Abstract
Members of the genus Ranavirus (family Iridoviridae) have been recognized as major viral pathogens of cold-blooded vertebrates. Ranaviruses have been associated with amphibians, fish, and reptiles. At this time, the relationships between ranavirus species are still unclear. Previous studies suggested that ranaviruses from salamanders are more closely related to ranaviruses from fish than they are to ranaviruses from other amphibians, such as frogs. Therefore, to gain a better understanding of the relationships among ranavirus isolates, the genome of epizootic hematopoietic necrosis virus (EHNV), an Australian fish pathogen, was sequenced. Our findings suggest that the ancestral ranavirus was a fish virus and that several recent host shifts have taken place, with subsequent speciation of viruses in their new hosts. The data suggesting several recent host shifts among ranavirus species increase concern that these pathogens of cold-blooded vertebrates may have the capacity to cross numerous poikilothermic species barriers and the potential to cause devastating disease in their new hosts.
Iridoviruses are large, double-stranded DNA viruses that infect both vertebrate and invertebrate hosts (9, 64). The family Iridoviridae currently contains five genera, the Iridovirus and Chloriridovirus genera, associated with insects, the Lymphocystivirus and Megalocytivirus genera, which infect fish species, and the genus Ranavirus, whose members have been associated with mortality events in amphibians, fish, and reptiles (64). At this time, the type isolates for each genus in the family Iridoviridae have been sequenced (Table 1).
TABLE 1.
Completely sequenced iridoviruses
| Genus | Virus | Known host | Genome size (kb) | GC content (%) | No. of potential genes | GenBank accession no. |
|---|---|---|---|---|---|---|
| Ranavirus | ATV | Salamander | 106,332 | 54 | 92 | AY150217 |
| EHNV | Fish | 127,011 | 54 | 100 | FJ433873 | |
| FV3 | Frog | 105,903 | 55 | 97 | AY548484 | |
| TFV | Frog | 105,057 | 55 | 103 | AF389451 | |
| SGIV | Fish | 140,131 | 48 | 139 | AY521625 | |
| GIV | Fish | 139,793 | 49 | 139 | AY666015 | |
| Megalocytivirus | ISKNV | Fish | 111,362 | 55 | 117 | AF371960 |
| OSGIV | Fish | 112,636 | 54 | 116 | AY894343 | |
| RBIV | Fish | 112,080 | 53 | 116 | AY532606 | |
| Lymphocystivirus | LCDV-1 | Fish | 102,653 | 29 | 108 | L63545 |
| LCDV-C | Fish | 186,247 | 27 | 178 | AY380826 | |
| Iridovirus | IIV-6 (CIV) | Insect | 212,482 | 29 | 211 | AF303741 |
| Chloriridovirus | IIV-3 (MIV) | Insect | 190,132 | 48 | 126 | DQ643392 |
Members of the genus Ranavirus have been recognized as major pathogens of economically and ecologically important cold-blooded vertebrates (8, 64). For example, ranaviruses (RVs) have been isolated from amphibians in North America (6, 18, 24, 34, 35), Asia (27, 66), Australia (56), and the United Kingdom (10, 19), from fish (2, 41, 46), and from reptiles (3, 14, 30, 37, 42, 43). In fact, ranaviruses are now considered agents of emerging infectious disease (9). As interest in RVs has grown, the number of ranaviruses that have been completely sequenced has also increased. These include frog virus 3 (FV3) (58), the type virus of the genus Ranavirus; tiger frog virus (TFV) (27), an RV closely related to FV3 that was isolated from frogs in Asia; and Ambystoma tigrinum virus (ATV) (36), an RV associated with salamander mortalities in North America. In addition, two grouper iridoviruses which are also members of the genus Ranavirus, the grouper iridovirus (GIV) (62) and the Singapore grouper iridovirus (SGIV) (53), were recently sequenced. In addition, at the time of preparation of the manuscript, the genomic sequence of the soft-shelled turtle ranavirus (STIV) became available (29). Information obtained by comparing ranavirus genomic sequences offers insight into RV evolutionary history, identifies core groups of genes, and gives insight into the genes responsible for viral immune evasion and pathogenesis.
Previous studies have shown that RV isolates can be translocated across large distances in infected salamanders that are used as bait for sport fishing (35, 44, 51). Phylogenetic analysis was used to compare the major capsid protein (MCP) sequences from salamander RV isolates from the southern Arizona border to Canada to other RV MCP sequences (35). The data suggest that salamander RV isolates are more closely related to fish RV isolates, such as epizootic hematopoietic necrosis virus (EHNV), than to other amphibian (frog) RV isolates, such as FV3 (35). Dot plot analysis comparing the genomic sequence of ATV to those of FV3 and TFV showed two major genomic inversions (36), while the FV3 and TFV genomes showed complete colinearity. These data suggest that at some point in virus evolutionary history, an ancestral virus diverged into the salamander virus and frog virus lineages. A genomic rearrangement occurred in one of the lineages at the time of divergence or after. Subsequent host-specific evolution occurred, limiting cross transmission among isolates, in such a way that frog RVs do not cause disease during laboratory infection of salamanders and vice versa (34). There is some evidence that salamander RV isolates can be isolated from or detected in laboratory-infected frogs (52) and that a pathogen host shift is the result of the movement of these pathogens (35). Thus, the ecological and economic consequences of RVs moving in the environment include the potential of these pathogens infecting and decimating new amphibian, fish, or reptile populations. Therefore, a more complete understanding of the genetic determinants that make up RVs would help to predict future transmission events.
EHNV was isolated in Australia from redfin perch (Perca fluviatilis) and rainbow trout (Oncorhynchus mykiss) (38, 39). EHNV can be classified as an indiscriminate pathogen of freshwater finfish, as it readily kills juvenile redfin perch and rainbow trout in inland water bodies throughout Australia (63). In addition, challenge experiments showed that following bath inoculation, other fish species are also susceptible to infection with EHNV, including the Macquarie perch (Macquaria australasica), silver perch (Bidyanus bidyanus), mosquito fish (Gambusia affinis), and mountain galaxias (Galaxias olidus). In contrast, Murray cod (Maccullochella peeli), golden perch (Macquaria ambigua), Australian bass (Macquaria novemaculeata), Macquarie perch, silver perch, and Atlantic salmon (Salmo salar) were susceptible only by intraperitoneal (i.p.) injection of virus. Serological surveys (A. Hyatt, unpublished data) show that redfin perch and rainbow trout can also be carriers of EHNV. Virus was reisolated from animals not showing clinical signs of disease, making them likely vehicles for the translocation and introduction of EHNV into naïve host populations. Preliminary and unpublished data have shown that i.p. inoculation of Australian frogs or the cane toad Bufo marinus with EHNV results in seroconversion but no signs of clinical disease (68). While EHNV has not been identified in fish populations in North America, it is possible that this pathogen could be translocated via movement of animals for food, bait, or scientific purposes, thereby infecting and potentially decimating naïve fish populations. In fact, the disease caused by EHNV is recognized by the World Organization for Animal Health (Office International Epizootics [OIE]) as a major cause of finfish mortalities (www.oie.int). In addition, both an EHNV disease in fish and a ranavirus infection in amphibians are notifiable diseases to OIE. Since the recognition of disease due to EHNV in Australia in 1986, similar systemic necrotizing iridovirus syndromes in farmed fish have been reported. These include catfish (Ictalurus melas) in France (European catfish virus) (45), sheatfish (Silurus glanis) in Germany (European sheatfish virus) (1), turbot (Scophthalmus maximus) in Denmark (5), and pike perch (Stizostedion lucioperca) in Finland (59). In addition, while EHNV has been classified as an RV, the relationship between this fish pathogen and amphibian RVs is poorly understood. Therefore, in order to better understand the relationships among RV isolates, the complete sequence of EHNV genomic DNA was determined. The characteristics of the EHNV genome, its relatedness to other iridoviruses, and insights into RV evolution are the focus of this study.
MATERIALS AND METHODS
Generation of EHNV genomic DNA library.
EHNV DNA was isolated as previously described (65) from cell culture-amplified virus stocks of the original EHNV isolated in Australia (39). The EHNV shotgun library was constructed by kinetically shearing 10 μg of viral DNA in 200 μl of TE (10 mM Tris-HCl, 1 mM EDTA) buffer. The sheared DNA was ethanol precipitated, and the pellet containing DNA was end repaired using T4 DNA polymerase and Klenow polymerase, concentrated by ethanol precipitation, and quantified. BstXI adaptors were ligated to the end-repaired viral DNA and then size selected by gel electrophoresis. DNAs of 2 to 4 kbp were extracted from the gel and ligated into the pOTWI3 plasmid. Plasmid DNA was transformed into DH10B competent cells by electroporation, plated on prewarmed agar plates containing 50 μg/ml chloramphenicol, and incubated overnight at 37°C. Colonies containing plasmid were selected using automated equipment, and plasmid DNA was isolated using solid-phase reversible immobilization (SPRI) technology. Isolated plasmids were sequenced from both ends of the insert by use of automated equipment (ABI 3730XL; Applied Biosystems). Sequences were aligned and assembled using Phred/Phrap (http://www.phrap.org) and finished using standard methods, with the aid of Consed (23).
