (eBioscience, San Diego, CA), and CD11b-PE-Cy7 (BD Biosciences). Stained cells were run on an LSRII flow cytometer (BD Biosciences) and analyzed with FacsAria software.
Histology.
Dissected lungs lobes were either embedded in 50% OCT (Tissue-Tek, Torrance, CA) and snap-frozen or perfused with 10% buffered formalin (Fisher), formalin fixed, and paraffin embedded. Paraffin blocks were sectioned and stained with hematoxylin and eosin (H&E). OCT-embedded lung sections were sectioned with a cryostat, fixed with 2% formaldehyde (PolySciences, Warrington, PA), and stained for immunohistochemistry with monoclonal antibodies CD4-FITC, CD4-Cy5 (eBioscience), B220-PE, CD8a-PE, CD11c-FITC, CD11b-PE, and CD11b-Cy5 (BD Pharmingen). Briefly, sections were permeabilized with 0.1% Triton X-100 (Sigma) for 20 min and washed three times with Dulbecco's PBS (DPBS)-0.05% Triton. Background fluorescence was reduced by a 15-min incubation with 50 mM NH4Cl in DPBS-Triton, followed by 1 h of incubation with 8% goat serum (Sigma) in DPBS-Triton. Slides were stained with specific antibodies diluted in PBS-Triton-0.8% goat serum before the addition of ProLong Gold AntiFade with 4′,6-diamidino-2-phenylindole (DAPI; Invitrogen). Epifluorescent images were acquired with a Zeiss Axioplan II microscope. Series of horizontal optical sections (0.3 μm each) were collected using Slidebook 4.0 software (Intelligent Imaging Innovations, Denver, Co) and subsequently deconvolved and processed with Volocity 4.4 software (Improvision Inc., Waltham, MA).
Plaque assay.
Dissected lungs were weighed and kept on ice. Lungs were teased apart in 1 ml of PBS-1% BSA supplemented with 100 U/ml antibiotic-antimycotic solution. The obtained lung homogenates were stored at −80°C until used in viral plaque assays. A standard 96-well plaque assay was performed as described previously (48). Briefly, 50 μl of lung homogenate samples or serial dilutions were added to confluent MDCK cells and incubated at 35°C for 1 h, swirling the plate every 15 min. Cells were covered with 100 μl of Avicel-581 overlay (FMC Corp., Newark, DE) containing equal parts 2× minimal essential medium-1% BSA, 1% tosylsulfonyl phenylalanyl chloromethyl ketone-trypsin (Pierce), and 2.4% Avicel-581. After incubation for 24 to 36 h, plates were aspirated, fixed with 4% paraformaldehyde for 30 min at 4°C, and washed with PBS. Cells were permeabilized and background was reduced by a15-min incubation with 50 μl/well PBS-0.5% Triton-20 mM l-glycine. After aspiration, cells were stained with anti-influenza virus A-horseradish peroxidase (1:1,000; Biodesign Int., Saco, ME) in PBS-0.05% Tween 80-8% goat serum for 1 h at room temperature. After three 5-min washes with 100 μl PBS-Tween 80, 50 μl/well of TrueBlue peroxidase substrate (KPL, Gaithersburg, MD) was added until color developed. The reaction was stopped with distilled water and plaques were counted as PFU.
Data analysis.
Statistical analysis was performed using either Prism or SPSS software. Survival analysis was performed using the log-rank test. Correlation analysis was performed with Pearson's two-tailed test. All other analyses were determined by Mann-Whitney U test for unequal variance. A P value of <0.05 was accepted as being significant.
RESULTS
Immunomodulator treatments alter the steady-state immune status of the lung.
Previous published reports indicated that delivery of immunomodulators by the intranasal or intrapulmonary route is a very effective means of inducing local responses for controlling lung infections. Specifically, nasal administration has often proved more successful than subcutaneous, intradermal, or intraperitoneal administration when used to promote protective responses against pulmonary disease (46, 65, 78, 80). Consequently, for our studies we chose to treat and infect animals nasally. In this technique, the administration of 30 to 50 μl of solution or infectious inoculum into the nares of anesthetized, vertically suspended animals allows the solution to reach various internal organs, including the lungs, where it localizes in airway passages and lung parenchyma within minutes of administration (unpublished observations) (79). Preliminary studies in our laboratory demonstrated that either one or two treatments with immunomodulators given 1 week apart result in significant changes to the pulmonary immunological environment in BALB/c mice, including cellular recruitment and altered cytokine expression. These changes peak approximately 24 h after the second treatment and return to normal levels after 2 to 3 weeks (data not shown). Therefore, for all but one of the studies reported here, we infected animals with influenza virus 24 h after the second application of the immunomodulator.
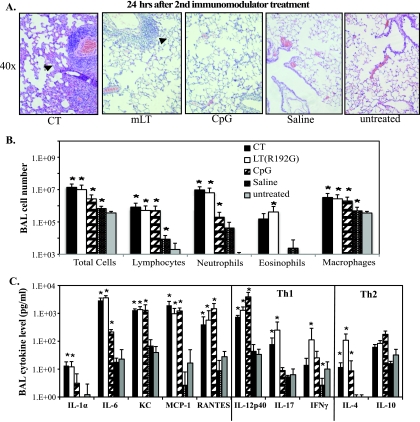
To analyze the host response to immunomodulatory treatments immediately before viral challenge, BALB/c mice were treated as above with 5 μg of either CT, LT(R192G), or CpG, while controls received saline or were left untreated. Animals were sacrificed 24 h later and samples collected for cellular, cytokine, and histological analysis. Treatment with CT induced a significant level of histopathology in the lungs as evidenced by changes in tissue architecture, including inflamed septae, peribronchial hemorrhage, diffuse alveolar damage, and dilated pulmonary blood vessels (Fig. 1A). Lungs of animals treated with LT(R192G) revealed some areas of peribronchial hemorrhage and inflammation, but these changes were more moderate than those observed after CT treatment. By comparison, there were minimal histological changes in the lungs of animals treated with CpG or saline compared with the untreated group (Fig. 1A). Differential leukocyte counts of cells recovered from BAL fluid showed that all immunomodulator treatments significantly increased the number of total airway cells, lymphocytes, neutrophils, and macrophages compared to naive mice (Fig. 1B). CT and LT(R192G) treatments also resulted in increased eosinophils; the increase was significant only for LT(R192G) compared to untreated mice. Surprisingly, treatment with saline also significantly increased the number of total cells, lymphocytes, and macrophages compared to untreated mice (Fig. 1B). Consistent with these cellular changes, all treatments significantly altered levels of several inflammatory cytokines/chemokines present in the BAL fluid (Fig. 1C). Both CT and LT(R912G) treatments increased IL-1α levels (Fig. 1C), as previously reported (5, 62). All immunomodulator treatments significantly increased levels of the pleiotropic inflammatory cytokine IL-6, neutrophil attractant KC (mouse IL-8 homolog; also known as CXCL1), monocyte attractant and NK cell activator MCP-1 (monocyte chemotactic protein-1, or CCL2), and leukocyte attractant, monocyte attractant and NK cell activator RANTES (CCL5). Immunomodulator treatments also altered Th1/Th2 cytokine expression in the BAL fluid. As seen in Fig. 1C, compared to saline or untreated groups, the three immunomodulators significantly increased the antibody-promoting cytokine IL-4, while CT and LT(R192G) increased IL-17 levels and only LT(R192G) significantly increased IFN-γ expression. Saline treatment slightly decreased levels of several chemokines and cytokines (i.e., MCP-1, RANTES, IL-17, IL-10, and IFN-γ), but this was statistically significant only for IFN-γ (Fig. 1C).
FIG. 1.
Nasal immunomodulator treatments alter the steady-state immune status in the lung. BALB/c mice were nasally treated twice, 1 week apart, with 5 μg of CT, LT(R192G), or CpG. Controls received saline or were untreated. Mice were sacrificed 24 h later and samples collected for cellular, cytokine, and histological analyses. (A) Representative H&E-stained lung sections. Magnification, ×40. Arrows indicate areas with inflammatory infiltrates and inflamed septae. (B) Recovered BAL cells were characterized for the number of immune system cells, based on hemacytometer cell counts and cytospin/Hema3 differentiation analysis. (C) BAL inflammatory cytokine and chemokine levels, including Th1/Th2 cytokines, were detected using a Bioplex cytokine assay. Bars represent standard deviations of the means (n ≥ 4), with significance values indicated with an asterisk for P values of <0.05 by Mann-Whitney U test.
Prophylactic treatment with immunomodulators improves survival to influenza virus challenge and differentially affects weight loss.
