Abstract
Murine gammaherpesvirus 68 (γHV68) provides an important experimental model for understanding mechanisms of immune control of the latent human gammaherpesviruses. Antiviral CD8 T cells play a key role throughout three separate phases of the infection: clearance of lytic virus, control of the latency amplification stage, and prevention of reactivation of latently infected cells. Previous analyses have shown that T-cell responses to two well-characterized epitopes derived from ORF6 and ORF61 progress with distinct kinetics. ORF6487-specific cells predominate early in infection and then decline rapidly, whereas ORF61524-specific cells continue to expand through early latency, due to sustained epitope expression. However, the paucity of identified epitopes to this virus has limited our understanding of the overall complexities of CD8 T-cell immune control throughout infection. Here we screened 1,383 predicted H-2b-restricted peptides and identified 33 responses, of which 21 have not previously been reported. Kinetic analysis revealed a spectrum of T-cell responses based on the rapidity of their decline after the peak acute response that generally corresponded to the expression patterns of the two previously characterized epitopes. The slowly declining responses that were maintained during latency amplification proliferated more rapidly and underwent maturation of functional avidity over time. Furthermore, the kinetics of decline was accelerated following infection with a latency-null mutant virus. Overall, the data show that γHV68 infection elicits a highly heterogeneous CD8 T-cell response that segregates into two distinctive kinetic patterns controlled by differential epitope expression during the lytic and latency amplification stages of infection.
Murine gammaherpesvirus 68 (γHV68) is a mouse pathogen closely related to the human gammaherpesviruses Epstein-Barr virus (EBV) and Kaposi's sarcoma-associated herpesvirus (KSHV). Intranasal infection of mice with γHV68 leads to an acute infection in lung epithelial cells that is ultimately cleared and the concurrent establishment of latency in B cells, dendritic cells, and macrophages that undergoes amplification in the spleen and is maintained lifelong (11, 12). Even though γHV68 has the capacity to downregulate major histocompatibility complex class I (MHC-I) molecules (36), CD8 T cells specific for γHV68 are generated and have been shown to proliferate in response to cognate antigen, protect naive mice from γHV68 infection, lyse peptide-pulsed target cells in vivo and in vitro, and maintain the ability to produce antiviral cytokines (5, 6, 13, 27, 35). Until recently, knowledge of the antiviral CD8 T-cell repertoire in C57BL/6 mice was largely limited to two well-characterized epitopes derived from ORF6 and ORF61. T-cell responses to these epitopes have been shown to progress with distinct kinetics, with ORF6487-specific cells predominating early in infection and ORF61524-specific cells continuing to expand through early latency before declining and then persisting at higher levels late in infection (33). The difference in response kinetics correlates with the differential presentation of the epitopes, with the ORF6487 epitope being expressed only during lytic infection and the ORF61524 epitope being expressed both during lytic infection and during the latency amplification phase (22, 28). Additionally, the latency amplification phase is associated with the expansion of CD8 T cells with a Vβ4 T-cell receptor (TCR) component in several mouse strains (17), presumably due to a superantigen-like effect of the γHV68 M1 protein (4, 9).
To better understand the breadth of the anti-γHV68 T-cell response, we used an enzyme-linked immunospot (ELISpot) approach to identify new epitopes. We identified a large number of epitopes derived from 26 proteins that drive the acute CD8 T-cell response to γHV68, which then narrowed over time, resulting in a limited antiviral response during latency. We did not observe inflation of any of the responses, as has been demonstrated for some murine cytomegalovirus (MCMV)-specific responses (20, 26). There was no evidence for functional exhaustion, as all detectable CD8 T-cell responses maintained functionality, but the responses declined in numbers over time. The decline in responses occurred over a broad kinetic range, which segregated into two general groups that correlated precisely with those previously described for ORF6 and ORF61. Thus, some responses declined rapidly after the acute phase of infection, while others declined more slowly.