Genome annotation.
The newly sequenced genome was annotated using similar procedures to those described previously (36). Using the BLASTP, BLASTX, and TBLASTX procedures (49, 50), all open reading frames (ORFs) with sequence similarity to any other closely related viral ORF and/or containing a domain(s) or homology with any known protein were identified. Identified ORFs were confirmed using the Genome Annotation Transfer Utility (GATU) (http://www.biovirus.org/), a program that uses previously annotated genomic DNA as a reference for annotating a newly sequenced genomic DNA, using all of the completely sequenced iridoviruses as reference sequences. The iridoviruses used in this analysis were as follows (also see Table 1): ATV (36), FV3 (58), TFV (27), GIV (62), SGIV (53), lymphocystis disease virus 1 (LCDV-1) (60), lymphocystis disease virus China (LCDV-C) (67), infectious spleen and kidney necrosis virus (ISKNV) (26), orange spotted grouper iridovirus (OSGIV) (40), rock bream iridovirus (RBIV) (15), insect iridovirus 6 (IIV-6) or Chilo iridovirus (CIV) (33), and invertebrate iridovirus 3 (IIV-3) or mosquito iridovirus (MIV) (13). The genome of a soft-shelled turtle ranavirus isolate was published during the preparation of the manuscript (29). However, due to the timing of this publication, the genomic information from this newly isolated RV was not used in our analysis. ORFs in the Iridoviridae family are presumed to be nonoverlapping; however, ORFs were considered overlapping if both ORFs had high sequence identity (i.e., a high BLASTP expect score) to other sequenced iridoviruses.
Phylogenetic and dot plot analysis.
Concatenated iridovirus phylogenetic analysis was conducted by obtaining the homologues of the EHNV ORFs 1L (myristylated membrane protein), 7R (RNA polymerase, α subunit), 8L (NTPase/helicase), 10L (DNA repair enzyme RAD2), 11R, 13L (ICP-46), 14L (MCP), 16L (thiol oxidoreductase), 18L (thymidine kinase), 19L (PCNA), 23L (transcription elongation factor SII), 24R (RNase III), 38R (ribonucleotide reductase, small subunit), 43R (RNA polymerase β subunit), 44L (DNA polymerase), 48L (CTD-phosphotransferase), 53L (myristylated membrane protein), 62R (tyrosine kinase), 72R, 77R, 85L (D5 NTPase), 86R, 89L (serine-threonine protein kinase), 92L (ABC ATPase), 95R, and 100R (putative replication factor) from representative members of the sequenced iridoviruses (see Tables S1 and S2 in the supplemental material) in GenBank by BLASTP analysis (http://www.ncbi.nlm.nih.gov/). All sequences were concatenated using BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html). The sequences were aligned and neighbor-joining analysis was conducted using MEGA4 software (57) with default options.
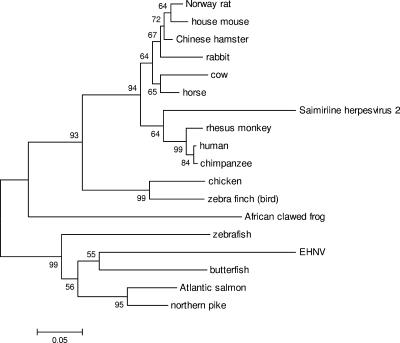
Phylogenetic analysis of the EHNV ORF 87L was performed by acquiring homologous sequences by BLASTP analysis (http://www.ncbi.nlm.nih.gov/). The homologous sequences used in this phylogeny were from butterfish (GenBank accession no. ACQ58597.1), Atlantic salmon (NP_001135373.1), Northern pike (ACO14357.1), zebrafish (CAM14042.1), Norway rat (NP_569084.1), Chinese hamster (BAE78431.1), house mouse (NP_034179.1), rabbit (ACO49549.1), cow (P00376.3), horse (XP_001504693.1), rhesus monkey (XP_001110551.1), human (NP_000782.1), chimpanzee (XP_001134992.1), African clawed frog (NP_001088506.1), chicken (NP_001006584.2), zebra finch (XP_002190476.1), and herpesvirus saimiri 2 (NP_040203.1). The sequences were aligned and neighbor-joining analysis was conducted using MEGA4 software (57) with default options.
Dot plots using whole genome sequences comparing all of the sequenced iridoviruses (Table 1) to EHNV were generated using JDotter (http://www.biovirus.org/) (54, 55), using the default settings.
Nucleotide sequence accession number.
The EHNV sequence was deposited in the GenBank database (http://www.ncbi.nlm.nih.gov/) under accession number FJ433873.
RESULTS AND DISCUSSION
EHNV genome characteristics.
An EHNV random genomic DNA library was successfully generated and produced over 1,800 EHNV-specific sequences. The sequences contributed to the assembly of the complete genomic sequence, with an average final sequence coverage of >4-fold.
The finished EHNV genomic sequence (127,011 bp) is larger than the genomes of the amphibian RVs ATV, TFV, and FV3 (average, 105,754 bp), but smaller than the genomes of the grouper RVs GIV and SGIV (average, 139,962 bp) (Table 1). In addition, the EHNV genome is larger than the genomes of the other fish pathogens ISKNV, OSGIV, and RBIV (average, 112,026 bp) but smaller than the insect viral genomes of CIV and MIV (average, 201,307 bp). The lymphocystiviruses LCDV-1 and LCDV-C have very differently sized genomes (Table 1). EHNV genomic DNA is smaller than the average of these two viral genomic sequences (average, 144,450 bp). EHNV has a similar G+C content (54%) to those of TFV, FV3, ATV, ISKNV, OSGIV, and RBIV (53 to 55%), a slightly higher G+C content than those of the grouper iridoviruses GIV and SGIV and the insect iridovirus MIV (48 to 49%), and a much higher G+C content than those of LCDV-1, LCDV-C, and CIV (27 to 29%).
Open reading frame analysis.
One hundred ORFs are predicted for the EHNV genome, based on the annotation criteria described in Materials and Methods (Fig. 1; Table 2; see Table S1 in the supplemental material). The number of ORFs predicted for EHNV (100) is similar to the number of ORFs predicted for ATV (92) and close to the numbers of ORFs predicted for FV3 (97) and TFV (103) as well; however, all of these RVs have considerably fewer ORFs than the 139 ORFs seen in the fish RVs GIV and SGIV (Table 1). In addition, the number of EHNV ORFs is relatively close to the numbers of ORFs predicted for the other fish iridoviruses ISKNV, OSGIV, RBIV, and LCDV-1, while the numbers of ORFs in LCDV-C, CIV, and MIV are much larger, corresponding to their larger genome sizes (Table 1).
FIG. 1.
Annotation of the EHNV genome showing the order and orientation of ORFs. The EHNV genome was annotated as described in Materials and Methods. Each arrow represents an ORF in the right or left orientation. Conserved iridovirus ORFs (C) and putative virulence ORFs (V) are indicated. The brackets enclose the region of consecutively oriented ORFs and the arrows indicate the locations of genomic inversions compared to FV3.
TABLE 2.