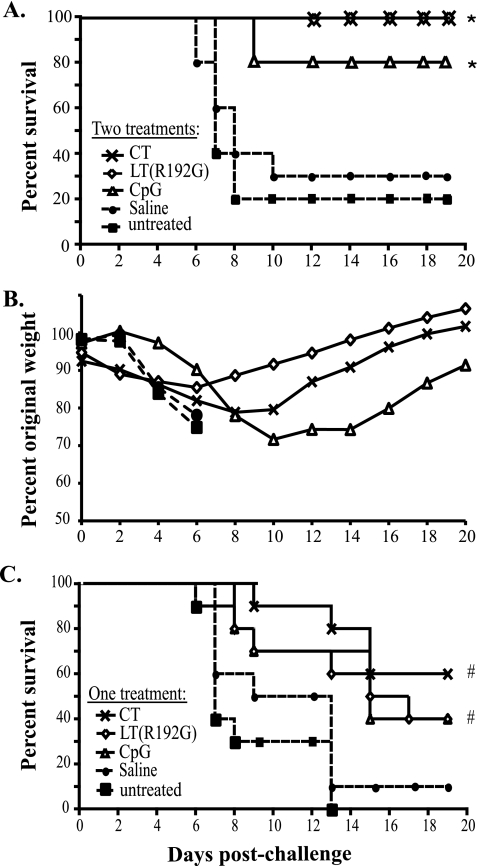
Previous studies demonstrated partial or full protection against influenza virus infection with antigen-free immunomodulator treatments. Intraperitoneal and intranasal CpG administration reduced or prevented mortality in mice infected with influenza virus, presumably by increasing Th1 immune responses (15, 82). Likewise, intranasal delivery of LT(S63K) or CT-B containing trace amounts of CT reduced the influenza viral burden in mice (49, 80). Although these studies highlight the potential use of immunomodulators to control influenza virus, studies directly comparing these agents have not been published, limiting a comprehensive understanding of how different strategies nonspecifically protect against pulmonary viral infections. Our first experiment indicated the induction of different patterns of inflammation and chemokine/cytokine expression after treatment with the three immunomodulators. Based on these observations and considering the ascribed immunological biases of CT, LT(R192G), and CpG toward Th2, mixed Th1/Th2, and Th1, respectively, we wanted to examine the effects of prophylactic treatment with these agents on the survival of mice subsequently challenged with influenza A virus. For these experiments, we treated BALB/c mice nasally twice, 1 week apart, with 5 μg of CT, LT(R192G), or CpG; controls included mice treated with saline or were untreated. Twenty-four hours after the second treatment, mice were challenged with a lethal dose of influenza A/PR/8/34 virus and were monitored for survival and weight loss. The results shown in Fig. 2A demonstrate that two treatments with CT, LT(R192G), or CpG prior to infection significantly improved survival compared to untreated mice (P < 0.001, P < 0.001, and P = 0.002, respectively). Twenty days after infection, all mice treated with CT or LT(R192G) were alive, while 8 out of 10 mice treated with CpG survived. Saline-treated mice demonstrated an insignificant trend of improved survival compared to the untreated infected control group, as has been reported in other studies (67). Surprisingly, there was no significant difference in survival between groups treated with the different immunomodulators, except for a nonsignificant pattern of improved survival of CT- and LT(R192G)-treated mice over those treated with CpG.
FIG. 2.
CT, LT(R192G), and CpG improve survival and differentially affect weight loss after influenza virus challenge. Groups of BALB/c mice (n ≥ 10) were untreated or nasally treated twice with saline or with 5 μg of immunomodulator [CT, LT(R192G), or CpG]. Twenty-four hours after the last treatment mice were infected with influenza A/PR/8/34 virus and monitored for survival (A) or weight loss (B). Data shown are group averages. Significance values indicated by asterisks represent log-rank test results for survival for CT, LT(R192G), and CpG treatment groups compared to untreated mice (P < 0.001, P < 0.001, and P = 0.002, respectively). Significance values for percent original weight loss are described in the text. (C) Alternatively, mice were infected 24 h after a single immunomodulator treatment and monitored for survival. Significance values (indicated by #) represent log-rank test results for survival for CT, LT(R192G), and CpG groups compared to untreated mice (P < 0.001, P = 0.006, and P = 0.001, respectively).
CT and LT(R192G) treatments resulted in significant initial weight loss after infection compared to untreated or saline-treated mice; however, after a week the trend began to reverse and on day 14 postchallenge all these mice had returned to their original weight [P < 0.05 for CT or LT(R192G) versus untreated on days 1, 2, and 6] (Fig. 2B). Treatment with CpG significantly delayed the onset of weight loss after challenge compared to untreated mice (P < 0.005 on days 3 and 5 to 7); however, these mice started to lose weight after about 6 days. By the second week of infection, CT- and LT(R192G)-treated animals had regained significantly more weight than CpG-treated mice [LT(R192G) versus CpG, P < 0.005 for days 13 to 20; CT versus CpG, P < 0.01 days 14 to 16] (Fig. 2B). Saline-treated mice had a single-day significant delay in initial weight loss compared to untreated mice (day 2; P = 0.015) but lost weight quickly after day 3. By day 8 postinfection, most of the mice that were untreated or treated with saline had succumbed to the infection.
Given that two preinfection treatments with immunomodulators significantly enhanced survival after viral challenge, we next determined whether similar results could be obtained after a single treatment. To test this, we challenged mice with a lethal dose of influenza A/PR/8/34 virus 24 h after a single treatment with 5 μg of CT, LT(R192G), or CpG. As seen in Fig. 2C, a single treatment with immunomodulators significantly improved survival compared with control mice (P < 0.001, P = 0.006, and P = 0.001, respectively). No statistical differences were observed between the survival rates of these three groups, although there was a perceived improvement in survival of CT-treated animals compared to mice given LT(R192G) or CpG. The percentage of animals that survived challenge was notably reduced compared to those that received two treatments (Fig. 2A). Before infection, single treatments with CT, LT(R192G), and CpG resulted in increased lymphocytes, neutrophils, and chemokine expression in the airways compared to untreated groups (data not shown) but at a significantly lower level than observed after two treatments (Fig. 1B and C). In addition, single immunomodulator treatments did not significantly alter lung histology, total number of airway cells, or Th1 and Th2 cytokine levels (data not shown). Thus, it is likely that the higher magnitudes of cytokine expression and cellular infiltration observed after two treatments better primed the animals to fight viral infection than the single treatment.
These results indicate that protection from challenge begins as soon as 24 h after the first immunomodulator treatment and increases after the second immunomodulator treatment, potentially giving at least a full week of enhanced immune system “preparedness” for influenza virus infection with either CT, LT(R192G), or CpG treatment. However, the cost of improved survival with immunomodulator treatments might be initial morbidity, exemplified by weight loss and inflammatory changes in the lung. These experiments also revealed that two treatments with the immunomodulators were superior to a single treatment in enhancing survival to influenza virus challenge; therefore, for all subsequent studies mice were prophylactically given two doses, 1 week apart.
Altered viral load and histology in lungs of infected mice treated with immunomodulators.
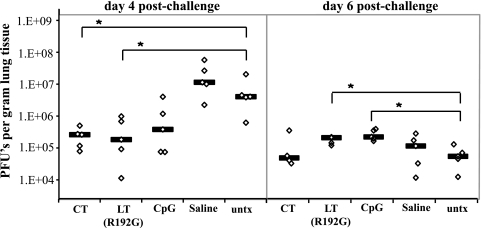
Since all immunomodulator treatments improved influenza virus infection survival but differentially affected weight loss, we wanted to compare how the treatments altered the disease process. BALB/c mice were treated twice with immunomodulators, challenged with influenza A/PR/8/34 virus 24 h later, and sacrificed 4, 6, or 21 days postinfection. Lungs were harvested and analyzed for histological changes and virus levels. Prophylaxis with CT, LT(R192G), or CpG did not prevent virus replication, as evidenced by detectable virus in lung homogenates of all groups; however, on day 4 postinfection the viral loads in treated groups were 1 to 2 log10 lower than those in untreated mice (Fig. 3). This decrease was significant only in the CT- and LT(R192G)-treated groups (P = 0.008 and P = 0.032, respectively). On day 6 postinfection this reduction in lung viral load was no longer evident, and all groups of infected animals, including saline and untreated groups, had titers around 1 × 105 PFU per gram of tissue. At this time LT(R192G)- and CpG-treated mice had viral lung PFU levels that were slightly but significantly higher than in untreated mice (P = 0.036 and P = 0.009, respectively). Noticeably, the lung viral loads in the immunomodulator-treated mice never reached the high levels (0.5 ×107 to 1 ×107 PFU/gram) seen in saline-treated and untreated mice on day 4 postchallenge (Fig. 3). None of the animals that survived challenge had detectable levels of virus in lung tissue 21 days after infection (data not shown).