We examined two epitope-specific responses from each of the two patterns in detail over time for functional and phenotypic characteristics and found the responses to be highly heterogeneous, differing in TCR affinity, functional avidity, and proliferation rates. Importantly, slowly declining responses were not maintained as efficiently after infection with a latency-deficient virus, consistent with a role for epitope expression in driving the heterogeneous rate of decline in cell number after the acute infection. The data show that the response kinetics seen for the ORF6487 and ORF61524 responses are broadly applicable to multiple CD8 T-cell epitopes.
MATERIALS AND METHODS
Mice and viral infections.
Female 6- to 12-week-old C57BL/6 mice were obtained from the Trudeau Institute animal facility and kept under specific-pathogen-free conditions. Mice were anesthetized with 2,2,2-tribromoethanol and infected intranasally (i.n.) with 400 PFU murine γHV68 (strain WUMS) or AC-RTA (18a) in 30 μl Hanks' balanced salt solution (HBSS). All animal experiments were approved by the Institutional Animal Care and Use Committee of the Trudeau Institute.
Tetramers and flow cytometry.
Allophycocyanin-conjugated MHC-I tetramers specific for γHV68 epitopes Db/ORF6487-495 (p56, AGPHNDMEI), Kb/ORF61524-531 (p79, TSINFVKI), Kb/ORF75c940-947 (KSLTYYKL), and Kb/ORF48148-155 (TNYKFSLV) were obtained from the Trudeau Institute Molecular Biology Core Facility. Cells were treated with Fc Block and then stained with tetramers for 1 h at room temperature, followed by incubation with appropriate antibodies. Fluorochrome-conjugated antibodies against CD8α, CD27, CD43 (1B11), CD44, CD127, KLRG1, and Vβ4 TCR were purchased as needed. To measure intracellular Bcl-2, cells were permeabilized following surface staining using the BD Pharmingen Cytofix/Cytoperm kit according to the manufacturer's instructions. All samples were collected on a BD FACSCanto II cytometer and analyzed using FlowJo software (TreeStar).
Intracellular cytokine assay.
Cells were incubated with congenic splenocytes and 10 μg/ml appropriate peptide for 5 h at 37°C in the presence of brefeldin-A and then washed, labeled, and permeabilized using the BD Pharmingen Cytofix/Cytoperm kit according to the manufacturer's instructions. Antibodies to gamma interferon (IFN-γ) and tumor necrosis factor alpha (TNF-α) were purchased from BD Pharmingen. For the functional avidity assay, 100-fold dilutions of each peptide were used and maximum IFN-γ was set as the amount produced with 10 μg/ml peptide.
In vivo cytotoxicity assay.
Splenocytes from congenic (CD45.1 or Thy1.1) uninfected mice were pulsed with 10 μg/ml of the appropriate peptide for 5 h at 37°C. Experimental peptide-pulsed splenocytes were labeled with differential concentrations of carboxyfluorescein diacetate succinimidyl ester (CFSE), and control peptide (influenza NP366)-pulsed cells were left unlabeled. Cells were mixed in a 1:1:1 ratio in two groups (Thy1.1 donors, ORF6487/ORF48148/NP366; CD45.1 donors, ORF61524/ORF75c940/NP366), and 6 × 107 cells from each donor were intravenously injected into mice that had been previously infected with γHV68 (or into naive mice to calculate specific lysis). Spleens were harvested from recipient mice approximately 16 h postinjection, and CD45.1 or Thy1.1 donor cells were identified and enumerated using flow cytometry. Lysis was calculated in individual mice using the formula (1 − ratio uninfected/ratio infected) × 100.
BrdU incorporation assay.
To measure proliferation, mice were treated with 0.8 mg/ml 5-bromo-2-deoxyuridine (BrdU) in their drinking water for 4 consecutive days at various times postinfection (p.i.). After 4 days, splenocytes were harvested and BrdU incorporation was measured using the BD Pharmingen BrdU kit with DNase in accordance with the manufacturer's instructions.
Tetramer affinity assay.
