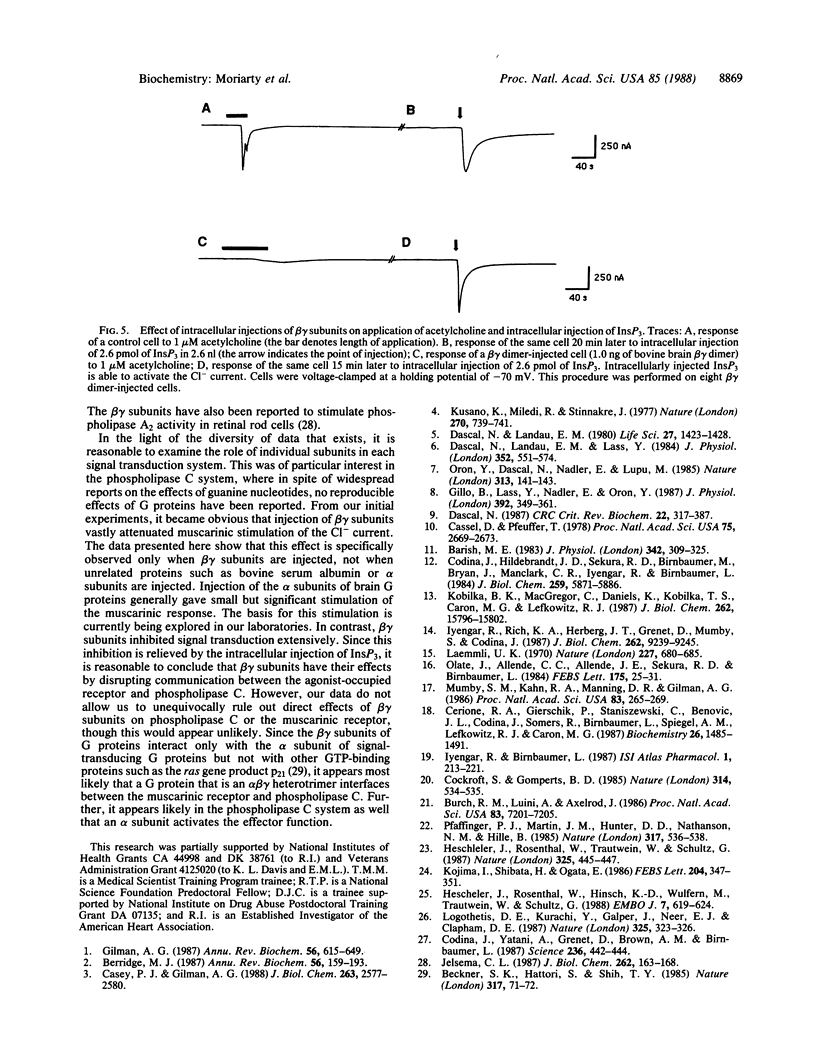
Abstract
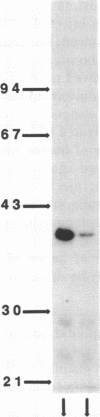
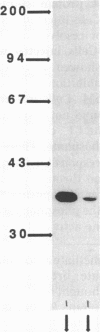
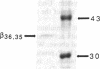
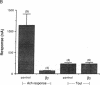
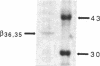
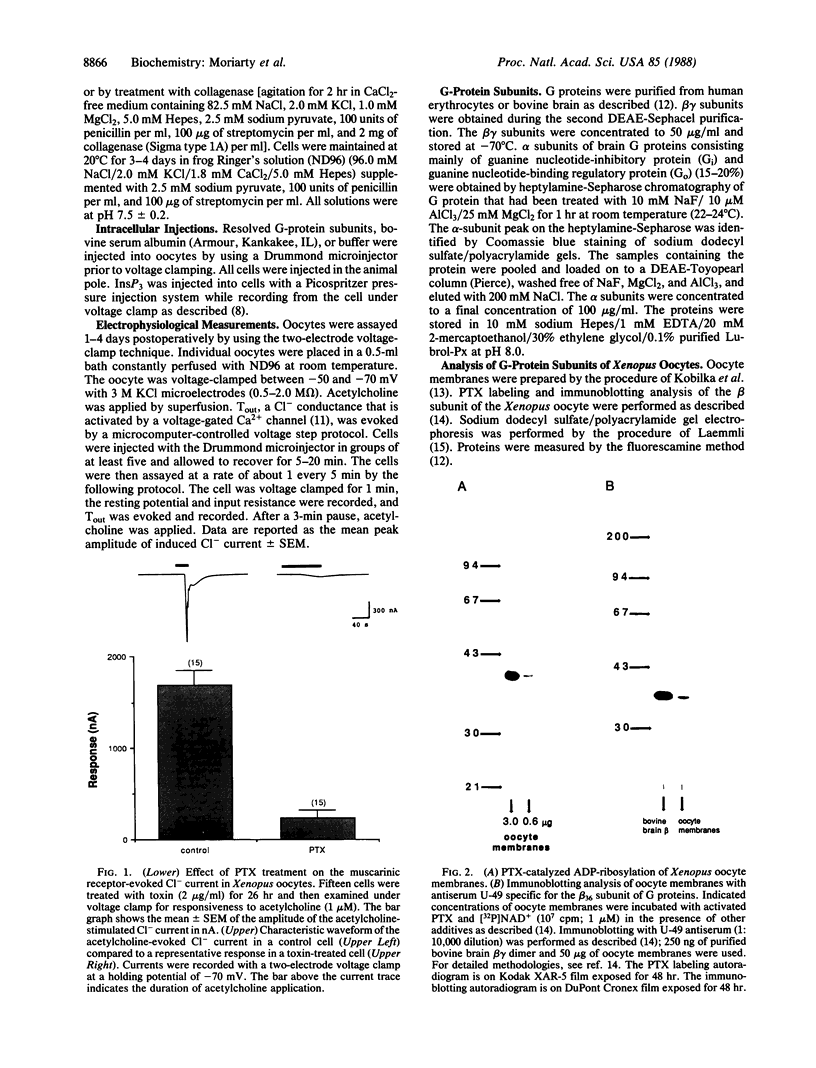
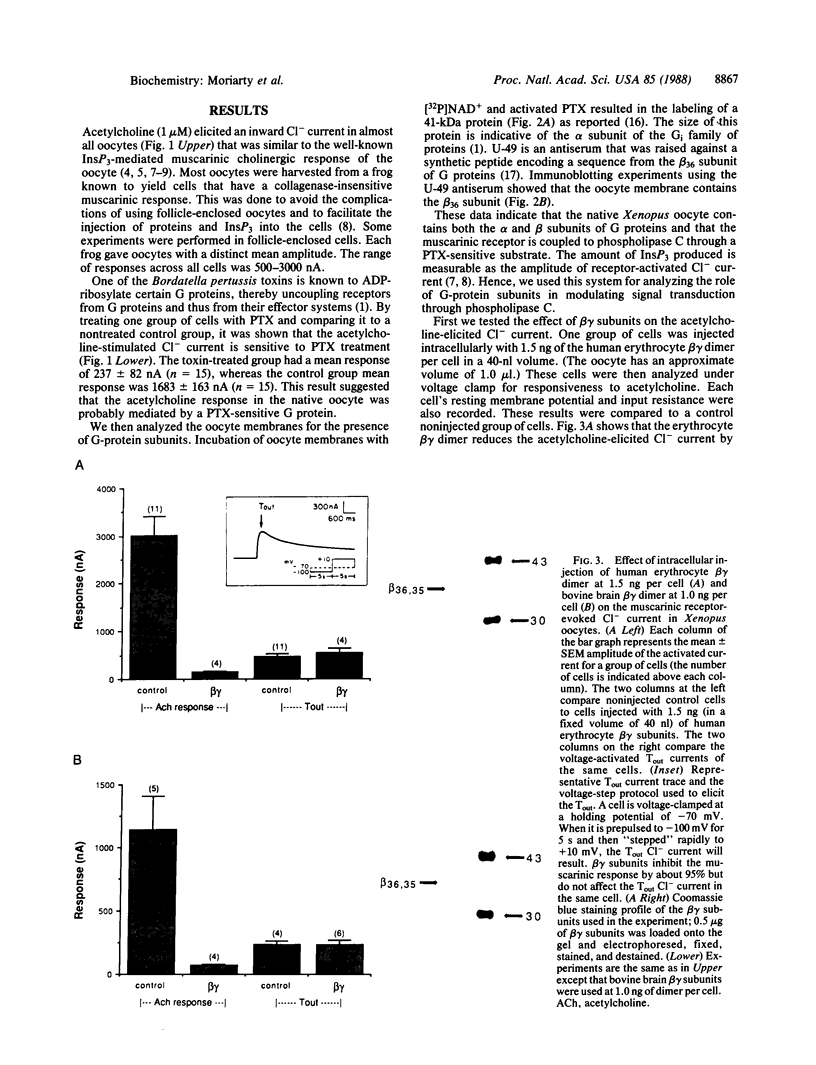
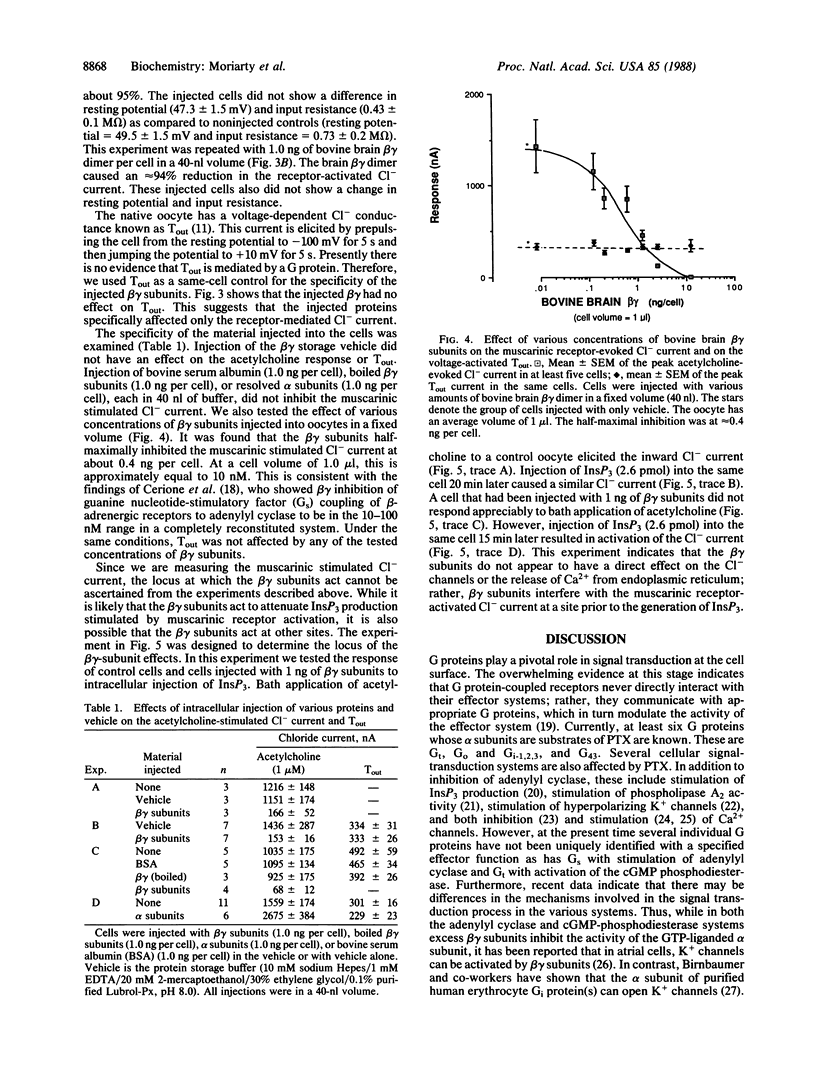
This study examines the mechanism of guanine nucleotide-binding protein (G protein) coupling of receptors to phospholipase C. The Xenopus oocyte has a muscarinic receptor-activated Cl- current that is mediated by inositol 1,4,5-trisphosphate. Modulation of the muscarinic receptor-evoked Cl- current was examined under voltage clamp in oocytes injected with resolved G-protein subunits. The presence of an alpha subunit of G proteins in oocytes was shown by pertussis toxin-labeling of a 41-kDa band in oocyte membranes. The presence of the beta subunit of G proteins was demonstrated by immunoblotting experiments with an antiserum (U-49) that is specific for the beta subunit. Pertussis toxin treatment of oocytes resulted in the uncoupling of muscarinic receptors from activation of the Cl- current. Cells microinjected with 1.5 ng of human erythrocyte beta gamma-subunit complex or 1.0 ng of bovine brain beta gamma-subunit complex showed approximately a 95% reduction in the evoked Cl- current. Cells injected with equal volumes of protein storage vehicle showed no change in response. Cells injected with boiled beta gamma subunits, bovine serum albumin, or resolved alpha subunits also showed no reduction in response. Cells injected with various concentrations of beta gamma subunits showed a concentration dependence with