FIG. 3.
Immunomodulators alter viral burden in mouse lungs during influenza virus infection. Groups of BALB/c mice (n = 5) untreated or nasally treated twice with CT, LT(R192G), CpG, or saline were challenged with influenza A/PR/8/34 virus and sacrificed 4 or 6 days postchallenge. Recovered lung tissue was weighed and homogenized. Virus levels in lung homogenates were detected by plaque assay in MDCK cells. Values for individual animals (diamonds) and group medians (lines) are represented. Significance values (indicated by *) for Mann-Whitney U test were P = 0.008, P = 0.032, P = 0.036, and P = 0.009 for day 4 CT, day 4 LT(R192G), day 6 LT(R192G), and day 6 CpG, respectively. untx, untreated.
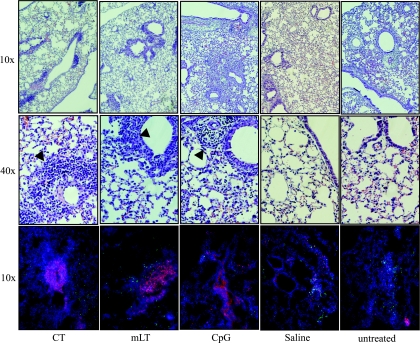
Unlike human infection, which often presents as an upper respiratory infection, murine influenza virus infection results in interstitial pneumonia with lung consolidation (71, 74). Histological analysis of H&E-stained lungs from untreated mice on day 4 and 6 postchallenge demonstrated this typical influenza virus pathology, including architectural distortion of lung parenchyma with indications of hemorrhage and areas of consolidation (Fig. 4 and data not shown). Consistent with the observed weight loss in all groups, none of the treatments [CT, LT(R192G), CpG, or saline] prevented these pathological changes (Fig. 4 and data not shown). Normal lung tissue architecture was not fully restored in surviving mice by day 21 postchallenge, regardless of the treatment administered. Therefore, we conclude that although preinfection treatment with CT, LT(R192G), or CpG initially decreases lung virus levels, this does not prevent infection or viral replication from occurring. Instead, it is likely that the immunomodulators control the infection by altering the initial local host response and inducing more robust innate immune responses to the virus.
FIG. 4.
Immunomodulator treatment induces cellular aggregates in influenza virus-infected mouse lungs, suggestive of iBALT. Groups of BALB/c mice (n = 5) were nasally treated and infected as explained in the text. Recovered lung tissue on day 4 postchallenge was H&E stained (magnification for rows indicated on left margin); cellular aggregates are also indicated (arrows). Other lung sections were immunofluorescently stained with B220 (red), CD4 (green), and DAPI (blue) (10× magnification). Pictures are representative of group images.
One major histological difference between immunomodulator-treated and control groups was the presence of many dense perivascular and peribronchial immune cell aggregates, which was most prominent at day 4 postchallenge (Fig. 4 and data not shown). In an effort to discern the nature of these cell aggregates, we performed microscopic analysis of lung sections. Immunofluorescent staining for B cells, CD4 T cells, and antigen-presenting cells (APCs) (with anti-B220, anti-CD4, and anti-CD11b or -CD11c, respectively) identified these cellular aggregates as mainly B cells with some dispersed CD4 T cells and APCs (Fig. 4 and data not shown). This pattern is consistent with the description of inducible bronchus-associated lymphoid tissue (iBALT), an active site of antigen presentation and clonal expansion of antigen-specific cells found during both human and mouse respiratory infections (31, 43, 52). Saline and untreated mice developed a few small perivascular and peribronchial immune aggregates, but these appeared less dense and stained poorly for B cells. While other studies have reported iBALT appearance by day 10 of influenza virus infection in BALB/c mice (52), the detection of similar iBALT structures in mice treated with immunomodulators in our study by day 4 postchallenge strongly indicates that these treatments alter and perhaps accelerate a natural pulmonary immune response against influenza virus infection.
Altered airway cell populations in mice treated with immunomodulators and infected with influenza virus.
During influenza virus infection in mice, resident and recruited airway cells play specific and diverse roles in the immune response to influenza virus, which ultimately leads to survival or death. In humans, CD4 T-cell deficiency increases influenza disease severity and prevents influenza virus vaccine efficacy (18). In murine models, while no single lymphocyte population is completely responsible for controlling infection, important roles in viral clearance have been demonstrated for antigen-specific B cells, CD4 T cells, and CD8 T cells (14, 19). Additionally, while innate cell populations like alveolar macrophages produce important cytokines and chemokines, enabling clearance of infected cells (66), these cells have also been linked to pathology and tissue damage (13). Thus, immune system cells responsible for clearance of viral infection can also contribute to the development of immunopathology via a complex interplay that is still not clear.
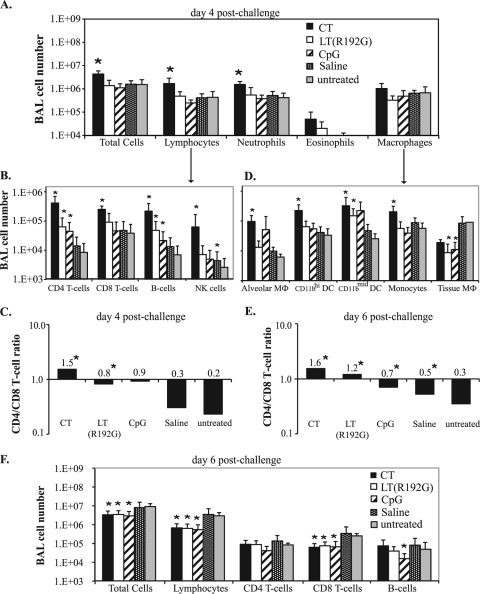
To further understand how immunomodulators alter the natural host response to infection, we examined the effects of CT, LT(R192G), and CpG treatments on airway cell populations during influenza virus infection. BALB/c mice were treated twice with immunomodulators, challenged with influenza virus 24 h later, and sacrificed 4 days after infection. Cells collected from the BAL fluid were enumerated by cell counting and analyzed by cytospin/Hema3 differentiation and flow cytometry. Treatments with CT, LT(R192G), and CpG altered the distribution of cells recovered from the BAL fluid (Fig. 5A to D). Mice treated with CT were the only group to have significantly increased total BAL cells, lymphocytes, and neutrophils (all P = 0.009) (Fig. 5A). Functionally distinct lymphocyte populations are known to play key roles during influenza virus infection; therefore, we examined the BAL fluid for the presence of CD4 T cells (CD4+ CD3+), CD8 T cells (CD8+ CD3+), B cells (CD19+ CD3−), NKT cells (NK1.1+ CD3+), and NK cells (NK1.1+ CD3−). All immunomodulator treatments increased the number of airway CD4 T cells and B cells; although the magnitude of this increase varied for different treatments, the differences were significant (all P < 0.009 except for CpG CD4 cells [P = 0.028] and CpG B cells [P = 0.016]) (Fig. 5B and C). There was also a dramatic increased of the CD4/CD8 T-cell ratio compared to saline and untreated mice, although this was only significant for CT- and LT(R192G)-treated groups (both P = 0.008) (Fig. 5C). CT-treated mice also displayed significantly higher levels of CD8 T cells and NK cells (both P = 0.009) (Fig. 5B) compared to untreated mice. NKT cells were not detected in any mouse group (data not shown). This differs from at least one other influenza virus study with immunomodulators (25a) and may indicate that NKT-cell recruitment is not required for a successful influenza virus immunomodulation strategy. Mice treated with saline had essentially the same cell numbers as those untreated, except that NK cells were elevated (P = 0.047) (Fig. 5B).
FIG. 5.
Immunomodulator treatments alter airway cell composition during influenza virus infection. Groups of BALB/c mice (n = 5) were nasally treated and infected as explained in the text. Recovered BAL cells on day 4 or 6 postchallenge were characterized based on hemacytometer cell counts and cytospin/Hema3 differentiation analysis (A and F) and/or flow cytometry analysis of lymphocyte subtypes (B and F), APC subtypes (D), and CD4/CD8 T-cell ratios (C and E). Bars represent group means ± standard deviations, with significance values indicated by asterisks (P < 0.05, by Mann-Whitney U test, comparing treated to untreated groups).