Splenocytes were isolated 12 days p.i. and labeled with MHC-I tetramers for 1 h at room temperature. Cells were then washed and incubated with unlabeled anti-H-2Kb/Db antibody (Ab) for various times at 37°C. Cells were then washed and labeled with Ab to CD8α. Maximum tetramer binding was set to the percentage of tetramer+ CD8 T cells without incubation with anti-MHC Ab.
Bioinformatics and MHC peptide binding assays.
To identify potential CD8 T-cell epitopes from throughout the γHV68 genome, protein sequences were analyzed using previously described algorithms (25) that predict the affinity of peptides for H-2Kb (8- and 9-mers) and Db (9- and 10-mers) class I molecules. Peptides scoring in the top 1% range for each allele/size combination were selected for further study and were synthesized as crude material by Mimotopes (Clayton, Victoria, Australia).
MHC purification and quantitative assays to measure the affinity for binding of peptides to purified H-2Kb and H-2Db molecules were performed as previously described (21, 31). Briefly, 0.1 to 1 nM radiolabeled peptide was coincubated at room temperature with 1 μM to 1 nM purified MHC in the presence of 1 to 3 μM human β2-microglubulin (Scripps Laboratories) and a cocktail of protease inhibitors. After a 2-day incubation, binding of the radiolabeled peptide to the corresponding MHC-I molecule was determined by capturing MHC/peptide complexes on Lumitrac 600 microplates (Greiner Bio-One) coated with either the Y3 (anti-H-2Kb) or 28-14-8s (anti-H-2 Db, Ld, and Dq) antibody and measuring bound cpm using the TopCount microscintillation counter (Packard Instrument Co.).
For competition assays, the concentration of peptide yielding 50% inhibition of the binding of the radiolabeled peptide (IC50) was calculated. Peptides were typically tested at 6 different concentrations covering a 100,000-fold dose range and in 3 or more independent assays. Under the conditions utilized, where the concentration of the radiolabeled peptide is <MHC concentration and IC50 is ≥MHC concentration, the measured IC50s are reasonable approximations of true binding affinities (3, 16).
IFN-γ ELISpot assay.
Splenocytes from infected mice and irradiated naive splenocytes (3,000 rads) were incubated for 48 h at 37°C in a standard enzyme-linked immunospot (ELISpot) assay. For all assays, 1 μg/ml of appropriate peptide or a no-peptide control was used. Purified capture Ab and biotinylated detection Ab were purchased from BD Pharmingen, and Fast BCIP/NBT (Sigma) substrate and streptavidin-alkaline phosphatase (Dako) enzyme were used.
RESULTS
Robust early CD8 T-cell response to γHV68.
To better understand the T-cell response to γHV68, we characterized the breadth of the antiviral CD8 T-cell response. Using an MHC-I binding prediction algorithm for H-2Kb and H-2Db, we identified and synthesized 1,383 potential CD8 T-cell epitopes from coding sequences throughout the γHV68 genome (25). Each of these peptides was screened for its ability to induce IFN-γ release by splenocytes from mice that had been infected with γHV68 (12 days p.i.) in a standard ELISpot assay (Fig. 1A). We identified 33 peptides that reproducibly induced IFN-γ responses, including some that had been published previously (14, 23, 33, 34). The 33 responses we detected were derived from 26 proteins. In general, the larger the protein the more predicted epitopes they contain (Fig. 1B), but 6 genes (ORF38, ORF45, ORF52, M10b, M12, and M14) were not predicted to encode any MHC-I restricted epitopes.
FIG. 1.
Robust early CD8 T-cell response to γHV68. (A) Mice were infected with 400 PFU γHV68; at the given times, splenocytes were harvested, and 106 cells were stimulated for 48 h with 5 × 105 irradiated APCs pulsed with peptides or the medium control in a standard ELISpot assay for IFN-γ. The numbers of spots per 106 cells minus the medium control (±standard deviations [SD]) are shown. The dashed line is at 30 spots above background at 12 days p.i. Samples were run in duplicate per experiment, and data are from 3 or 4 experiments per time point. (B) The number of predicted MHC-I binding epitopes per open reading frame (ORF) is shown versus the amino acid length of the protein. r2 is 0.6904.