Predicted EHNV open reading frames and best-matching iridovirus homologues
| ORFa | Position (bp) | Size (no. of amino acids) | MW | Predicted function | Best-matching iridovirus ORF | Expect score | % Identity | % Positive | Gap % | GenBank accession no. |
|---|---|---|---|---|---|---|---|---|---|---|
| 1L | 11-1063 | 350 | 38,082 | Myristylated membrane protein | FV3 2L | 1e−145 | 98 | 98 | 0 | YP_031580 |
| 2L | 1101-1940 | 279 | 31,302 | FV3 2.5R | 6e−163 | 98 | 99 | 0 | AY548484 | |
| 3R | 1970-3184 | 404 | 44,679 | TFV 4R | 0.0 | 97 | 99 | 0 | ABB92272 | |
| 4R | 3225-3407 | 60 | 6,615 | ATV 4R | 8e−18 | 95 | 95 | 0 | YP_003775 | |
| 5L | 4077-4715 | 212 | 24,359 | FV3 6R | 4e−37 | 97 | 100 | 0 | YP_031584 | |
| 6L | 5931-6353 | 140 | 14,881 | ATV 5L | 5e−48 | 92 | 94 | 0 | YP_003776 | |
| 7R | 6433-10344 | 1,303 | 141,815 | RNA polymerase α subunit | ATV 6R | 0.0 | 97 | 98 | 0 | YP_003777 |
| 8L | 11025-13871 | 948 | 106,432 | NTPase or helicase | ATV 7L | 0.0 | 97 | 98 | 0 | AAP33184 |
| 9R | 13887-14300 | 137 | 15,018 | TFV 10R | 2e−70 | 99 | 99 | 0 | ABB92276 | |
| 10L | 15273-16367 | 364 | 40,646 | DNA repair enzyme RAD2 | ATV 10L | 0.0 | 97 | 99 | 0 | AAP33187 |
| 11R | 16461-16928 | 155 | 17,886 | ATV 11R | 7e−78 | 97 | 97 | 0 | AAP33188 | |
| 12R | 17041-17208 | 55 | 5,686 | TFV 99L | 2e−09 | 96 | 98 | 0 | ABB92345 | |
| 13L | 18518-19705 | 395 | 45,578 | Immediate-early protein ICP-46 | FV3 91R | 0.0 | 97 | 98 | 0 | AAT09751 |
| 14L | 19829-21220 | 463 | 50,105 | Major capsid protein | ATV 14L | 0.0 | 98 | 99 | 0 | AAP33191 |
| 15L | 21313-22419 | 368 | 41,742 | TFV 95R | 1e−135 | 79 | 80 | 14 | ABB92343 | |
| 16L | 22487-22939 | 150 | 16,652 | Thiol oxidoreductase | ATV 16L | 2e−83 | 99 | 100 | 0 | AAP33193 |
| 17R | 22972-24825 | 617 | 66,505 | TFV 93L | 0.0 | 96 | 97 | 0 | ABB92341 | |
| 18L | 26068-26655 | 195 | 22,132 | Deoxyribonucleoside kinase/thymidine kinase | TFV 91.5R | 7e−104 | 97 | 98 | 0 | ABB92339 |
| 19L | 26730-27512 | 260 | 27,685 | Proliferating cell nuclear antigen (PCNA) | ATV 20L | 3e−133 | 96 | 98 | 0 | AAP33197 |
| 20L | 27933-28577 | 214 | 24,897 | Cytosine DNA methyltransferase | ATV 21L | 9e−119 | 97 | 98 | 0 | AAP33198 |
| 21L | 29728-30621 | 297 | 33,872 | Thymidylate synthase | ATV 22L | 4e−145 | 94 | 96 | 0 | AAP33199 |
| 22L | 30900-31373 | 157 | 17,405 | Putative immediate-early protein | ATV 23L | 5e−89 | 99 | 100 | 0 | AAP33200 |
| 23L | 31502-31780 | 92 | 10,427 | Transcription elongation factor SII | ATV 24L | 8e−41 | 98 | 98 | 0 | AAP33201 |
| 24R | 31836-32954 | 372 | 40,481 | RNase III | ATV 25R | 0.0 | 98 | 99 | 0 | AAP33202 |
| 25L | 33681-35618 | 645 | 71,712 | ATV 26L | 0.0 | 82 | 85 | 10 | AAP33203 | |
| 26R* | 36724-37491 | 255 | 29,090 | TFV 83L | 2e−118 | 94 | 95 | 0 | ABB92336 | |
| 27R | 38298-38645 | 115 | 12,821 | ATV 27R | 1e−54 | 97 | 99 | 0 | AAP33204 | |
| 28L | 38642-38863 | 73 | 7,952 | ATV 28L | 3e−35 | 98 | 98 | 0 | AAP33205 | |
| 29R | 38926-39180 | 84 | 9,228 | ATV 29R | 8e−30 | 95 | 96 | 0 | AAP33206 | |
| 30R | 39237-40418 | 393 | 42,021 | ATV 30R | 2e−164 | 92 | 95 | 0 | AAP33207 | |
| 31R | 40638-41612 | 324 | 36,090 | NTPase/helicase | ATV 31R | 1e−177 | 98 | 99 | 0 | AAP33208 |
| 32R | 42423-42938 | 171 | 18,773 | FV3 72L | 8e−78 | 97 | 99 | 0 | AAT09732 | |
| 33L | 42429-42911 | 160 | 17,275 | TFV 77L | 2e−89 | 98 | 100 | 0 | ABB92331 | |
| 34L | 43196-43432 | 78 | 8,381 | ATV 34L | 4e−24 | 92 | 94 | 0 | AAP33211 | |
| 35L | 43473-43841 | 122 | 13,201 | ATV 34.5L | 9e−60 | 95 | 96 | 1 | AY150217 | |
| 36L | 43859-44125 | 88 | 9,360 | FV3 69R | 7e−37 | 98 | 98 | 0 | AAT09729 | |
| 37R | 44233-44937 | 234 | 25,229 | ATV 37R | 6e−112 | 90 | 95 | 0 | AAP33214 | |
| 38R | 45326-46489 | 387 | 44,030 | Ribonucleotide reductase, small subunit | ATV 38R | 0.0 | 98 | 99 | 0 | AAP33216 |
| 39R | 46547-47098 | 183 | 18,610 | ATV38.5L | 3e−49 | 96 | 97 | 0 | AY150217 | |
| 40R | 47337-48059 | 240 | 22,522 | FV3 65L | 2e−07 | 97 | 97 | 0 | AAT09725 | |
| 41L | 48535-48822 | 95 | 10,372 | CARD-like caspase; putative interleukin-1 beta convertase | ATV 40L | 7e−42 | 94 | 97 | 0 | YP_003812 |
| 42L | 48917-49411 | 164 | 17,426 | dUTPase | FV3 63R | 8e−79 | 96 | 98 | 0 | YP_031642 |
| 43R | 49791-53474 | 1227 | 133,829 | DNA-dependent RNA polymerase, β subunit | FV3 62L | 0.0 | 97 | 98 | 0 | YP_031641 |
| 44L | 54549-57590 | 1013 | 114,508 | DNA polymerase | TFV 63R | 0.0 | 99 | 99 | 0 | AAL77804 |
| 45R | 57756-58814 | 352 | 39,781 | FV3 59L | 0.0 | 97 | 98 | 0 | YP_031638 | |
| 46L | 59343-59897 | 184 | 20,461 | FV3 58.5R | 2e−121 | 98 | 99 | 0 | AY548484 | |
| 47L | 59912-60250 | 112 | 10,518 | SGIV 45L | 6e−11 | 51 | 59 | 0 | YP_164140 | |
| 48L | 61123-62619 | 498 | 53,522 | CTD-phosphotransferase | ATV 47L | 0.0 | 97 | 98 | 0 | YP_003820 |
| 49L | 62661-63065 | 134 | 15,244 | TFV 58R | 6e−66 | 97 | 98 | 0 | ABB92316 | |
| 50R | 63102-63251 | 49 | 5,208 | TFV 57L | 4e−09 | 97 | 100 | 0 | ABB92315 | |
| 51R | 63259-64554 | 431 | 47,190 | Helicase | ATV 50R | 0.0 | 97 | 98 | 0 | YP_003823 |
| 52R | 64592-64822 | 76 | 8,757 | Putative nuclear calmodulin-binding protein | FV3 54L | 1e−29 | 93 | 96 | 0 | YP_031632 |
| 53L | 65456-67027 | 523 | 54,869 | Myristylated membrane protein | ATV 51L | 0.0 | 98 | 98 | 0 | YP_003824 |
| 54R | 67366-68433 | 355 | 39,399 | 3-β-Hydroxy-d-5-C27-steroid oxidoreductase-like protein | FV3 52L | 0.0 | 96 | 98 | 0 | YP_031630 |
| 55R | 68611-69672 | 353 | 39,507 | FV3 23R | 7e−46 | 32 | 50 | 7 | YP_031601 | |
| 56R | 70259-71407 | 382 | 42,567 | FV3 23R | 0.0 | 97 | 97 | 0 | YP_031601 | |
| 57R | 71804-72901 | 365 | 40,928 | FV3 24R | 0.0 | 96 | 99 | 0 | YP_031602 | |
| 58L | 73215-73724 | 169 | 19,443 | FV3 23R | 2e−14 | 31 | 51 | 4 | YP_031601 | |
| 59L | 74255-74989 | 244 | 27,214 | TFV 23R | 8e−33 | 34 | 56 | 2 | ABB92288 | |
| 60R | 76159-77073 | 304 | 34,545 | p31K | ATV 55R | 2e−148 | 96 | 98 | 0 | YP_003828 |
| 61R | 77399-78178 | 259 | 28,304 | eIF2α homolog | TFV 27R | 4e−134 | 94 | 96 | 0 | AAL77798 |
| 62R | 78765-81677 | 970 | 106,902 | Tyrosine kinase | ATV 58R | 0.0 | 96 | 98 | 0 | YP_003831 |
| 63R | 81726-82235 | 169 | 18,912 | ATV 59R | 8e−84 | 92 | 93 | 4 | YP_003832 | |
| 64R* | 82345-83310 | 321 | 35,189 | Putative capsid maturation protease (herpesvirus) | ||||||
| 65R* | 84057-84881 | 275 | 30,740 | |||||||
| 66R | 84937-85422 | 161 | 17,937 | SGIV 158L | 2e−08 | 32 | 50 | 8 | YP_164253 | |
| 67R | 86177-86596 | 139 | 15,143 | ATV 60R | 7e−73 | 98 | 99 | 0 | YP_003833 | |
| 68R | 86646-88622 | 658 | 73,571 | Neurofilament triplet H1-like protein | TFV 33R | 0.0 | 80 | 83 | 10 | ABB92295 |
| 69R | 88707-88898 | 63 | 6,613 | TFV 34R | 8e−12 | 95 | 96 | 0 | ABB92296 | |
| 70R | 89046-89369 | 107 | 11,546 | ATV 62.5R | 9e−70 | 97 | 98 | 0 | AY150217 | |
| 71L | 89432-89932 | 166 | 17,840 | FV3 35L | 7e−66 | 94 | 94 | 0 | ABB92299 | |
| 72R | 90144-90779 | 211 | 23,478 | TFV 40R | 1e−109 | 98 | 99 | 0 | ABB92302 | |
| 73R | 90918-92615 | 565 | 62,186 | Ribonucleotide reductase, large subunit | TFV 41R | 0.0 | 98 | 99 | 0 | AAL77800 |
| 74R | 92722-93072 | 116 | 12,710 | TFV 42R | 6e−39 | 93 | 95 | 0 | ABB92303 | |