To determine changes in lymphocyte populations at a time when incipient adaptive immune responses start playing a role in virus control, a similar analysis was performed on day 6 postchallenge. As infection progressed, the CD4/CD8 T-cell ratios remained significantly elevated in treated mice, in a pattern similar to that seen on day 4 postchallenge (for immunomodulator groups, P = 0.009; for saline, P = 0.047) (Fig. 5E). However, the number of total BAL cells, lymphocytes, and CD8 T cells in immunomodulator-treated mice significantly decreased compared to untreated mice (Fig. 5F), suggestive of decreased cellular inflammation in these groups.
Various APC populations, phenotypically distinguished based on surface markers, are known to play specific roles in the responses induced by influenza virus infection (13, 20, 66, 70). We differentiated macrophage populations in the lung based on surface expression of F4/80, CD11c, CD11b (high or mid levels), and the ability to autofluoresce, with phenotypes outlined in Table 1, using a strategy similar to that published in other reports (20a, 21). With the exception of tissue macrophages, CT treatment significantly increased the numbers of macrophages and dendritic cells compared to untreated mice by day 4 postchallenge (all P = 0.009) (Fig. 5D). All three immunomodulator treatments resulted in higher levels of CD11bmid DCs (CD11bmid CD11chi F4/80− cells), but the increase was significant only for CT- and LT(R192G)-treated mice (both P = 0.009). A similar DC subset (defined as CD103+ CD11b− CD11c+) was recently shown to play an important role in murine anti-influenza virus T-cell responses and disease severity (20a). Levels of tissue macrophages (F4/80+ CD11c− CD11b−) were also lowered by all immunodulators (Fig. 5D). By day 6 postchallenge, macrophage populations in immunomodulator-treated groups were about 0.5 log10 lower than in untreated or saline-treated mice (data not shown). To identify cell populations that might directly correlate with lung viral loads, we performed correlation analyses. The recovered BAL tissue macrophages at day 4 postchallenge was the only cell type to significantly correlate to lung virus levels (data not shown). Tissue macrophages are normally only found in homogenized lung tissue, as they are embedded in the lung parenchyma (26). Thus, the presence of tissue macrophages in the BAL fluid would suggest lung tissue disruption due to severe influenza virus infection.
TABLE 1.
Immunomodulators alter CD80 and CD86 costimulatory levels in airway APCs during influenza virus infection
| Flow cytometry population | Cell type(s) | MFI on day 4 posthcallengea |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| CD80 |
CD86 |
||||||||||
| CT | LT(R192G) | CpG | Saline | Untx | CT | LT(R192G) | CpG | Saline | Untx | ||
| All cells | Mixed | 1,306* | 1,281* | 1,777* | 765 | 776 | 1,539 | 1,450 | 1,490 | 2,331 | 2,082 |
| Autofluorescing, CD11c+ F4/80+ | Alveolar macrophages | 10,616* | 8,598* | 5,522* | 3,065 | 3,646 | 6,472* | 6,071 | 3,264* | 7,681 | 9,679 |
| CD11b− CD11c− F4/80+ | Tissue macrophages | 4,866 | 3,482 | 4,185 | 1,083 | 1,728 | 2,930 | 1,784 | 1,633 | 5,613 | 6,102 |
| CD11bhi CD11chi F4/80− | Dendritic cells | 4,627 | 5,085 | 5,053 | 3,949 | 3,454 | 6,231 | 6,394 | 6,303 | 8,045 | 9,068 |
| CD11bmid CD11chi F4/80− | Dendritic cells | 2,602 | 2,130 | 2,007 | 2,401 | 2,364 | 1,151 | 1,205 | 1,239 | 1,149 | 1,647 |
| CD11bmid CD11cmid F4/80− | Monocytes | 1,558 | 1,417 | 2,119 | 1,165 | 1,406 | 4,739* | 4,569* | 6,884 | 8,474 | 9,303 |
| CD11bhi CD11c− F4/80− | Neutrophils | 916 | 870 | 1,386 | 952 | 1,018 | 1,145* | 1,094* | 1,179* | 3,088 | 3,134 |
| CD19+ | B cells | 3,622 | 3,253 | 5,031 | 4,052 | 4,685 | 2,927* | 2,436* | 2,829* | 4,934 | 6,881 |
Numbers represent group average MFI levels detected by flow cytometry analysis of recovered BAL cells on day 4 postchallenge. Untx, untreated. *, significantly (P < 0.05) different by Mann-Whitney U test compared to untreated mice.
CD80 and CD86 costimulatory marker expression indicates activation of APCs, a necessary step to priming antigen-specific T-cell and B-cell responses (26). In addition, high CD80 expression has been linked to generation of Th1-polarized immune responses (36), and low monocyte CD80/86 levels directly correlate with decreased effectiveness of the trivalent influenza vaccine (75). APCs, including DCs, macrophages, neutrophils, and B cells (20, 26, 44, 55, 57), were evaluated for CD80/CD86 cell surface expression. Table 1 details the changes of levels of expression in these surface markers, measured as changes in the mean fluorescent intensity (MFI). As seen, CT, LT(R192G), and CpG treatment resulted in significantly increased CD80 expression in all BAL APCs and an insignificant reduction in CD86 expression compared to saline-treated and untreated control groups. Other cells from treated mice displaying lower levels of CD86 expression included monocytes, neutrophils, and B cells. No changes in surface marker expression were observed on DCs in any of the groups.
Treatment with immunomodulators reduces chemokine expression in mice infected with influenza virus.
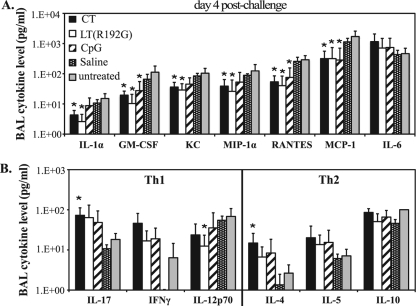
Cytokine and chemokine secretion is integral to recovery from influenza virus infection, but it has also been linked to detrimental host responses to infection (13, 27, 37, 59). Given this dichotomy, we wanted to explore how the immunomodulators in our study influenced cytokine and chemokine levels during early infection. BAL supernatants recovered from mice on day 4 postchallenge were analyzed for cytokine and chemokine levels with a mouse 23-plex cytokine assay. Strikingly, treatment with CT, LT(R192G), or CpG reduced levels of inflammatory cytokines and chemokines compared to untreated mice, including IL-1α, granulocyte-macrophage colony-stimulating factor (GM-CSF), KC, macrophage inflammatory protein 1α (MiP-1α), RANTES, and monocyte chemoattractant protein 1 (MCP-1), but not tumor necrosis factor alpha (TNF-α) (not shown) or IL-6 (Fig. 6A). These reductions were consistently larger in CT and LT(R192G) groups than in CpG-treated mice. Importantly, the chemokine levels observed in mice treated with immunomodulators and subsequently infected with influenza virus (Fig. 6A) displayed an opposite trend than the one identified at the time of infection (Fig. 1C). These observations suggest that administration of immunoenhancers prior to infection induces an early onset of protective responses against influenza virus challenge while limiting potentially damaging levels of inflammatory mediators during infection.
FIG. 6.
Immunomodulator treatments decrease potentially damaging chemokine levels during influenza virus infection. Groups of BALB/c mice (n = 5) were nasally treated [twice with CT, LT(R192G), CpG, or saline] or left untreated, challenged 24 h later with influenza A/PR/8/34 virus, and sacrificed 4 days postchallenge. Supernatants from BAL samples were analyzed with a mouse cytokine/chemokine 23 Bio-plex array to detect levels of inflammatory cytokines and chemokines (A) and Th1/Th2 cytokines (B). Bars represent means ± standard deviations, with significance values indicated (*, P < 0.05 by Mann-Whitney U test).
The cytokine panel we tested also included a number of Th1- and Th2-associated cytokines, which demonstrated considerably less variability between the samples. Similar to the time of infection (Fig. 1C), all immunomodulator-treated groups had more detectable IL-4, a potent antibody-promoting cytokine, but only in CT-treated mice was this significant compared to untreated mice (Fig. 6B). This pattern was also seen for levels of IL-17 (Fig. 6B), a cytokine involved in neutrophil activation and inflammatory Th17 responses (26). In fact, in the samples obtained 4 days after challenge, IL-17 was the only cytokine tested which correlated with the number of airway neutrophils (Pearson's test; P = 0.036) (data not shown), unlike other neutrophil-associated chemokines (KC, G-CSF, and GM-CSF). Although several studies have indicated the importance of type I IFN production during influenza virus infection (27), levels of IFN-α or IFN-β in BAL samples obtained on day 4 postchallenge were not different in treated compared to untreated groups (data not shown). This observation does not preclude the possibility that differences in type I interferons exist at earlier time points or that the differences are beyond the limits of detection of the ELISA we used.