half-maximal inhibition of the muscarinic activated Cl- current at about 10 nM. Cells injected with 1.0 ng of bovine brain beta gamma subunits could not respond to bath-applied agonist but could generate the Cl- current on intracellular injection of inositol 1,4,5-trisphosphate. These observations suggest that there is a G protein responsible for muscarinic receptor-mediated signal transduction through phospholipase C and that it is an alpha beta gamma heterotrimer. It appears that the mode of action of the G protein in the phospholipase C system may be similar to that of the hormone-activated adenylyl cyclase.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barish M. E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J Physiol. 1983 Sep;342:309–325. doi: 10.1113/jphysiol.1983.sp014852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckner S. K., Hattori S., Shih T. Y. The ras oncogene product p21 is not a regulatory component of adenylate cyclase. Nature. 1985 Sep 5;317(6032):71–72. doi: 10.1038/317071a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Burch R. M., Luini A., Axelrod J. Phospholipase A2 and phospholipase C are activated by distinct GTP-binding proteins in response to alpha 1-adrenergic stimulation in FRTL5 thyroid cells. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7201–7205. doi: 10.1073/pnas.83.19.7201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. J., Gilman A. G. G protein involvement in receptor-effector coupling. J Biol Chem. 1988 Feb 25;263(6):2577–2580. [PubMed] [Google Scholar]