In the mouse model of the betaherpesvirus murine cytomegalovirus (MCMV), several patterns of antiviral T-cell responses develop in response to infection, including a delayed but prolonged accumulation of some epitope-specific cells termed “memory inflation” (20, 26). To determine whether memory inflation was also driven by γHV68 infection, we investigated whether any of the 33 T-cell responses we identified at 12 days p.i. inflated over time. As shown in Fig. 1A (solid bars), we found no evidence for inflation of any of the antiviral CD8 T-cell responses. On the contrary, there was a dramatic reduction in the breadth of the response, as many of the peptides failed to induce IFN-γ release at the late time point. This could be due to impairment in the ability of the cells to synthesize or release IFN-γ, a reduction in the number of IFN-γ-producing cells, or a combination of both.
During the course of our study, a separate report identified 19 previously unknown γHV68-specific CD8 T-cell epitopes using a caged MHC-I tetramer approach (14). In addition to three well-characterized epitopes (ORF61524, ORF6487, and ORF8604) and 9 of the 19 epitopes recently identified using the caged tetramer approach, our screen identified 21 novel CD8 T-cell epitopes (Table 1). Comparative analysis of the affinity of binding to MHC-I by the epitopes identified in our screen and those identified by the caged tetramer technique showed that the binding affinity of the epitopes identified by the ELISpot method was significantly lower (Fig. 2).
TABLE 1.
Novel CD8 T-cell epitopes identified by ELISpot assay
| ORF | Amino acids | Sequence | Function | Gene expressiona | MHC restriction | IC50 (nM)b |
|---|---|---|---|---|---|---|
| ORF40 | 599-606 | ILSNYETL | Helicase-primase | L | Kb | 423 |
| ORF21 | 586-595 | FCYELSKPHM | Thymidine kinase | E | Db | 6,323 |
| ORF25 | 1208-1216 | YSPENAAKL | Major capsid protein | L | Db | 10 |
| ORF74 | 250-258 | VSFICTTIL | G-protein coupled receptor | L | Kb | 80 |
| ORF75a | 807-814 | SHIIWVSL | Tegument protein/FGARAT | IE | Kb | 313 |
| ORF67 | 148-157 | NSMVVCRTYI | Tegument protein | L | Db | 417 |
| ORF60 | 227-234 | VEYSFIAA | Ribonucleotide reductase, small subunit | E | Kb | 671 |
| ORF22 | 175-182 | KYVEYGSL | Glycoprotein H | E | Kb | 90 |
| ORF24 | 447-456 | YSVKEPSFTI | Unknown function | E | Db | 21,558 |
| ORF7 | 83-92 | LSHENTYDHV | Transport protein | L | Db | 930 |
| ORF66 | 20-28 | RSFCIYGHL | Unknown function | L | Kb | 75 |
| ORF7 | 496-504 | YKLLNGPLI | Transport protein | L | Db | 11 |
| ORF9 | 283-291 | SSWPPYNIL | DNA polymerase | E | Kb | 121 |
| ORF40 | 323-330 | ITSMWPLL | Helicase-primase | L | Kb | 102 |
| ORF19 | 386-924 | FSFLMENYL | Tegument protein | L | Kb | 510 |
| M4 | 136-143 | TIYSVVPV | Unknown function | E | Kb | 67 |
| ORF39 | 209-216 | LVLFYRPI | Glycoprotein M | L | Kb | 149 |
| ORF22 | 410-418 | VSAHAFTFM | Glycoprotein H | E | Kb | 99 |
| ORF58 | 322-329 | NGVYFVYL | Unknown function | E | Kb | 147 |
| ORF64 | 1604-1611 | ISTNSAKL | Tegument protein | L | Kb | 973 |
| ORF69 | 193-201 | IFFEETNGM | Unknown function | E | Kb | 471 |
IE, immediate early; E, early; L, late.
IC50, 50% inhibitory concentration.
FIG. 2.