DISCUSSION
The objective of this study was to compare how prophylactic treatment with bacterially derived immunomodulators known for promoting different Th1/Th2 profiles would change the course of influenza virus infection in a murine model. For this study, we evaluated CT, LT(R192G), and CpG, which are well-characterized adjuvants known for their distinct immunomodulatory properties. Our study revealed that prior to infection, nasal administration of these immune-enhancing agents altered the steady-state immune status of the lung, increasing cellular recruitment and cytokine expression. When prophylactically treated animals are infected, instead of a pulmonary anti-inflammatory/Th2 profile, the virus encounters an immunologically primed landscape with increased numbers of activated lymphocytes, neutrophils, and macrophages and elevated levels of proinflammatory cytokines and chemokines. Some of these recruited cells are professional APCs likely to have enhanced antigen-presenting properties and upon infection can quickly travel to the draining lymph nodes to prime T and B cells, accelerating the initiation of adaptive immunity.
Upon lethal influenza virus challenge, all three prophylactic treatments prevented or decreased influenza virus mortality. This was a surprising finding given the different immune phenotypes induced by the three immunomodulators. CpG is a TLR agonist known for promoting strong Th1 responses, while LT(R192G) and CT are enterotoxins which, respectively, induce mixed Th1/Th2- or Th2-polarized responses. Yet, compared to CpG, treatment with CT or LT(R192G) resulted in equal or better survival in our model of influenza disease. An interesting observation in this study was that mice treated nasally with saline had inflammatory changes in their lungs and a slight delay in mortality. These data agree with recent findings reporting that in vitro or in vivo application of saline solutions helps to prevent microbial infection (33, 67). When developing anti-influenza virus therapies or prophylaxis for human patients, the inherent advantage of an intranasal or pernasal route should be considered.
The two enterotoxins induced notable histological changes, higher neutrophil infiltration, and elevated levels of IL-1α and IL-17, indicative of initial inflammatory, Th1, and Th17 responses. Although previous publications have reported that CT induces early inflammation (29, 79), the induction of Th17 responses after intranasal CT administration has only been noted in a recent publication (40). Little is known about the role of IL-17 in viral pulmonary infections; it has been suggested that an IL-17 receptor agonist antagonizes the response to influenza virus challenge (12), but our results and those of others (22, 50) indicate that Th17 cells can be involved in protection against influenza virus. Whereas other studies using immunosuppressive agents report exacerbated influenza disease (7, 46), in this study, CT-treated mice appeared to develop initial Th1/Th17 inflammatory responses that directly contributed to enhanced survival. In our experiments, the viral challenge was given 24 h after immunomodulator administration. It is possible that a more pronounced polarization of the immune response occurs at a later time and that the outcome of a delayed primary or a secondary challenge would be different, as observed in some studies using enterotoxin administration (79).
Another explanation for our results could be the very nature of acute influenza virus infection in the pulmonary mucosa. As infection is controlled by integrated interaction between Th1 and Th2 factors, it could be that induction of a Th1-dominated response would not be superior to a response with a strong Th2 component. A study by Harker et al. (24) using RSV also demonstrated unexpected results when comparing Th1 versus Th2 strategies. Mice that were infected with recombinant RSV expressing IL-4 (rRSV/IL-4) recovered faster from primary and secondary challenge than mice that received rRSV expressing IFN-γ (rRSV/IFN-γ). In addition, when mice infected with rRSV/IL-4 or rRSV/IFN-γ were challenged 1 month later with nonlethal influenza A virus, rRSV/IL-4-primed mice recovered faster than their rRSV/IFN-γ counterparts, despite significantly fewer airway lymphocytes and increased eosinophils during infection. Thus, the authors of that study concluded that skewing the response toward Th1 was not always beneficial in pulmonary viral infections. Our results would tend to agree with their assessment, but we have only just begun to understand the consequences of Th1/Th2-biased immunomodulation strategies on influenza disease. It is possible that altering challenge and/or treatment schedules or using a different infection model could dramatically change the results of similar experimental setups. For example, immunomodulation studies with the highly virulent H5N1 influenza virus strain suggest that suppression of inflammation after infection improves mouse survival (84).
In the current study, the enhanced survival might be based not only on the generation of specific Th1/Th2 responses but also on the magnitude and kinetics of an initial inflammatory and activated immune response. Several studies would support this conclusion, including a recent report by Tuvim et al. that showed that lung inflammation induced by aerosolized bacterial lysate from nontypeable Haemophilus influenzae protects mice from mortality and reduces viral titers after lethal influenza virus challenge (72). In addition, other studies have shown that depletion of inflammatory cytokine IL-1, neutrophils, or TLR3 during influenza virus infection in mice significantly increases influenza disease severity (41, 59, 68, 69). We observed that both CT and LT(R192G), and to a lesser extent CpG, initially increased airway cellularity, including macrophages and neutrophils, as well as levels of various chemokines and cytokines. However, histology sections of CT-treated mice indicated more severe inflammation and immunopathology than with LT(R192G) and especially with CpG-treated mice, and levels of IL-1α secretion, neutrophil infiltration, and TLR3 expression followed this pattern (data not shown). The magnitude of the inflammatory reaction could explain both the enhanced survival seen after two treatments, compared to a single treatment, and the initial weight loss observed in infected mice treated with either CT or LT(R192G) but not with CpG. Yet, CpG-treated mice experienced increased morbidity reflected by greater weight loss and higher pulmonary viral loads. The changes induced by CT appeared to last longer, as on day 4 postchallenge these mice continued to have significantly higher numbers of total cells, lymphocytes, and neutrophils as well as IL-17 levels. A surprising observation was the decreased chemokine levels observed by day 4 of infection in treated mice. It is likely that the reduction of these inflammatory mediators played a role in preventing the immunopathology and poor disease outcome generally associated with high inflammation and an infection-induced cytokine storm (37). It was also noteworthy that the reduction in lymphocytes and CD8 T cells observed at day 6 did not have an impact on the CD4/CD8 ratios but coincided with the point when treated mice started to reverse their weight loss. Further experiments are needed to elucidate the precise contribution of the implicated effector molecules and cells in prophylactic strategies for influenza disease.
CT, LT(R192G), and CpG are known to enhance both systemic and mucosal responses, thus it was not surprising to find elevated numbers of lymphocytes and DCs in the lung at day 4 postinfection. Furthermore, all immunomodulator-treated mice maintained a higher CD4/CD8 ratio in airway-recovered cells than control groups. While CD8 T cells are important in viral clearance (reviewed in reference 64), several groups have suggested that CD4 T cells are the main protective immune cell during influenza virus infection (6). Although we have not performed effector function analyses, the significant alterations in cell populations, specifically in CD4 T cells, B cells, and CD11bmid DCs, is consistent with the development of iBALT structures seen in the lungs of infected immunomodulator-treated animals. Transient BALT formation has been reported in respiratory infection in both mice and humans (31, 43, 52). During influenza virus infection, iBALT formation correlates with decreased disease severity and can substitute for secondary lymphoid tissue (52). The role of iBALT-like formations in our study is unclear, but it is interesting that these immune cell-dense structures were found in mice treated with immunomodulators that also demonstrated decreased disease severity.
Collectively, our results indicate that prophylactic immunomodulation with CT, LT(R192G), or CpG induces a local initial inflammatory process that results in decreased morbidity and mortality upon influenza virus challenge. The observation that CT and LT(R192G) induce better protection than CpG leads us to believe that the inclusion of Th17 and Th2 components in influenza virus prophylaxis could provide additional benefits for rapid clearance of infection. Immunomodulation strategies offer great potential to bolster immune responses for the control of seasonal and pandemic influenza virus outbreaks. Administration of immunomodulators combined with antiviral therapies could broaden the clinical platform of anti-influenza virus strategies. Understanding both the requirements of protection and the consequences of immune system manipulation are critical to the success of any disease prevention or therapeutic strategy. Our studies provide important insights into the influence of different kinds of immunomodulation strategies for the control of influenza virus infection, and the results presented here might be broadly applicable for immune modulation of other pulmonary viral infections.
Acknowledgments
This work was supported, in part, by the Louisiana Vaccine Center and the South Louisiana Institute for Infectious Disease Research sponsored by the Louisiana Board of Regents.
We gratefully acknowledge Christina Pettus and Louise Lawson for critical reading of the manuscript and Rebecca Brocato and Brian Kaplan for technical assistance with influenza virus assays.
We have no financial conflict of interest.
Footnotes
Published ahead of print on 6 January 2010.