- Cassel D., Pfeuffer T. Mechanism of cholera toxin action: covalent modification of the guanyl nucleotide-binding protein of the adenylate cyclase system. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2669–2673. doi: 10.1073/pnas.75.6.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerione R. A., Gierschik P., Staniszewski C., Benovic J. L., Codina J., Somers R., Birnbaumer L., Spiegel A. M., Lefkowitz R. J., Caron M. G. Functional differences in the beta gamma complexes of transducin and the inhibitory guanine nucleotide regulatory protein. Biochemistry. 1987 Mar 10;26(5):1485–1491. doi: 10.1021/bi00379a041. [DOI] [PubMed] [Google Scholar]
- Cockcroft S., Gomperts B. D. Role of guanine nucleotide binding protein in the activation of polyphosphoinositide phosphodiesterase. Nature. 1985 Apr 11;314(6011):534–536. doi: 10.1038/314534a0. [DOI] [PubMed] [Google Scholar]
- Codina J., Hildebrandt J. D., Sekura R. D., Birnbaumer M., Bryan J., Manclark C. R., Iyengar R., Birnbaumer L. Ns and Ni, the stimulatory and inhibitory regulatory components of adenylyl cyclases. Purification of the human erythrocyte proteins without the use of activating regulatory ligands. J Biol Chem. 1984 May 10;259(9):5871–5886. [PubMed] [Google Scholar]
- Codina J., Yatani A., Grenet D., Brown A. M., Birnbaumer L. The alpha subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987 Apr 24;236(4800):442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- Dascal N., Landau E. M., Lass Y. Xenopus oocyte resting potential, muscarinic responses and the role of calcium and guanosine 3',5'-cyclic monophosphate. J Physiol. 1984 Jul;352:551–574. doi: 10.1113/jphysiol.1984.sp015310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dascal N., Landau E. M. Types of muscarinic response in Xenopus oocytes. Life Sci. 1980 Oct 13;27(15):1423–1428. doi: 10.1016/0024-3205(80)90407-5. [DOI] [PubMed] [Google Scholar]