| 75R | 93161-93967 | 268 | 28,876 | ATV 67R | 1e−62 | 76 | 79 | 5 | YP_003841 | |
| 76R | 94224-94409 | 61 | 7,474 | ATV 68R | 2e−−08 | 72 | 72 | 24 | YP_003842 | |
| 77R | 94481-97978 | 1165 | 129,034 | ATV 69R | 0.0 | 97 | 98 | 0 | YP_003843 | |
| 78L | 98486-99343 | 285 | 29,429 | TFV 46L | 5e−74 | 69 | 70 | 21 | ABB92307 | |
| 79L | 99469-99879 | 136 | 15,551 | TFV 47L | 2e−−72 | 99 | 100 | 0 | ABB92308 | |
| 80L | 99933-100544 | 203 | 23,004 | Neurofilament triplet H1-like protein | ATV 72L | 4e−36 | 65 | 70 | 26 | YP_003846 |
| 81L | 100670-101086 | 138 | 15,572 | FV3 47L | 1e−−60 | 97 | 99 | 0 | YP_031625 | |
| 82L | 101089-101340 | 83 | 9,547 | TFV 50L | 9e−−35 | 96 | 98 | 0 | ABB92310 | |
| 83L | 101459-103084 | 541 | 60,677 | ATV 75L | 5e−147 | 77 | 83 | 5 | YP_003850 | |
| 84R | 103165-104850 | 561 | 61,508 | ATV 76R | 0.0 | 97 | 99 | 0 | YP_003851 | |
| 85L | 105693-108614 | 973 | 108,742 | D5 family NTPase | FV3 22R | 0.0 | 98 | 99 | 0 | ABB92287 |
| 86R | 108744-109403 | 219 | 25,327 | ATV 78R | 1e−104 | 99 | 99 | 0 | YP_003853 | |
| 87L* | 110079-110648 | 189 | 21,084 | Dihydrofolate reductase | ||||||
| 88L | 111114-111563 | 149 | 16,142 | ATV 79L | 1e−62 | 97 | 97 | 0 | YP_003854 | |
| 89L | 111611-114334 | 907 | 98,760 | Serine/threonine protein kinase | TFV 19R | 0.0 | 88 | 89 | 7 | ABB92284 |
| 90R | 114659-116167 | 502 | 53,345 | ATV 81R | 0.0 | 99 | 99 | 0 | YP_003856 | |
| 91R | 116276-117151 | 291 | 31,665 | ATV 82.5L | 9e−147 | 96 | 98 | 0 | YP_003857 | |
| 92L | 117540-118466 | 308 | 34,686 | ABC-ATPase | ATV 83L | 3e−178 | 98 | 98 | 0 | YP_003858 |
| 93L | 118563-118919 | 118 | 13,354 | FV3 14R | 3e−44 | 97 | 98 | 0 | YP_031592 | |
| 94R | 119046-119288 | 80 | 9,267 | ATV 85.5L | 4e−31 | 91 | 96 | 0 | YP_003860 | |
| 95R | 119947-120840 | 297 | 32,649 | ATV 87R | 2e−132 | 96 | 98 | 0 | YP_003862 | |
| 96L | 120906-121118 | 70 | 7,885 | ATV 88L | 9e−24 | 95 | 98 | 0 | YP_003863 | |
| 97L | 121917-122354 | 145 | 16,101 | LCDV-C 71L | 0.22 | 29 | 52 | 6 | YP_073578 | |
| 98R | 123028-123714 | 228 | 24,474 | ATV 89R | 8e−121 | 96 | 97 | 0 | YP_003864 | |
| 99R | 123780-124193 | 137 | 15,166 | ATV 90R | 3e−70 | 94 | 97 | 0 | YP_003865 | |
| 100R | 124882-125652 | 256 | 29,693 | Putative replication factor | FV3 1R | 5e−144 | 98 | 98 | 0 | YP_031579 |
*, EHNV-specific ORFs.
Of the 100 EHNV ORFs, 26 ORFs are conserved throughout the family Iridoviridae (see Tables S2 and S3 in the supplemental material). These ORFs can be defined as the core iridovirus genes, since they are present in every iridovirus sequenced to date and confirm published reports that all iridoviruses contain these 26 ORFs (20). The majority of these conserved ORFs (21/26 ORFs) have a predicted function, based on sequence homology to other characterized proteins, or have been identified based on experimental data (see Table S2 in the supplemental material). In contrast, only 4 of the 27 additional ORFs that are conserved throughout the genus Ranavirus have a predicted function (see Tables S1 and S4 in the supplemental material), and only 1 of the 13 amphibian RV-specific genes has a predicted function (the viral homologue of eukaryotic translation initiation factor 2α [vIF2αH; ORF 61R]) (see Table S5 in the supplemental material).
Interestingly, there are three unique EHNV ORFs, namely, ORFs 64R and 65R, which have no homology to any known protein sequence, and ORF 87L, which is predicted to encode a dihydrofolate reductase (DHFR) (Table 2; see Table S1 in the supplemental material). DHFRs are thought to be involved in nucleotide metabolism and have been described for herpesviruses (61). BLAST search analysis of the EHNV ORF 87L showed the highest sequence similarity with butterfish, Atlantic salmon, and northern pike DHFRs (data not shown). Phylogenetic analysis of this EHNV ORF compared to homologous sequences suggests that EHNV acquired this unique gene from a fish host, thereby allowing the virus to replicate and cause disease in finfish (Fig. 2). There is evidence of host-derived gene transfer in poxviruses (7), a group of closely related DNA viruses (31, 32), so it is reasonable to hypothesize that similar events have occurred with iridoviruses. In fact, iridoviruses may have a higher rate of horizontal gene transfer from their host due to the nuclear stage of iridovirus DNA replication (9, 64).
FIG. 2.
Phylogenetic analysis of EHNV ORF 87L. Homologous sequences to the EHNV DHFR gene (ORF 87L) were obtained by BLASTP analysis. The neighbor-joining tree was determined using MEGA4, and it is shown with statistical support indicating the robustness of the inferred branching pattern, as assessed using the bootstrap test. The accession number for each gene in the phylogeny is given in Materials and Methods.
There appear to be nine ORF clusters, containing a minimum of four consecutively oriented ORFs (COOs), throughout the genome. These clusters of COOs all have similar orientations, either right or left, with the majority of the COOs having the same orientation. While the genomes of iridoviruses are circularly permuted and terminally redundant (64), and therefore the orientation of these ORFs relative to the orientation of the start of the genome was an arbitrary decision, the amount of conservation among RV isolates within these regions is surprising. For example, the region between EHNV ORFs 54 and 77 contains 21 of the 24 predicted ORFs in the right orientation, while in ATV 19 of the 20 ORFs in this region (ATV ORFs 52 to 69) are oriented in the same direction (36). FV3 and TFV also have similarly oriented ORFs in this region (27, 58).
In overall appearance, the EHNV COOs are reminiscent of pathogenesis islands (PAIs) found in pathogenic bacteria. The bacterial PAIs, mobile genetic elements that contribute to rapid changes in virulence potential (16, 21, 22, 25), contain ORFs that are in the same orientation and code for proteins that have been correlated with increased pathogenesis. Poxviruses contain groups of ORFs at the hairpin ends of their genomes that are associated with virulence and have been suggested to be analogous to PAIs (11). It is interesting to note the similarity between these related vertebrate pathogens in that ORFs correlating with pathogenesis are in close proximity to each other. Further analysis of this region may help to shed light on the specific function of these ORFs.
Another interesting region of the EHNV genome is between ORFs 55R and 59L. The five ORFs within this region share homology with two ORFs from ATV (53R and 54R) and two ORFs from FV3 and TFV (23R and 24R), as well as sharing homology with each other (see Table S1 in the supplemental material). In addition, these EHNV ORFs also share homology with SGIV and GIV ORFs. EHNV 57R has homology with GIV 53L, while EHNV 56R has homology to five GIV ORFs (GIV ORFs 50L, 51L, 53L, 91L, and 94L) (see Table S1 in the supplemental material). Alignments of these EHNV ORFs (data not shown) suggest that these five EHNV ORFs may be the result of gene duplication events.
Phylogenetic analysis.