REFERENCES
- 1.Anonymous. 2008. Influenza fact sheet no. 211. World Health Organization, Geneva, Switzerland.
- 2.Bagley, K. C., S. F. Abdelwahab, R. G. Tuskan, and G. K. Lewis. 2006. Cholera toxin indirectly activates human monocyte-derived dendritic cells in vitro through the production of soluble factors, including prostaglandin E(2) and nitric oxide. Clin. Vaccine Immunol. 13:106-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Banerjee, B., K. J. Kelly, J. N. Fink, J. D. Henderson, Jr., N. K. Bansal, and V. P. Kurup. 2004. Modulation of airway inflammation by immunostimulatory CpG oligodeoxynucleotides in a murine model of allergic aspergillosis. Infect. Immun. 72:6087-6094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bowman, C. C., and J. D. Clements. 2001. Differential biological and adjuvant activities of cholera toxin and Escherichia coli heat-labile enterotoxin hybrids. Infect. Immun. 69:1528-1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bromander, A., J. Holmgren, and N. Lycke. 1991. Cholera toxin stimulates IL-1 production and enhances antigen presentation by macrophages in vitro. J. Immunol. 146:2908-2914. [PubMed] [Google Scholar]
- 6.Brown, D. M., A. M. Dilzer, D. L. Meents, and S. L. Swain. 2006. CD4 T cell-mediated protection from lethal influenza: perforin and antibody-mediated mechanisms give a one-two punch. J. Immunol. 177:2888-2898. [DOI] [PubMed] [Google Scholar]
- 7.Buchweitz, J. P., P. W. Karmaus, J. R. Harkema, K. J. Williams, and N. E. Kaminski. 2007. Modulation of airway responses to influenza A/PR/8/34 by Δ9-tetrahydrocannabinol in C57BL/6 mice. J. Pharmacol. Exp. Ther. 323:675-683. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, E., L. Cardenas-Freytag, and J. D. Clements. 1999. The role of cAMP in mucosal adjuvanticity of Escherichia coli heat-labile enterotoxin (LT). Vaccine 18:38-49. [DOI] [PubMed] [Google Scholar]
- 9.Choi, J. H., H. M. Ko, S. J. Park, K. J. Kim, S. H. Kim, and S. Y. Im. 2007. CpG oligodeoxynucleotides protect mice from lethal challenge with Candida albicans via a pathway involving tumor necrosis factor-alpha-dependent interleukin-12 induction. FEMS Immunol. Med. Microbiol. 51:155-162. [DOI] [PubMed] [Google Scholar]
- 10.Clements, J. D., and R. A. Finkelstein. 1979. Isolation and characterization of homogeneous heat-labile enterotoxins with high specific activity from Escherichia coli cultures. Infect. Immun. 24:760-769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cluff, C. W., J. R. Baldridge, A. G. Stover, J. T. Evans, D. A. Johnson, M. J. Lacy, V. G. Clawson, V. M. Yorgensen, C. L. Johnson, M. T. Livesay, R. M. Hershberg, and D. H. Persing. 2005. Synthetic toll-like receptor 4 agonists stimulate innate resistance to infectious challenge. Infect. Immun. 73:3044-3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe, C. R., K. Chen, D. A. Pociask, J. F. Alcorn, C. Krivich, R. I. Enelow, T. M. Ross, J. L. Witztum, and J. K. Kolls. 2009. Critical role of IL-17RA in immunopathology of influenza infection. J. Immunol. 183:5301-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dawson, T. C., M. A. Beck, W. A. Kuziel, F. Henderson, and N. Maeda. 2000. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am. J. Pathol. 156:1951-1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty, P. C., D. J. Topham, R. A. Tripp, R. D. Cardin, J. W. Brooks, and P. G. Stevenson. 1997. Effector CD4+ and CD8+ T-cell mechanisms in the control of respiratory virus infections. Immunol. Rev. 159:105-117. [DOI] [PubMed] [Google Scholar]
- 15.Dong, L., I. Mori, M. J. Hossain, B. Liu, and Y. Kimura. 2003. An immunostimulatory oligodeoxynucleotide containing a cytidine-guanosine motif protects senescence-accelerated mice from lethal influenza virus by augmenting the T helper type 1 response. J. Gen. Virol. 84:1623-1628. [DOI] [PubMed] [Google Scholar]
- 16.DuBois, A. B., L. C. Freytag, and J. D. Clements. 2007. Evaluation of combinatorial vaccines against anthrax and plague in a murine model. Vaccine 25:4747-4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Edwards, L., A. E. Williams, A. M. Krieg, A. J. Rae, R. J. Snelgrove, and T. Hussell. 2005. Stimulation via Toll-like receptor 9 reduces Cryptococcus neoformans-induced pulmonary inflammation in an IL-12-dependent manner. Eur. J. Immunol. 35:273-281. [DOI] [PubMed] [Google Scholar]
- 18.Evans, K. D., and M. W. Kline. 1995. Prolonged influenza A infection responsive to rimantadine therapy in a human immunodeficiency virus-infected child. Pediatr. Infect. Dis. J. 14:332-334. [DOI] [PubMed] [Google Scholar]
- 19.Gerhard, W. 2001. The role of the antibody response in influenza virus infection. Curr. Top. Microbiol. Immunol. 260:171-190. [DOI] [PubMed] [Google Scholar]
- 20.GeurtsvanKessel, C. H., and B. N. Lambrecht. 2008. Division of labor between dendritic cell subsets of the lung. Mucosal Immunol. 1:442-450. [DOI] [PubMed] [Google Scholar]
- 20a.GeurtsvanKessel, C. H., M. A. Willart, L. S. van Rijt, F. Muskens, M. Kool, C. Baas, K. Thielemans, C. Bennett, B. E. Clausen, H. C. Hoogsteden, A. D. Osterhaus, G. F. Rimmelzwaan, and B. N. Lambrecht. 2008. Clearance of influenza virus from the lung depends on migratory langerin+CD11b− but not plasmacytoid dendritic cells. J. Exp. Med. 205:1621-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Juarrero, M., T. S. Shim, A. Kipnis, A. P. Junqueira-Kipnis, and I. M. Orme. 2003. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J. Immunol. 171:3128-3135. [DOI] [PubMed] [Google Scholar]
- 22.Hamada, H., L. Garcia-Hernandez Mde, J. B. Reome, S. K. Misra, T. M. Strutt, K. K. McKinstry, A. M. Cooper, S. L. Swain, and R. W. Dutton. 2009. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J. Immunol. 182:3469-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hammerbeck, D. M., G. R. Burleson, C. J. Schuller, J. P. Vasilakos, M. Tomai, E. Egging, F. R. Cochran, S. Woulfe, and R. L. Miller. 2007. Administration of a dual toll-like receptor 7 and toll-like receptor 8 agonist protects against influenza in rats. Antivir. Res. 73:1-11. [DOI] [PubMed] [Google Scholar]
- 24.Harker, J., A. Bukreyev, P. L. Collins, B. Wang, P. J. Openshaw, and J. S. Tregoning. 2007. Virally delivered cytokines alter the immune response to future lung infections. J. Virol. 81:13105-13111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris, T. H., J. M. Mansfield, and D. M. Paulnock. 2007. CpG oligodeoxynucleotide treatment enhances innate resistance and acquired immunity to African trypanosomes. Infect. Immun. 75:2366-2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25a.Ho, L. P., L. Denney, K. Luhn, D. Teoh, C. Clelland, and A. J. McMichael. 2008. Activation of invariant NKT cells enhances the innate immune response and improves the disease course in influenza A virus infection. Eur. J. Immunol. 38:1913-1922. [DOI] [PubMed] [Google Scholar]
- 26.Holt, P. G., D. H. Strickland, M. E. Wikstrom, and F. L. Jahnsen. 2008. Regulation of immunological homeostasis in the respiratory tract. Nat. Rev. Immunol. 8:142-152. [DOI] [PubMed] [Google Scholar]
- 27.James, C. M., M. Y. Abdad, J. P. Mansfield, H. K. Jacobsen, A. R. Vind, P. A. Stumbles, and E. J. Bartlett. 2007. Differential activities of alpha/beta IFN subtypes against influenza virus in vivo and enhancement of specific immune responses in DNA vaccinated mice expressing haemagglutinin and nucleoprotein. Vaccine 25:1856-1867. [DOI] [PubMed] [Google Scholar]
- 28.Jia, W., H. Li, and Y. W. He. 2008. Pattern recognition molecule mindin promotes intranasal clearance of influenza viruses. J. Immunol. 180:6255-6261. [DOI] [PubMed] [Google Scholar]
- 29.Jones, H. P., L. M. Hodge, K. Fujihashi, H. Kiyono, J. R. McGhee, and J. W. Simecka. 2001. The pulmonary environment promotes Th2 cell responses after nasal-pulmonary immunization with antigen alone, but Th1 responses are induced during instances of intense immune stimulation. J. Immunol. 167:4518-4526. [DOI] [PubMed] [Google Scholar]
- 30.Jurk, M., and J. Vollmer. 2007. Therapeutic applications of synthetic CpG oligodeoxynucleotides as TLR9 agonists for immune modulation. BioDrugs 21:387-401. [DOI] [PubMed] [Google Scholar]