Comparison of MHC-I binding affinities. The log10 concentrations of peptide yielding 50% inhibition of binding of radiolabeled peptide (IC50s) are shown for 21 previously published epitopes and the 21 epitopes newly identified in this report (Table 1). ***, P < 0.0001 (Student's t test).
Dynamic range of CD8 T-cell response kinetics.
Although we did not observe inflation of any of the 33 responses by 6 months p.i. and although all responses declined over time, we observed variation in the patterns of decline among the responses. To investigate this in more detail as well as to independently confirm the identified epitopes, we resynthesized 13 of the peptides from Fig. 1 and used them to stimulate CD8 T cells from γHV68-infected mice at various times after infection in an intracellular cytokine stain assay. All resynthesized peptides induced IFN-γ production, and some responses showed a prolonged ability to produce IFN-γ, maintaining greater than 40% of their 12-day IFN-γ production at 49 days p.i. (Fig. 3A). Other responses showed a faster decline in their ability to synthesize IFN-γ, such that at 49 days, less than 40% of their 12-day IFN-γ production level remained intact (Fig. 3B). The kinetic patterns did not correlate with the affinity of each peptide for MHC-I (Fig. 3C). As previously reported, the well-characterized ORF61524- and ORF6487-specific responses segregated into the two patterns, consistent with their differential expression throughout infection (22, 33).
FIG. 3.
Two patterns of CD8 T-cell response kinetics. (A and B) CD8 T cells from infected mice were stimulated for 5 h in the presence of brefeldin-A with congenic splenocytes pulsed with the indicated peptides and then analyzed by flow cytometry. The percentage of CD8 T cells that produced IFN-γ was normalized to the percentage at 12 days after infection. (A) Pattern 1 responses maintained 40% or greater of the 12-day value at 49 days after infection (n = 3/group). (B) Pattern 2 responses maintained less than 40% of the 12-day value at 49 days after infection (n = 3/group). (C) The IC50 values for the 11 pattern 1 and 4 pattern 2 responses are shown. ns, not significant (Student's t test).
Multifunctionality of γHV68-specific responses.
The ability of T cells to exhibit multiple effector functions correlates with their protective efficacy (7). In chronic infections such as HIV and hepatitis C virus (HCV) infections in humans and lymphocytic choriomeningitis virus (LCMV) infection in mice, CD8 T cells can lose their effector functions in a stepwise manner and become functionally exhausted (15, 30, 41). Using peptide-specific stimulation and intracellular cytokine staining, we examined the ability of CD8 T cells specific for 6 different epitopes to produce IFN-γ and TNF-α at 12 and 49 days p.i. (Fig. 4A). Although the total production of IFN-γ and the ratio of IFN-γ+ TNF-α+ cells to IFN-γ+ TNF-α− cells dropped for each response at the later time point, there was still a considerable proportion of the IFN-γ+ cells that can also synthesize TNF-α at 49 days after infection. Additionally, at all times examined, all IFN-γ+ cells were also positive for CD107a, a marker for lytic granule release (data not shown) (1). As targeted release of lytic granules is closely correlated with the ability of CD8 T cells to kill virally infected cells, we measured the ability of T cells specific for each of the epitopes to kill CFSE-labeled target cells pulsed with cognate antigen in vivo in a 16-h cytotoxicity assay (Fig. 4B). CD8 T cells specific for all 6 epitopes could specifically lyse target cells 12 days after infection, and most of the responses maintained their cytotoxic ability as late as 6 months after infection. Given the low frequency of IFN-γ+ cells at 6 months p.i. in the ELISpot assay for each epitope (Fig. 1A), the prolonged ability to kill target cells in vivo suggests that these responses maintain functionality as they decline in numbers.
FIG. 4.