- Dascal N. The use of Xenopus oocytes for the study of ion channels. CRC Crit Rev Biochem. 1987;22(4):317–387. doi: 10.3109/10409238709086960. [DOI] [PubMed] [Google Scholar]
- Gillo B., Lass Y., Nadler E., Oron Y. The involvement of inositol 1,4,5-trisphosphate and calcium in the two-component response to acetylcholine in Xenopus oocytes. J Physiol. 1987 Nov;392:349–361. doi: 10.1113/jphysiol.1987.sp016784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins: transducers of receptor-generated signals. Annu Rev Biochem. 1987;56:615–649. doi: 10.1146/annurev.bi.56.070187.003151. [DOI] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Hinsch K. D., Wulfern M., Trautwein W., Schultz G. Angiotensin II-induced stimulation of voltage-dependent Ca2+ currents in an adrenal cortical cell line. EMBO J. 1988 Mar;7(3):619–624. doi: 10.1002/j.1460-2075.1988.tb02855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hescheler J., Rosenthal W., Trautwein W., Schultz G. The GTP-binding protein, Go, regulates neuronal calcium channels. 1987 Jan 29-Feb 4Nature. 325(6103):445–447. doi: 10.1038/325445a0. [DOI] [PubMed] [Google Scholar]
- Iyengar R., Rich K. A., Herberg J. T., Grenet D., Mumby S., Codina J. Identification of a new GTP-binding protein. A Mr = 43,000 substrate for pertussis toxin. J Biol Chem. 1987 Jul 5;262(19):9239–9245. [PubMed] [Google Scholar]
- Jelsema C. L. Light activation of phospholipase A2 in rod outer segments of bovine retina and its modulation by GTP-binding proteins. J Biol Chem. 1987 Jan 5;262(1):163–168. [PubMed] [Google Scholar]