- 31.Karrer, U., A. Althage, B. Odermatt, C. W. Roberts, S. J. Korsmeyer, S. Miyawaki, H. Hengartner, and R. M. Zinkernagel. 1997. On the key role of secondary lymphoid organs in antiviral immune responses studied in alymphoplastic (aly/aly) and spleenless (Hox11−/−) mutant mice. J. Exp. Med. 185:2157-2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kawamura, Y. I., R. Kawashima, Y. Shirai, R. Kato, T. Hamabata, M. Yamamoto, K. Furukawa, K. Fujihashi, J. R. McGhee, H. Hayashi, and T. Dohi. 2003. Cholera toxin activates dendritic cells through dependence on GM1-ganglioside which is mediated by NF-κB translocation. Eur. J. Immunol. 33:3205-3212. [DOI] [PubMed] [Google Scholar]
- 33.Kim, H. J., J. G. Lee, J. W. Kang, H. J. Cho, H. S. Kim, H. K. Byeon, and J. H. Yoon. 2008. Effects of a low concentration hypochlorous acid nasal irrigation solution on bacteria, fungi, and virus. Laryngoscope 118:1862-1867. [DOI] [PubMed] [Google Scholar]
- 34.Kline, J. N., T. J. Waldschmidt, T. R. Businga, J. E. Lemish, J. V. Weinstock, P. S. Thorne, and A. M. Krieg. 1998. Modulation of airway inflammation by CpG oligodeoxynucleotides in a murine model of asthma. J. Immunol. 160:2555-2559. [PubMed] [Google Scholar]
- 35.Krieg, A. M., S. M. Efler, M. Wittpoth, M. J. Al Adhami, and H. L. Davis. 2004. Induction of systemic TH1-like innate immunity in normal volunteers following subcutaneous but not intravenous administration of CPG 7909, a synthetic B-class CpG oligodeoxynucleotide TLR9 agonist. J. Immunother. 27:460-471. [DOI] [PubMed] [Google Scholar]
- 36.Kuchroo, V. K., M. P. Das, J. A. Brown, A. M. Ranger, S. S. Zamvil, R. A. Sobel, H. L. Weiner, N. Nabavi, and L. H. Glimcher. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 80:707-718. [DOI] [PubMed] [Google Scholar]
- 37.La Gruta, N. L., K. Kedzierska, J. Stambas, and P. C. Doherty. 2007. A question of self-preservation: immunopathology in influenza virus infection. Immunol. Cell Biol. 85:85-92. [DOI] [PubMed] [Google Scholar]
- 38.Lavelle, E. C., A. Jarnicki, E. McNeela, M. E. Armstrong, S. C. Higgins, O. Leavy, and K. H. Mills. 2004. Effects of cholera toxin on innate and adaptive immunity and its application as an immunomodulatory agent. J. Leukoc. Biol. 75:756-763. [DOI] [PubMed] [Google Scholar]
- 39.Lavelle, E. C., E. McNeela, M. E. Armstrong, O. Leavy, S. C. Higgins, and K. H. Mills. 2003. Cholera toxin promotes the induction of regulatory T cells specific for bystander antigens by modulating dendritic cell activation. J. Immunol. 171:2384-2392. [DOI] [PubMed] [Google Scholar]
- 40.Lee, J. B., J. E. Jang, M. K. Song, and J. Chang. 2009. Intranasal delivery of cholera toxin induces th17-dominated T-cell response to bystander antigens. PLoS One 4:e5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Le Goffic, R., J. Pothlichet, D. Vitour, T. Fujita, E. Meurs, M. Chignard, and M. Si-Tahar. 2007. Cutting edge: influenza A virus activates TLR3-dependent inflammatory and RIG-I-dependent antiviral responses in human lung epithelial cells. J. Immunol. 178:3368-3372. [DOI] [PubMed] [Google Scholar]
- 42.Lu, X., J. D. Clements, and J. M. Katz. 2002. Mutant Escherichia coli heat-labile enterotoxin [LT(R192G)] enhances protective humoral and cellular immune responses to orally administered inactivated influenza vaccine. Vaccine 20:1019-1029. [DOI] [PubMed] [Google Scholar]
- 43.Lund, F. E., S. Partida-Sanchez, B. O. Lee, K. L. Kusser, L. Hartson, R. J. Hogan, D. L. Woodland, and T. D. Randall. 2002. Lymphotoxin-alpha-deficient mice make delayed, but effective, T and B cell responses to influenza. J. Immunol. 169:5236-5243. [DOI] [PubMed] [Google Scholar]
- 44.Lyons, C. R., and M. F. Lipscomb. 1983. Alveolar macrophages in pulmonary immune responses. I. Role in the initiation of primary immune responses and in the selective recruitment of T lymphocytes to the lung. J. Immunol. 130:1113-1119. [PubMed] [Google Scholar]
- 45.Maier, M., T. J. Seabrook, and C. A. Lemere. 2005. Modulation of the humoral and cellular immune response in Abeta immunotherapy by the adjuvants monophosphoryl lipid A (MPL), cholera toxin B subunit (CTB) and E. coli enterotoxin LT(R192G). Vaccine 23:5149-5159. [DOI] [PubMed] [Google Scholar]
- 46.Marsolais, D., B. Hahm, K. H. Edelmann, K. B. Walsh, M. Guerrero, Y. Hatta, Y. Kawaoka, E. Roberts, M. B. Oldstone, and H. Rosen. 2008. Local not systemic modulation of dendritic cell S1P receptors in lung blunts virus-specific immune responses to influenza. Mol. Pharmacol. 74:896-903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Martin, M., D. J. Metzger, S. M. Michalek, T. D. Connell, and M. W. Russell. 2001. Distinct cytokine regulation by cholera toxin and type II heat-labile toxins involves differential regulation of CD40 ligand on CD4(+) T cells. Infect. Immun. 69:4486-4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matrosovich, M., T. Matrosovich, W. Garten, and H. D. Klenk. 2006. New low-viscosity overlay medium for viral plaque assays. Virol. J. 3:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsuo, K., T. Yoshikawa, H. Asanuma, T. Iwasaki, Y. Hagiwara, Z. Chen, S. E. Kadowaki, H. Tsujimoto, T. Kurata, and S. I. Tamura. 2000. Induction of innate immunity by nasal influenza vaccine administered in combination with an adjuvant (cholera toxin). Vaccine 18:2713-2722. [DOI] [PubMed] [Google Scholar]
- 50.McKinstry, K. K., T. M. Strutt, A. Buck, J. D. Curtis, J. P. Dibble, G. Huston, M. Tighe, H. Hamada, S. Sell, R. W. Dutton, and S. L. Swain. 2009. IL-10 deficiency unleashes an influenza-specific Th17 response and enhances survival against high-dose challenge. J. Immunol. 182:7353-7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miyagi, K., K. Kawakami, Y. Kinjo, K. Uezu, T. Kinjo, K. Nakamura, and A. Saito. 2005. CpG oligodeoxynucleotides promote the host protective response against infection with Cryptococcus neoformans through induction of interferon-gamma production by CD4+ T cells. Clin. Exp. Immunol. 140:220-229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Moyron-Quiroz, J. E., J. Rangel-Moreno, K. Kusser, L. Hartson, F. Sprague, S. Goodrich, D. L. Woodland, F. E. Lund, and T. D. Randall. 2004. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 10:927-934. [DOI] [PubMed] [Google Scholar]
- 53.Mozdzanowska, K., D. Zharikova, M. Cudic, L. Otvos, and W. Gerhard. 2007. Roles of adjuvant and route of vaccination in antibody response and protection engendered by a synthetic matrix protein 2-based influenza A virus vaccine in the mouse. Virol. J. 4:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mutsch, M., W. Zhou, P. Rhodes, M. Bopp, R. T. Chen, T. Linder, C. Spyr, and R. Steffen. 2004. Use of the inactivated intranasal influenza vaccine and the risk of Bell's palsy in Switzerland. N. Engl. J. Med. 350:896-903. [DOI] [PubMed] [Google Scholar]
- 55.Potter, N. S., and C. V. Harding. 2001. Neutrophils process exogenous bacteria via an alternate class I MHC processing pathway for presentation of peptides to T lymphocytes. J. Immunol. 167:2538-2546. [DOI] [PubMed] [Google Scholar]
- 56.Quan, F. S., R. W. Compans, H. H. Nguyen, and S. M. Kang. 2008. Induction of heterosubtypic immunity to influenza virus by intranasal immunization. J. Virol. 82:1350-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez-Pinto, D. 2005. B cells as antigen presenting cells. Cell Immunol. 238:67-75. [DOI] [PubMed] [Google Scholar]
- 58.Roux, X., C. Dubuquoy, G. Durand, T. L. Tran-Tolla, N. Castagne, J. Bernard, A. Petit-Camurdan, J. F. Eleouet, and S. Riffault. 2008. Sub-nucleocapsid nanoparticles: a nasal vaccine against respiratory syncytial virus. PLoS One 3:e1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmitz, N., M. Kurrer, M. F. Bachmann, and M. Kopf. 2005. Interleukin-1 is responsible for acute lung immunopathology but increases survival of respiratory influenza virus infection. J. Virol. 79:6441-6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwerbrock, N. M., E. A. Karlsson, Q. Shi, P. A. Sheridan, and M. A. Beck. 2009. Fish oil-fed mice have impaired resistance to influenza infection. J. Nutr. 139:1588-1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Shin, K., H. Wakabayashi, K. Yamauchi, S. Teraguchi, Y. Tamura, M. Kurokawa, and K. Shiraki. 2005. Effects of orally administered bovine lactoferrin and lactoperoxidase on influenza virus infection in mice. J. Med. Microbiol. 54:717-723. [DOI] [PubMed] [Google Scholar]
- 62.Soriani, M., L. Bailey, and T. R. Hirst. 2002. Contribution of the ADP-ribosylating and receptor-binding properties of cholera-like enterotoxins in modulating cytokine secretion by human intestinal epithelial cells. Microbiology 148:667-676. [DOI] [PubMed] [Google Scholar]
- 63.Reference deleted.