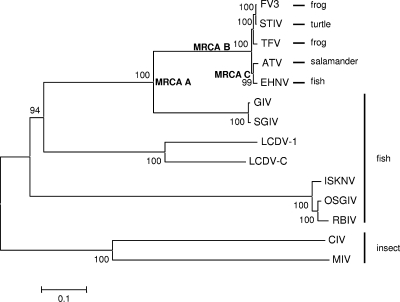
Twenty-six EHNV ORFs, the core iridovirus genes, and the orthologous genes from the 12 completely sequenced iridoviruses were used to generate a concatenated phylogeny (Fig. 3). The phylogenetic tree shows high bootstrap support (100%) for EHNV being a member of the genus Ranavirus in the family Iridoviridae. In addition, EHNV is more closely related to ATV, a salamander RV, than it is to FV3 and TFV, which are frog RVs, supporting previously published analysis (35). EHNV is more distantly related to LCDV-1 and LVDC-C, to ISKNV, to OSGIV, and to RBIV, MIV, and CIV (members of the Lymphocystivirus, Megalocytivirus, Chloriridovirus, and Iridovirus genera, respectively). The grouper iridoviruses GIV and SGIV are clearly more divergent from the ATV/EHNV and FV3/TFV ranaviruses. Based on these data and that of others (53, 62), the grouper iridoviruses or GIV-like isolates could be considered a distantly related species within the genus Ranavirus, or perhaps a subspecies of ranaviruses. Therefore, we suggest that the RVs be divided into two subspecies, the GIV-like RV and the amphibian-like ranavirus (ALRV) subspecies, based on our observations and those of others (20). The ALRVs can then be classified further as being ATV-like or FV3-like. It is interesting that the branch lengths of the ALRVs are very short, suggesting evolutionarily recent speciation of these viral isolates.
FIG. 3.
Concatenated phylogeny of 26 conserved iridovirus sequences. Phylogenetic relationships of 26 conserved open reading frames from the 13 completely sequenced iridovirus genomes are shown. The neighbor-joining tree obtained using MEGA4 is shown, with statistical support indicating the robustness of the inferred branching pattern, as assessed using the bootstrap test. The sequences used for this analysis are described in Tables S1 and S3 in the supplemental material. The most recent common ancestors (MRCAs) are indicated at particular branch points on the phylogeny.
Whole-genome alignments.
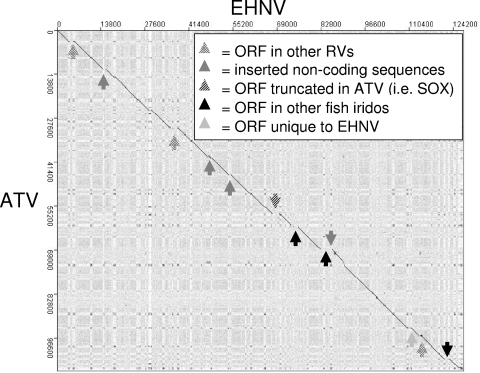
Dot plot comparisons of whole genomic sequences can reveal a large amount of information on the entire genome and how genomic sequences are organized (e.g., colinearity, inversions, and repeat sequences). Comparing the sequence of EHNV to that of ATV, a −45° line can be observed (Fig. 4). Breaks in this line reveal inserted sequences, i.e., ORFs present in one genome and not in the other (Fig. 4; see Table S1 in the supplemental material). In addition, repeated “dots” can be seen running vertically and horizontally throughout the dot plot (Fig. 4). Interestingly, one can almost map out the EHNV ORFs between these “dots,” suggesting that these repeated regions may be involved in the regulation of gene expression (Fig. 4). The “dots” also appear to be running in the lighter streaks running vertically and horizontally in the dot plot (Fig. 4). These lighter streaks represent regions of different G/C or A/T content, a phenomenon also observed with poxviruses (11). The EHNV genome has an overall G+C content of 54% (Table 1), but this does not mean that the entire genome has a uniform %GC, as some regions are more G/C rich than others. The A/T-rich regions appear as lighter streaks on the dot plot, with the off-vertical dots indicating similar A/T-rich regions at multiple places in the genomes. Similar patterns have been observed previously in dot plots for ATV, TFV, and FV3 (20, 36, 58).
FIG. 4.
Dot plot analysis of EHNV versus ATV. The genomic sequence of EHNV was compared to that of ATV by dot plot analysis (JDotter [www.biovirus.org/]). The dot plot comparison of EHNV with ATV shows unique sequences in the EHNV genome.
As observed in other dot plot comparisons (20, 36), the typical RV repeat patterns can be observed by comparing EHNV to ATV, FV3, and TFV (Fig. 4 and 5). Comparing EHNV to ATV by dot plot analysis showed colinearity between these two RVs (Fig. 4). This is a surprising result, as these two viruses infect very different hosts and have been isolated on different continents. This observation suggests that these two different RV pathogens are very closely related, confirming the phylogenetic analysis of 26 ORFs (Fig. 3). There are regions of the dot plot that show unique sequences in EHNV compared to ATV, and these regions correlate with the unique EHNV ORFs (Fig. 4; see Table S1 in the supplemental material) or with extra noncoding DNA sequences. These extra sequences are visualized as breaks in the −45° colinear line and a shift in this line to the right. This shift represents a sequence that is in EHNV but not present in ATV (Fig. 4). There are no sequences present in ATV that are missing from EHNV.
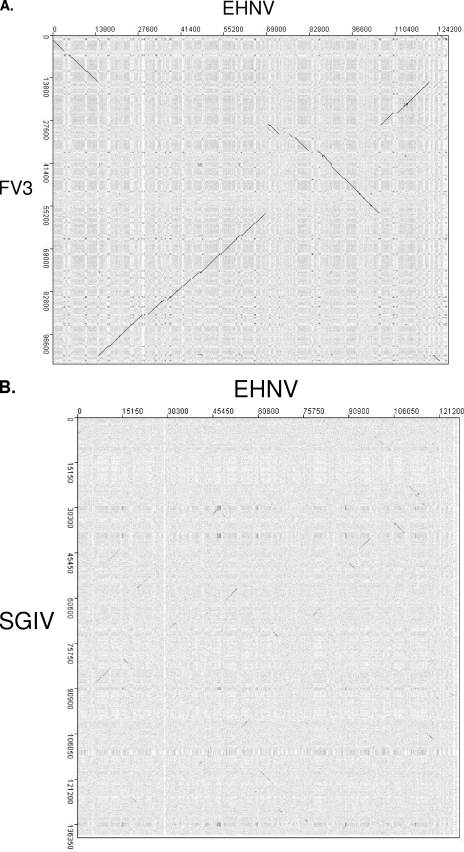
FIG. 5.
Dot plot analysis of EHNV versus other ranaviruses. The genomic sequence of EHNV was compared to those of FV3 and SGIV by dot plot analysis (JDotter [www.biovirus.org/]). (A) Comparison of the EHNV genome to the FV3 genome. (B) Dot plot comparison of EHNV and SGIV genomic sequences.
In contrast to previous reports (58), no inversions were observed between FV3 and TFV, using the same program (MacVector) or the dot plot program used in this study (JDotter; data not shown). In addition, dot plots comparing FV3 and STIV revealed colinearity between these two ranaviruses (29), and dot plots comparing SGIV with GIV also revealed complete colinearity, although the starts of these RV genomes differ (data not shown). Therefore, EHNV and ATV, FV3, STIV, and TFV, and SGIV and GIV were each grouped together for dot plot analysis (Fig. 5A and B). Two major genomic inversions in the FV3/TFV lineage compared to the EHNV/ATV lineage can be visualized on the dot plot as a +45° line (Fig. 5A). This is similar to previously published reports comparing FV3/TFV with ATV (20, 36). In comparing EHNV/ATV to GIV/SGIV, long stretches of colinearity are not observed between these sequences, although small sections of colinearity can be observed in the dot plot (Fig. 5B). The short diagonal lines on the dot plot are indicative of groups of ORFs (2 to 4 ORFs) that are scattered throughout the genome, suggesting that major genomic rearrangements have taken place among RV species. The dot plot correlates with the phylogeny in that EHNV is more closely related to the amphibian RVs than it is to the GIV-like viruses that infect fish.
Very little colinearity was observed in comparing EHNV to all other completely sequenced iridovirus isolates (data not shown). Short stretches of colinearity were observed, but the numbers, intensities, and lengths of these lines were much smaller when comparing EHNV to all other iridovirus genomic sequences.