Multifunctionality of γHV68-specific responses. (A) CD8 T cells were analyzed by intracellular flow cytometry for IFN-γ and TNF-α synthesis following 5 h of stimulation with the indicated peptides. Numbers indicate the percentages of CD8 T cells in the quadrant. Data are representative of 3 experiments per time point. (B) T-cell cytotoxicity was measured in vivo. Infected mice were injected with CFSE-labeled congenic splenocytes that had been pulsed with a γHV68 peptide epitope or influenza virus NP366 epitope as a negative control. Spleens were harvested 16 h later, and specific killing was calculated as described in Materials and Methods. Data are representative of at least 3 experiments per time point.
Decline in numbers of CD8 T-cell responses.
To distinguish a loss of T-cell function from a decline in numbers, we made MHC-I tetramers with two new epitopes representative of each of the response patterns, ORF48148 and ORF75c940, and compared these with ORF6487 and ORF61524 in terms of tetramer-positive CD8 T-cell numbers, function, and phenotype (Table 2).
TABLE 2.
CD8 T-cell epitopes for MHC-I tetramer synthesis
| Pattern | ORF | Amino acids | Sequence | Function | Gene expressiona | MHC restriction | IC50 (nM)b | Tetramer |
|---|---|---|---|---|---|---|---|---|
| 1 | ORF9 | 283-291 | SSWPPYNIL | DNA polymerase | E | Kb | 120 | No |
| 1 | ORF17 | 308-316 | SAITNHAAF | Capsid protein | E | Db | 81 | No |
| 1 | ORF25 | 1208-1216 | YSPENAAKL | Major capsid protein | L | Db | 10 | No |
| 1 | ORF39 | 209-216 | LVLFYRPI | Glycoprotein M | L | Kb | 149 | No |
| 1 | ORF40 | 323-330 | ITSMWPLL | Helicase-primase | L | Kb | 102 | No |
| 1 | ORF48 | 148-155 | TNYKFSLV | Unknown function | E | Kb | 10 | Yes |
| 1 | ORF54 | 253-260 | AVVQFIRV | dUTPase | E | Kb | 45 | No |
| 1 | ORF61 | 524-531 | TSINFVKI | Ribonucleotide reductase, large subunit | E | Kb | 32 | Yes |
| 1 | ORF75c | 176-184 | SAIENYETF | Tegument protein/FGARAT | L | Db | 10 | No |
| 2 | ORF6 | 487-495 | AGPHNDMEI | Single-stranded DNA binding protein | E | Db | 14 | Yes |
| 2 | ORF21 | 304-311 | TGFRYSYM | Thymidine kinase | E | Kb | 11 | No |
| 2 | ORF39 | 167-174 | FVYLFHFM | Glycoprotein M | L | Kb | 16 | No |
| 2 | ORF75c | 940-947 | KSLTYYKL | Tegument protein/FGARAT | L | Kb | 11 | Yes |
E, early; L, late.
IC50, 50% inhibitory concentration.
At various times after infection, splenocytes from γHV68-infected mice were stained for binding of CD8 and a tetramer (Fig. 5A). The two pattern 1 responses were characterized by a lower percentage of lymphocytes at 12 days p.i. than at 21 days p.i., whereas the pattern 2 responses were higher earlier. By 4 months p.i., however, only the ORF61524-specific T cells were retained in sizable numbers. Thus, the kinetics of the ORF75c940- and ORF48148-specific responses are consistent with the previously characterized kinetics for ORF6487- and ORF61524-specific responses, respectively. We then compared the percentage of CD8s that were tetramer positive with the percentage of CD8s that were positive for IFN-γ synthesis following stimulation at each time point (Fig. 5B). There was significant correlation between the numbers of tetramer+ cells and IFN-γ production, indicating that the loss of cytokine production we observed by ELISpot (Fig. 1A) and intracellular staining (Fig. 3 and 4) was due to a decline in T-cell numbers and not to impaired functionality or exhaustion.
FIG. 5.