- Kobilka B. K., MacGregor C., Daniel K., Kobilka T. S., Caron M. G., Lefkowitz R. J. Functional activity and regulation of human beta 2-adrenergic receptors expressed in Xenopus oocytes. J Biol Chem. 1987 Nov 15;262(32):15796–15802. [PubMed] [Google Scholar]
- Kojima I., Shibata H., Ogata E. Pertussis toxin blocks angiotensin II-induced calcium influx but not inositol trisphosphate production in adrenal glomerulosa cell. FEBS Lett. 1986 Aug 18;204(2):347–351. doi: 10.1016/0014-5793(86)80841-9. [DOI] [PubMed] [Google Scholar]
- Kusano K., Miledi R., Stinnakre J. Acetylcholine receptors in the oocyte membrane. Nature. 1977 Dec 22;270(5639):739–741. doi: 10.1038/270739a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Logothetis D. E., Kurachi Y., Galper J., Neer E. J., Clapham D. E. The beta gamma subunits of GTP-binding proteins activate the muscarinic K+ channel in heart. Nature. 1987 Jan 22;325(6102):321–326. doi: 10.1038/325321a0. [DOI] [PubMed] [Google Scholar]
- Mumby S. M., Kahn R. A., Manning D. R., Gilman A. G. Antisera of designed specificity for subunits of guanine nucleotide-binding regulatory proteins. Proc Natl Acad Sci U S A. 1986 Jan;83(2):265–269. doi: 10.1073/pnas.83.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olate J., Allende C. C., Allende J. E., Sekura R. D., Birnbaumer L. Oocyte adenylyl cyclase contains Ni, yet the guanine nucleotide-dependent inhibition by progesterone is not sensitive to pertussis toxin. FEBS Lett. 1984 Sep 17;175(1):25–30. doi: 10.1016/0014-5793(84)80562-1. [DOI] [PubMed] [Google Scholar]
- Oron Y., Dascal N., Nadler E., Lupu M. Inositol 1,4,5-trisphosphate mimics muscarinic response in Xenopus oocytes. Nature. 1985 Jan 10;313(5998):141–143. doi: 10.1038/313141a0. [DOI] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]