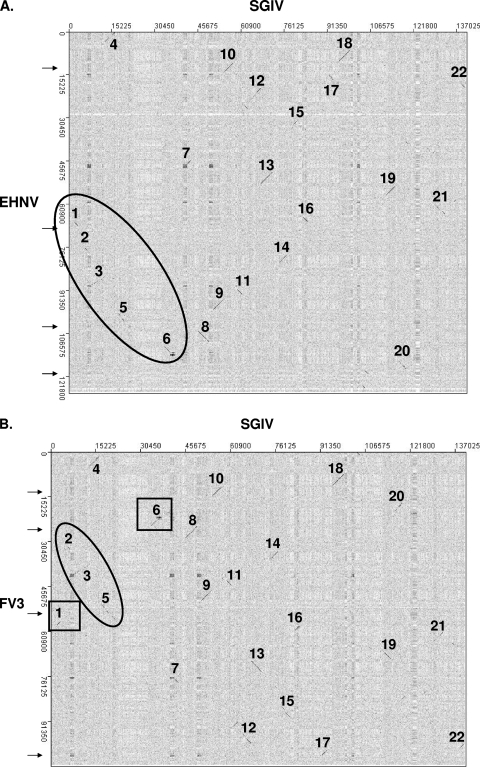
A closer examination of the genomic dot plots between SGIV and EHNV and between SGIV and FV3 revealed additional information on the evolution of the ranaviruses. In Fig. 6A and B, the small stretches of colinear ORFs along the SGIV genome are numbered consecutively. EHNV (and ATV) has segments 1 through 6 oriented together in consecutive order (Fig. 6A). However, the FV3 genome has a rearrangement of these segments (Fig. 6B). This rearrangement of segments corresponds to the inversion observed when comparing the EHNV/ATV and FV3/TFV genomic sequences (Fig. 5A and arrows in Fig. 6). Therefore, these data suggest that in EHNV/ATV, the gene order in this region is similar to the gene order in the most common recent ancestor (MRCA) of the ranaviruses (MRCA A) (Fig. 3) and that the inversion observed when comparing EHNV/ATV and FV3/TFV occurred in the FV3-like lineage (Fig. 7). There is not enough conservation of gene order in the region of the second inversion to be able to establish if the second inversion occurred in the ATV-like or FV3-like lineage.
FIG. 6.
Dot plot analysis of SGIV compared to EHNV and FV3. Dot plots were generated comparing SGIV to EHNV (A) and FV3 (B). Colinear segments were sequentially numbered along the SGIV genome. Consecutively ordered segments along the EHNV and FV3 genomes are circled, while inverted segments are boxed.
FIG. 7.
Model of ranavirus genomic rearrangements. Using the dot plot analysis shown in Fig. 5, consecutively ordered colinear segments were arranged diagrammatically. Comparing the order and orientation of the colinear segments, the rearrangements observed in Fig. 4 occurred in the FV3-like virus lineage and not in the ATV-like virus lineage.
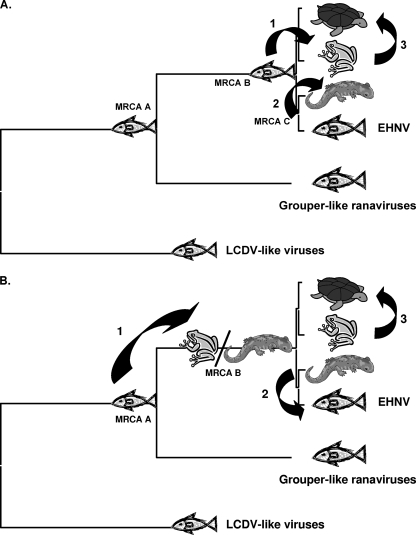
Since the ALRVs contain a virus isolated from fish (EHNV) and since all of the more distantly related vertebrate iridoviruses infect fish (GIV/SGIV, LCDV, and ISKNV), we hypothesize that the most recent common ancestor of the ALRVs was an ancestral fish virus (MRCA B) (Fig. 3 and 8A). Thus, an evolutionarily recent host shift from fish to amphibians, surmised by the shallowness of the ALRV branch lengths, must have occurred. In fact, our data suggest that there were two species jumps, one from fish to frogs (MRCA B) and a jump from fish to salamanders (MRCA C) (Fig. 8A). In addition, the rearrangement of the FV3-like virus genomic DNA relative to ATV/EHNV resulted in the speciation of the ALRVs into the ATV-like and FV3-like virus lineages. The newly acquired sequence of the soft-shelled turtle RV, which is completely colinear with FV3 (29), suggests that another host jump, from frogs to reptiles, also took place recently in evolutionary history (Fig. 3 and 8). However, an alternative hypothesis, where the most recent common ancestor for the ALRVs (MRCA B in Fig. 3 and 8B) infected a tetrapod amphibian, such that the species jump from fish to amphibians occurred prior to speciation of the ALRVs, is also consistent with the phylogeny in Fig. 3. This alternative hypothesis would require a more recent jump back from tetrapod amphibians into fish, yielding an EHNV-like virus. Both of these hypotheses suggest that for the majority of evolutionary time vertebrate iridoviruses were confined to fish, and much more recently, there appear to have been at least three species jumps, from fish to frogs, from fish to salamanders, and from frogs to reptiles, and perhaps as many as four species jumps, including a jump from tetrapod amphibians back to fish. It is tempting to speculate that activities associated with human harvesting of aquatic organisms during the past 40,000 years (28, 47, 48) led to the more common recent jumping of ranaviruses among aquatic organisms.
FIG. 8.
Ranavirus multiple-species jump hypotheses. Throughout the majority of evolutionary history, the iridoviruses have been restricted to fish species. Based on recent genomic sequence information, we hypothesize that the most recent common ancestor of the ranaviruses was a fish virus (MRCA A). In addition, we hypothesize that there have been evolutionarily recent host shifts. We propose two hypotheses to explain these multiple recent host shifts among the amphibian-like ranaviruses. (A) One hypothesis suggests that the most recent common ancestor of the ALRVs was a fish virus (MRCA B) and that a jump occurred from fish to frogs, with a subsequent jump from frogs to turtles. In addition, if the most recent common ancestor of the ATV-like viruses was a fish virus (MRCA C), then another jump from fish to salamanders occurred. (B) An alternative hypothesis suggests that the most recent common ancestor of the ranaviruses was a fish virus (MRCA A) and that a jump occurred from fish into tetrapod amphibians (MRCA B). At this time, it is unclear if the shift in host species was from fish to frogs, fish to salamanders, or both. A subsequent host shift occurred from frogs to turtles, as well as a jump from salamanders back into fish.
The sequencing of EHNV has allowed us to hypothesize that ranavirus host shifts are possible and that there have been evolutionarily recent ranavirus host shifts. In addition, the ability of this group of viruses to infect such a wide variety of host species suggests that more host shifts are likely. Therefore, it is important that we understand more of the evolutionary traits of this unique group of viruses, as there is no other closely related group of viruses that infect such a broad group of hosts, with the possible exception of the orthomyxoviruses (64).
Infectious diseases have become recognized as one of the most important threats to public, veterinary, and wildlife health over the past 30 years (4, 12). Combating infectious diseases is a key goal of public and veterinary health efforts, both nationally and internationally. Infectious diseases in insects, mammals, marsupials, amphibians, reptiles, and fish are not well understood. Global air travel, trade, tourism, immigration, and expansion of human settlements effectively increase the mixing of pathogens among humans as well as domestic and wild animals. From 1998 to 2000, the most-reported wildlife pathogens were viruses related to the anthropogenic movement of animals (12, 17). Recent reports have suggested that RVs move around the globe in host species used for bait, food, pets, and research (35, 44, 51). This phenomenon may increase the probability of new RV pathogens emerging in naïve populations and supports the need for controlling the movement of RV host species within and between geographical regions. Since RVs infect a wide variety of ecologically and economically important hosts, understanding RV evolution, including the importance of the unique genomic rearrangements found among RV isolates in relation to host specificity and viral evolution, will help to predict and perhaps to prevent further RV epizootics. While this study does give insight into RV evolution, more genomic sequence information is needed to continue our efforts to understand the role that RVs play in the environment.
Supplementary Material
Acknowledgments
This work was supported in part by Integrated Research Challenges in Environmental Biology (IBN-9977063) and Division of Environmental Biology (0213851) grants from the National Science Foundation.
Footnotes
Published ahead of print on 30 December 2009.
Supplemental material for this article may be found at http://jvi.asm.org/.