CD8 T-cell responses maintain functionality, but are reduced numerically over time. (A) Spleens were harvested from γHV68-infected mice at the indicated times, and cells were stained with an MHC-I tetramer and antibody to CD8. Zebra plots are representative of at least 3 experiments. Numbers indicate percentages of lymphocytes positive for CD8 and the tetramer. (B) Shown are the percentages of CD8 T cells that produced IFN-γ in response to peptide stimulation versus the percentages of CD8 T cells that bound that particular MHC-I tetramer at all time points tested. r2 values are as follows: ORF61524, 0.9515; ORF48148, 0.9441; ORF6487, 0.9518; ORF75c940, 0.9763 (n = 20/group). (C) At 41 days p.i., spleens were harvested and stained with antibodies to CD8, the tetramer, and TCR Vβ4. Numbers indicate percentages of CD8 T cells in each quadrant (n = 3).
It has been reported that γHV68 expresses a superantigen activity that drives a mononucleosis-like expansion of CD8 T cells expressing a Vβ4 TCR component (4). ORF61524- and ORF6487-specific cells have been shown previously to be excluded from this Vβ4 population (10, 33), and our current data show that neither the ORF48148- nor ORF75c940-specific cells were involved in the Vβ4 expansion (Fig. 5C). It remains to be determined whether the expanded Vβ4 population includes any of the other new epitopes we have identified.
To determine whether the distinct response patterns reflected differences in the rates of proliferation of T cells with different specificities, we analyzed the incorporation of a thymidine analog, 5-bromo-2-deoxyuridine (BrdU), into DNA. At each time tested, antigen-experienced CD44hi cells, but not naive CD44lo cells, underwent proliferation, but proliferation declined over time (Fig. 6A). We compared the rates of DNA synthesis in T cells with different specificities (Fig. 6B). A higher percentage of pattern 1 T cells proliferated at 20 days p.i., consistent with their slower decline in numbers at day 49 (Fig. 3A) compared to pattern 2 responses (Fig. 3B). We also measured intracellular levels of the antiapoptotic molecule Bcl-2. At 12 and 20 days after infection, we saw no differences in Bcl-2 expression between pattern 1 and pattern 2 responses (data not shown), suggesting that the rates of cell death for the responses are similar. Thus, it appears that the greater proliferation of pattern 1 responses at the peak of infection, and not increased cell death of pattern 2 responses, is responsible for the kinetic differences between the two patterns that we observe.
FIG. 6.
The two patterns exhibit differential proliferation rates. At various times after infection, mice were treated with BrdU in their drinking water for 4 consecutive days. At the end of treatment, spleens were harvested and stained with a tetramer and antibodies to CD8 and CD44. CD8 T cells were measured for BrdU incorporation. (A) Representative histograms show incorporation by CD44lo CD8 T cells and CD44hi CD8 T cells. (B) The percentages of tetramer+ cells that incorporated BrdU are shown (n = 5 to 10/group).
To further understand how the two kinetic patterns are regulated, we examined the surface expression of activation and memory markers on the four virus-specific T-cell populations (Fig. 7A). Consistent with immune responses to other respiratory infections (18), most cells exhibited a highly activated CD27hi CD43hi phenotype early after γHV68 infection. Over time, the populations shifted toward a less activated CD27+/− CD43lo phenotype. At 21 days p.i., a higher percentage of ORF61524-specific cells retained an activated phenotype than did cells of other specificities (Fig. 7B). We also examined the memory phenotype by examining surface expression of CD127 (interleukin-7Rα [IL-7Rα]), a marker that is expressed on naive cells, is downregulated during activation, and is reexpressed during memory (19, 29), and KLRG-1, a marker of terminal differentiation (Fig. 7C, D, and E) (29, 40). The populations progressed from low to higher CD127 expression, consistent with the development of memory (Fig. 7C and D). The cells adopted and maintained a KLRG-1hi phenotype, similar to cells from other persistent infections in mice and humans (32, 38), but ORF75c940-specific cells expressed lower levels of KLRG-1 than the other epitope-specific cells (Fig. 7C and E). These results suggest that T cells from different kinetic patterns express relatively similar levels of CD127 and KLRG1, and by 2 months p.i., there is a shift toward a less activated, memory phenotype, albeit one that is KLRG-1hi.
FIG. 7.