- 64.Stambas, J., C. Guillonneau, K. Kedzierska, J. D. Mintern, P. C. Doherty, and N. L. La Gruta. 2008. Killer T cells in influenza. Pharmacol. Ther. 120:186-196. [DOI] [PubMed] [Google Scholar]
- 65.Takabayashi, K., L. Libet, D. Chisholm, J. Zubeldia, and A. A. Horner. 2003. Intranasal immunotherapy is more effective than intradermal immunotherapy for the induction of airway allergen tolerance in Th2-sensitized mice. J. Immunol. 170:3898-3905. [DOI] [PubMed] [Google Scholar]
- 66.Tamura, S., and T. Kurata. 2004. Defense mechanisms against influenza virus infection in the respiratory tract mucosa. Jpn. J. Infect. Dis. 57:236-247. [PubMed] [Google Scholar]
- 67.Tano, L., and K. Tano. 2004. A daily nasal spray with saline prevents symptoms of rhinitis. Acta Otolaryngol. 124:1059-1062. [DOI] [PubMed] [Google Scholar]
- 68.Tate, M. D., A. G. Brooks, and P. C. Reading. 2008. The role of neutrophils in the upper and lower respiratory tract during influenza virus infection of mice. Respir. Res. 9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tate, M. D., Y. M. Deng, J. E. Jones, G. P. Anderson, A. G. Brooks, and P. C. Reading. 2009. Neutrophils ameliorate lung injury and the development of severe disease during influenza infection. J. Immunol. 183:7441-7450. [DOI] [PubMed] [Google Scholar]
- 70.Thitithanyanont, A., A. Engering, P. Ekchariyawat, S. Wiboon-ut, A. Limsalakpetch, K. Yongvanitchit, U. Kum-Arb, W. Kanchongkittiphon, P. Utaisincharoen, S. Sirisinha, P. Puthavathana, M. M. Fukuda, and S. Pichyangkul. 2007. High susceptibility of human dendritic cells to avian influenza H5N1 virus infection and protection by IFN-alpha and TLR ligands. J. Immunol. 179:5220-5227. [DOI] [PubMed] [Google Scholar]
- 71.Trammell, R. A., and L. A. Toth. 2008. Genetic susceptibility and resistance to influenza infection and disease in humans and mice. Expert Rev. Mol. Diagn. 8:515-529. [DOI] [PubMed] [Google Scholar]
- 72.Tuvim, M. J., S. E. Evans, C. G. Clement, B. F. Dickey, and B. E. Gilbert. 2009. Augmented lung inflammation protects against influenza A pneumonia. PLoS One 4:e4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Uddowla, S., L. C. Freytag, and J. D. Clements. 2007. Effect of adjuvants and route of immunizations on the immune response to recombinant plague antigens. Vaccine 25:7984-7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.van der Laan, J. W., C. Herberts, R. Lambkin-Williams, A. Boyers, A. J. Mann, and J. Oxford. 2008. Animal models in influenza vaccine testing. Expert Rev. Vaccines 7:783-793. [DOI] [PubMed] [Google Scholar]
- 75.van Duin, D., H. G. Allore, S. Mohanty, S. Ginter, F. K. Newman, R. B. Belshe, R. Medzhitov, and A. C. Shaw. 2007. Prevaccine determination of the expression of costimulatory B7 molecules in activated monocytes predicts influenza vaccine responses in young and older adults. J. Infect. Dis. 195:1590-1597. [DOI] [PubMed] [Google Scholar]
- 76.van Ginkel, F. W., R. J. Jackson, N. Yoshino, Y. Hagiwara, D. J. Metzger, T. D. Connell, H. L. Vu, M. Martin, K. Fujihashi, and J. R. McGhee. 2005. Enterotoxin-based mucosal adjuvants alter antigen trafficking and induce inflammatory responses in the nasal tract. Infect. Immun. 73:6892-6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waag, D. M., M. J. McCluskie, N. Zhang, and A. M. Krieg. 2006. A CpG oligonucleotide can protect mice from a low aerosol challenge dose of Burkholderia mallei. Infect. Immun. 74:1944-1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Waldman, R. H., J. O. Bond, L. P. Levitt, E. C. Hartwig, E. C. Prather, R. L. Baratta, J. S. Neill, and P. A. Small, Jr. 1969. An evaluation of influenza immunization: influence of route of administration and vaccine strain. Bull. W. H. O. 41:543-548. [PMC free article] [PubMed] [Google Scholar]
- 79.Wikstrom, M. E., E. Batanero, M. Smith, J. A. Thomas, C. von Garnier, P. G. Holt, and P. A. Stumbles. 2006. Influence of mucosal adjuvants on antigen passage and CD4+ T cell activation during the primary response to airborne allergen. J. Immunol. 177:913-924. [DOI] [PubMed] [Google Scholar]
- 80.Williams, A. E., L. Edwards, I. R. Humphreys, R. Snelgrove, A. Rae, R. Rappuoli, and T. Hussell. 2004. Innate imprinting by the modified heat-labile toxin of Escherichia coli (LTK63) provides generic protection against lung infectious disease. J. Immunol. 173:7435-7443. [DOI] [PubMed] [Google Scholar]
- 81.Wong, J. P., M. E. Christopher, A. M. Salazar, R. M. Dale, L. Q. Sun, and M. Wang. 2007. Nucleic acid-based antiviral drugs against seasonal and avian influenza viruses. Vaccine 25:3175-3178. [DOI] [PubMed] [Google Scholar]
- 82.Wong, J. P., L. P. Nagata, M. E. Christopher, A. M. Salazar, and R. M. Dale. 2005. Prophylaxis of acute respiratory virus infections using nucleic acid-based drugs. Vaccine 23:2266-2268. [DOI] [PubMed] [Google Scholar]
- 83.Zhao, Q., J. Temsamani, R. Z. Zhou, and S. Agrawal. 1997. Pattern and kinetics of cytokine production following administration of phosphorothioate oligonucleotides in mice. Antisense Nucleic Acid Drug Dev. 7:495-502. [DOI] [PubMed] [Google Scholar]
- 84.Zheng, B. J., K. W. Chan, Y. P. Lin, G. Y. Zhao, C. Chan, H. J. Zhang, H. L. Chen, S. S. Wong, S. K. Lau, P. C. Woo, K. H. Chan, D. Y. Jin, and K. Y. Yuen. 2008. Delayed antiviral plus immunomodulator treatment still reduces mortality in mice infected by high inoculum of influenza A/H5N1 virus. Proc. Natl. Acad. Sci. U. S. A. 105:8091-8096. [DOI] [PMC free article] [PubMed] [Google Scholar]