REFERENCES
- 1.Ahne, W., M. Bearzotti, M. Bremont, and S. Essbauer. 1989. Comparison of European systemic piscine and amphibian iridoviruses with epizootic haematopoietic necrosis virus and frog virus 3. Zentralbl. Veterinarmed. B 45:373-383. [DOI] [PubMed] [Google Scholar]
- 2.Ahne, W., M. Bremont, R. P. Hedrick, A. D. Hyatt, and R. J. Whittington. 1997. Iridoviruses associated with epizootic haematopoietic necrosis (EHN) in aquaculture. World J. Microbiol. Biotechnol. 13:367-373. [Google Scholar]
- 3.Allender, M. C., M. M. Fry, A. R. Irizarry, L. Craig, A. J. Johnson, and M. Jones. 2006. Intracytoplasmic inclusions in circulating leukocytes from an eastern box turtle (Terrapene carolina carolina) with iridoviral infection. J. Wildl. Dis. 42:677-684. [DOI] [PubMed] [Google Scholar]
- 4.Binder, S., A. M. Levitt, J. J. Sacks, and J. M. Hughes. 1999. Emerging infectious diseases: public health issues for the 21st century. Science 284:1311-1313. [DOI] [PubMed] [Google Scholar]
- 5.Bloch, B., and J. L. Larsen. 1993. An iridovirus-like agent associated with systemic infection in cultured turbot Scophthalmus-maximus fry in Denmark. Dis. Aquat. Organ. 15:235-240. [Google Scholar]
- 6.Bollinger, T. K., J. Mao, D. Schock, R. M. Brigham, and V. G. Chinchar. 1999. Pathology, isolation, and preliminary molecular characterization of a novel iridovirus from tiger salamanders in Saskatchewan. J. Wildl. Dis. 35:413-429. [DOI] [PubMed] [Google Scholar]
- 7.Bratke, K. A., and A. McLysaght. 2008. Identification of multiple independent horizontal gene transfers into poxviruses using a comparative genomics approach. BMC Evol. Biol. 8:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chinchar, V. G. 2002. Ranaviruses (family Iridoviridae): emerging cold-blooded killers. Arch. Virol. 147:447-470. [DOI] [PubMed] [Google Scholar]
- 9.Chinchar, V. G., A. Hyatt, T. Miyazaki, and T. Williams. 2009. Family Iridoviridae: poor viral relations no longer. Curr. Top. Microbiol. Immunol. 328:123-170. [DOI] [PubMed] [Google Scholar]
- 10.Cunningham, A. A., T. E. S. Langton, P. M. Bennett, J. F. Lewin, S. E. N. Drury, R. E. Gough, and S. K. MacGregor. 1996. Pathological and microbiological findings from incidents of unusual mortality of the common frog (Rana temporaria). Philos. Trans. R. Soc. Lond. B 351:1539-1557. [DOI] [PubMed] [Google Scholar]
- 11.Da Silva, M., and C. Upton. 2005. Host-derived pathogenicity islands in poxviruses. Virol. J. 2:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daszak, P., A. A. Cunningham, and A. D. Hyatt. 2000. Emerging infectious diseases of wildlife—threats to biodiversity and human health. Science 287:443-449. [DOI] [PubMed] [Google Scholar]
- 13.Delhon, G., E. R. Tulman, C. L. Afonso, Z. Lu, J. J. Becnel, B. A. Moser, G. F. Kutish, and D. L. Rock. 2006. Genome of invertebrate iridescent virus type 3 (mosquito iridescent virus). J. Virol. 80:8439-8449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Voe, R., K. Geissler, S. Elmore, D. Rotstein, G. Lewbart, and J. Guy. 2004. Ranavirus-associated morbidity and mortality in a group of captive eastern box turtles (Terrapene carolina carolina). J. Zoo Wildl. Med. 35:534-543. [DOI] [PubMed] [Google Scholar]
- 15.Do, J. W., C. H. Moon, H. J. Kim, M. S. Ko, S. B. Kim, J. H. Son, J. S. Kim, E. J. An, M. K. Kim, S. K. Lee, M. S. Han, S. J. Cha, M. S. Park, M. A. Park, Y. C. Kim, J. W. Kim, and J. W. Park. 2004. Complete genomic DNA sequence of rock bream iridovirus. Virology 325:351-363. [DOI] [PubMed] [Google Scholar]
- 16.Dobrindt, U., B. Hochhut, U. Hentschel, and J. Hacker. 2004. Genomic islands in pathogenic and environmental microorganisms. Nat. Rev. Microbiol. 2:414-424. [DOI] [PubMed] [Google Scholar]
- 17.Dobson, A., and J. Foufopoulos. 2001. Emerging infectious pathogens of wildlife. Philos. Trans. R. Soc. Lond. B 356:1001-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donnelly, T. M., E. W. Davidson, J. K. Jancovich, S. Borland, M. Newberry, and J. Gresens. 2003. What's your diagnosis? Ranavirus infection. Lab. Anim. (New York) 32:23-25. [DOI] [PubMed] [Google Scholar]
- 19.Drury, S. E. N., R. E. Gough, and A. A. Cunningham. 1995. Isolation of an iridovirus-like agent from common frogs (Rana-temporaria). Vet. Rec. 137:72-73. [DOI] [PubMed] [Google Scholar]
- 20.Eaton, H. E., J. Metcalf, E. Penny, V. Tcherepanov, C. Upton, and C. R. Brunetti. 2007. Comparative genomic analysis of the family Iridoviridae: re-annotating and defining the core set of iridovirus genes. Virol. J. 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gerlach, R. G., and M. Hensel. 2007. Salmonella pathogenicity islands in host specificity host pathogen-interactions and antibiotic resistance of Salmonella enterica. Berl. Munch. Tierarztl. Wochenschr. 120:317-327. [PubMed] [Google Scholar]
- 22.Gerlach, R. G., D. Jackel, B. Stecher, C. Wagner, A. Lupas, W. D. Hardt, and M. Hensel. 2007. Salmonella pathogenicity island 4 encodes a giant non-fimbrial adhesin and the cognate type 1 secretion system. Cell. Microbiol. 9:1834-1850. [DOI] [PubMed] [Google Scholar]
- 23.Gordon, D., C. Abajian, and P. Green. 1998. Consed: a graphical tool for sequence finishing. Genome Res. 8:195-202. [DOI] [PubMed] [Google Scholar]
- 24.Greer, A. L., M. Berrill, and P. J. Wilson. 2005. Five amphibian mortality events associated with ranavirus infection in south central Ontario, Canada. Dis. Aquat. Organ. 67:9-14. [DOI] [PubMed] [Google Scholar]
- 25.Hacker, J., B. Hochhut, B. Middendorf, G. Schneider, C. Buchrieser, G. Gottschalk, and U. Dobrindt. 2004. Pathogenomics of mobile genetic elements of toxigenic bacteria. Int. J. Med. Microbiol. 293:453-461. [DOI] [PubMed] [Google Scholar]
- 26.He, J. G., M. Deng, S. P. Weng, Z. Li, S. Y. Zhou, Q. X. Long, X. Z. Wang, and S. M. Chan. 2001. Complete genome analysis of the mandarin fish infectious spleen and kidney necrosis iridovirus. Virology 291:126-139. [DOI] [PubMed] [Google Scholar]
- 27.He, J. G., L. Lu, M. Deng, H. H. He, S. P. Weng, X. H. Wang, S. Y. Zhou, Q. X. Long, X. Z. Wang, and S. M. Chan. 2002. Sequence analysis of the complete genome of an iridovirus isolated from the tiger frog. Virology 292:185-197. [DOI] [PubMed] [Google Scholar]
- 28.Hu, Y. W., H. Shang, H. W. Tong, O. Nehlich, W. Liu, C. H. Zhao, J. C. Yu, C. S. Wang, E. Trinkaus, and M. P. Richards. 2009. Stable isotope dietary analysis of the Tianyuan 1 early modern human. Proc. Natl. Acad. Sci. USA 106:10971-10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang, Y. H., X. H. Huang, H. Liu, J. Gong, Z. L. Ouyang, H. C. Cui, J. H. Cao, Y. T. Zhao, X. J. Wang, Y. L. Jiang, and Q. W. Qin. 2009. Complete sequence determination of a novel reptile iridovirus isolated from soft-shelled turtle and evolutionary analysis of Iridoviridae. BMC Genomics 10:224-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hyatt, A. D., M. Williamson, B. E. H. Coupar, D. Middleton, S. G. Hengstberger, A. R. Gould, P. Selleck, T. G. Wise, J. Kattenbelt, A. A. Cunningham, and J. Lee. 2002. First identification of a ranavirus from green pythons (Chondropython viridis). J. Wildl. Dis. 38:239-252. [DOI] [PubMed] [Google Scholar]
- 31.Iyer, L. A., S. Balaji, E. V. Koonin, and L. Aravind. 2006. Evolutionary genomics of nucleo-cytoplasmic large DNA viruses. Virus Res. 117:156-184. [DOI] [PubMed] [Google Scholar]
- 32.Iyer, L. M., L. Aravind, and E. V. Koonin. 2001. Common origin of four diverse families of large eukaryotic DNA viruses. J. Virol. 75:11720-11734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jakob, N. J., K. Muller, U. Bahr, and G. Darai. 2001. Analysis of the first complete DNA sequence of an invertebrate iridovirus: coding strategy of the genome of Chilo iridescent virus. Virology 286:182-196. [DOI] [PubMed] [Google Scholar]
- 34.Jancovich, J. K., E. W. Davidson, J. F. Morado, B. L. Jacobs, and J. P. Collins. 1997. Isolation of a lethal virus from the endangered tiger salamander Ambystoma tigrinum stebbinsi. Dis. Aquat. Organ. 31:161-167. [Google Scholar]
- 35.Jancovich, J. K., E. W. Davidson, N. Parameswaran, J. Mao, V. G. Chinchar, J. P. Collins, B. L. Jacobs, and A. Storfer. 2005. Evidence for emergence of an amphibian iridoviral disease because of human-enhanced spread. Mol. Ecol. 14:213-224. [DOI] [PubMed] [Google Scholar]
- 36.Jancovich, J. K., J. Mao, V. G. Chinchar, C. Wyatt, S. T. Case, S. Kumar, G. Valente, S. Subramanian, E. W. Davidson, J. P. Collins, and B. L. Jacobs. 2003. Genomic sequence of a ranavirus (family Iridoviridae) associated with salamander mortalities in North America. Virology 316:90-103. [DOI] [PubMed] [Google Scholar]
- 37.Johnson, A. J., A. P. Pessier, and E. R. Jacobson. 2007. Experimental transmission and induction of ranaviral disease in western ornate box turtles (Terrapene ornata ornata) and red-eared sliders (Trachemys scripta elegans). Vet. Pathol. 44:285-297. [DOI] [PubMed] [Google Scholar]
- 38.Langdon, J. S., J. D. Humphrey, and L. M. Williams. 1988. Outbreaks of an EHNV-like iridovirus in cultured rainbow-trout, Salmo-gairdneri richardson, in Australia. J. Fish Dis. 11:93-96. [Google Scholar]
- 39.Langdon, J. S., J. D. Humphrey, L. M. Williams, A. D. Hyatt, and H. A. Westbury. 1986. 1st virus isolation from Australian fish—an iridovirus-like pathogen from redfin perch, Perca-fluviatilis L. J. Fish Dis. 9:263-268. [Google Scholar]
- 40.Lu, L., S. Y. Zhou, C. Chen, S. P. Weng, S. M. Chan, and J. G. He. 2005. Complete genome sequence analysis of an iridovirus isolated from the orange-spotted grouper, Epinephelus coioides. Virology 339:81-100. [DOI] [PubMed] [Google Scholar]
- 41.Mao, J. H., R. P. Hedrick, and V. G. Chinchar. 1997. Molecular characterization, sequence analysis, and taxonomic position of newly isolated fish iridoviruses. Virology 229:212-220. [DOI] [PubMed] [Google Scholar]
- 42.Marschang, R. E., P. Becher, H. Posthaus, P. Wild, H. J. Thiel, U. Muller-Doblies, E. F. Kalet, and L. N. Bacciarini. 1999. Isolation and characterization of an iridovirus from Hermann's tortoises (Testudo hermanni). Arch. Virol. 144:1909-1922. [DOI] [PubMed] [Google Scholar]
- 43.Marschang, R. E., S. Braun, and P. Becher. 2005. Isolation of a ranavirus from a gecko (Uroplatus fimbriatus). J. Zoo Wildl. Med. 36:295-300. [DOI] [PubMed] [Google Scholar]
- 44.Picco, A. M., and J. P. Collins. 2008. Amphibian commerce as a likely source of pathogen pollution. Conserv. Biol. 22:1582-1589. [DOI] [PubMed] [Google Scholar]
- 45.Pozet, F., M. Morand, A. Moussa, C. Torhy, and P. Dekinkelin. 1992. Isolation and preliminary characterization of a pathogenic icosahedral deoxyribovirus from the catfish Ictalurus-melas. Dis. Aquat. Organ. 14:35-42. [Google Scholar]
- 46.Qin, Q. W., S. F. Chang, G. H. Ngoh-Lim, S. Gibson-Kueh, C. Shi, and T. J. Lam. 2003. Characterization of a novel ranavirus isolated from grouper Epinephelus tauvina. Dis. Aquat. Organ. 53:1-9. [DOI] [PubMed] [Google Scholar]
- 47.Richards, M. P., P. B. Pettitt, M. C. Stiner, and E. Trinkaus. 2001. Stable isotope evidence for increasing dietary breadth in the European mid-Upper Paleolithic. Proc. Natl. Acad. Sci. USA 98:6528-6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Richards, M. P., and E. Trinkaus. 2009. Isotopic evidence for the diets of European Neanderthals and early modern humans. Proc. Natl. Acad. Sci. USA 106:16034-16039. doi: 10.1073/pnas.0903821106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schaffer, A. A., L. Aravind, T. L. Madden, S. Shavirin, J. L. Spouge, Y. I. Wolf, E. V. Koonin, and S. F. Altschul. 2001. Improving the accuracy of PSI-BLAST protein database searches with composition-based statistics and other refinements. Nucleic Acids Res. 29:2994-3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schaffer, A. A., Y. I. Wolf, C. P. Ponting, E. V. Koonin, L. Aravind, and S. F. Altschul. 1999. IMPALA: matching a protein sequence against a collection of PSI-BLAST-constructed position-specific score matrices. Bioinformatics 15:1000-1011. [DOI] [PubMed] [Google Scholar]
- 51.Schloegal, L. M., A. M. Picco, A. M. Kilpatrick, A. J. Davies, and A. D. Hyatt. 2009. Magnitude of the US trade in amphibians and presence of Batrachochytrium dendrobatidis and ranavirus infection in imported North American bullfrogs (Rana catesbeiana). Biol. Conserv. 142:1420-1426. [Google Scholar]
- 52.Schock, D. M., T. K. Bollinger, V. G. Chinchar, J. K. Jancovich, and J. P. Collins. 2008. Experimental evidence that amphibian ranaviruses are multi-host pathogens. Copeia 2008:133-143. [Google Scholar]
- 53.Song, W. J., Q. W. Qin, J. Qiu, C. H. Huang, F. Wang, and C. L. Hew. 2004. Functional genomics analysis of Singapore grouper iridovirus: complete sequence determination and proteomic analysis. J. Virol. 78:12576-12590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sonnhammer, E. L. L., and R. Durbin. 1995. A dot-matrix program with dynamic threshold control suited for genomic DNA and protein-sequence analysis. Gene 167:GC1-GC10. [DOI] [PubMed] [Google Scholar]
- 55.Sonnhammer, E. L. L., and J. C. Wootton. 2001. Integrated graphical analysis of protein sequence features predicted from sequence composition. Proteins 45:262-273. [DOI] [PubMed] [Google Scholar]
- 56.Speare, R., and J. R. Smith. 1992. An iridovirus-like agent isolated from the ornate burrowing frog Limnodynastes-ornatus in Northern Australia. Dis. Aquat. Organ. 14:51-57. [Google Scholar]
- 57.Tamura, K., J. Dudley, M. Nei, and S. Kumar. 2007. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol. Biol. Evol. 24:1596-1599. [DOI] [PubMed] [Google Scholar]
- 58.Tan, W. G. H., T. J. Barkman, V. G. Chinchar, and K. Essani. 2004. Comparative genomic analyses of frog virus 3, type species of the genus Ranavirus (family Iridoviridae). Virology 323:70-84. [DOI] [PubMed] [Google Scholar]
- 59.Tapiovaara, H., N. J. Olesen, J. Linden, E. Rimaila-Parnanen, and C. H. von Bonsdorff. 1998. Isolation of an iridovirus from pike-perch Stizostedion lucioperca. Dis. Aquat. Organ. 32:185-193. [DOI] [PubMed] [Google Scholar]
- 60.Tidona, C. A., and G. Darai. 1997. The complete DNA sequence of lymphocystis disease virus. Virology 230:207-216. [DOI] [PubMed] [Google Scholar]
- 61.Trimble, J. J., S. C. S. Murthy, A. Bakker, R. Grassmann, and R. C. Desrosiers. 1988. A gene for dihydrofolate-reductase in a herpesvirus. Science 239:1145-1147. [DOI] [PubMed] [Google Scholar]
- 62.Tsai, C. T., J. W. Ting, M. H. Wu, M. F. Wu, I. C. Guo, and C. Y. Chang. 2005. Complete genome sequence of the grouper iridovirus and comparison of genomic organization with those of other iridoviruses. J. Virol. 79:2010-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whittington, R. J., C. Kearns, A. D. Hyatt, S. Hengstberger, and T. Rutzou. 1996. Spread of epizootic haematopoietic necrosis virus (EHNV) in redfin perch (Perca fluviatilis) in southern Australia. Aust. Vet. J. 73:112-114. [DOI] [PubMed] [Google Scholar]
- 64.Williams, T., V. Barbosa-Solomieu, and V. G. Chinchar. 2005. A decade of advances in iridovirus research. Adv. Virus Res. 65:173-248. [DOI] [PubMed] [Google Scholar]
- 65.Yu, Y. X., M. Bearzotti, P. Vende, W. Ahne, and M. Bremont. 1999. Partial mapping and sequencing of a fish iridovirus genome reveals genes homologous to the frog virus 3 p31, p40 and human eIF2 alpha. Virus Res. 63:53-63. [DOI] [PubMed] [Google Scholar]
- 66.Zhang, Q. Y., F. Xiao, Z. Q. Li, J. F. Gui, J. H. Mao, and V. G. Chinchar. 2001. Characterization of an iridovirus from the cultured pig frog Rana grylio with lethal syndrome. Dis. Aquat. Organ. 48:27-36. [DOI] [PubMed] [Google Scholar]
- 67.Zhang, Q. Y., F. Xiao, J. Xie, Z. Q. Li, and J. F. Gui. 2004. Complete genome sequence of lymphocystis disease virus isolated from China. J. Virol. 78:6982-6994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zupanovic, Z., C. Musso, G. Lopez, C. L. Louriero, A. D. Hyatt, S. Hengstberger, and A. J. Robinson. 1998. Isolation and characterization of iridoviruses from the giant toad Bufo marinus in Venezuela. Dis. Aquat. Organ. 33:1-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.