Prolonged activation of ORF61524-specific responses. At various times after infection spleens were harvested and stained with a tetramer and the indicated antibodies. Representative zebra plots show staining of tetramer+ CD8 T cells with CD27 and CD43 (A) or CD127 and KLRG-1 (C). Numbers in plots show the percentages of tetramer+ cells in each quadrant. Plots are representative of at least 3 experiments. The percentages of tetramer+ cells that are CD27hi CD43hi (B), CD127hi (D), or KLRG1hi (E) are shown.
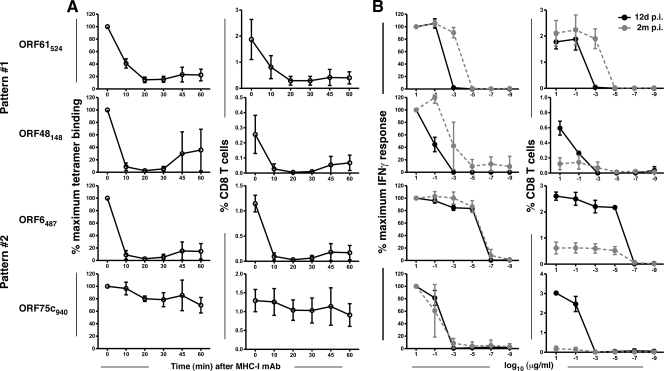
It has been shown previously that the ORF6487-specific T-cell population does not change TCR affinity (affinity of the TCR for cognate peptide-MHC complexes) or functional avidity (the ability to produce IFN-γ in response to a given concentration of peptide-MHC complexes) over time, as determined from the tetramer dissociation rate and IFN-γ production at low antigen densities, respectively (5). Whether this is also the case for the other epitope-specific responses is currently unknown. At day 12 after infection, we performed a standard tetramer dissociation assay to measure TCR affinity (Fig. 8A). Our data show that this parameter does not correlate with the kinetic patterns. Rather, only ORF75c940-specific cells appear to have a strong affinity for their cognate antigen-MHC complex. To measure functional avidity, we stimulated splenocytes with target cells pulsed with dilutions of peptide in an intracellular cytokine staining assay and measured IFN-γ accumulation (Fig. 8B). As has been shown previously, it was found that the ORF6487-specific response did not undergo maturation of functional avidity when measurements were made 2 months after infection (5). Interestingly, the other pattern 2 response we measured, that of ORF75c940, also maintained its level of functional avidity. Alternatively, the two pattern 1 responses tested both showed a shift of functional avidity over time toward higher avidity. Thus, it appears that functional avidity maturation may be a correlate of prolonged persistence in the overall antiviral T-cell pool.
FIG. 8.
Functional avidity maturation occurs for pattern 1 responses. (A) TCR affinity was measured by staining cells with a tetramer for 1 h followed by incubation with anti-H-2Kb/Db antibody at 37°C to observe tetramer dissociation. Cells were then stained with anti-CD8 and analyzed by flow cytometry. Data represent the percentages of tetramer+ CD8 T cells expressed as percentages (±SD) of maximum binding at time zero (left column) or as raw data showing the percentages (±SD) of CD8 T cells (right column). (B) Functional avidity was measured by stimulating cells for 5 h in the presence of the designated concentration of antigen and brefeldin-A and then staining for intracellular accumulation of IFN-γ. For each time point, data represent the percentages of cells positive for IFN-γ expressed as percentages (±SD) of the 10-μg/ml value (left column) or as raw data showing the percentages (±SD) of CD8 T cells (right column) (n = 2 or 3/concentration).
Role for antigen in maintaining T-cell responses.
The maturation of functional avidity of pattern 1 cells over the first 2 months of infection, along with their slower decline and higher rates of proliferation, suggests that these cells may be continually stimulated by persistent virus during the latency amplification phase. To test whether pattern 1 responses require sustained virus during latency amplification for their persistence we utilized a recently developed latency-null recombinant γHV68, termed AC-RTA (18a). Intranasal infection of mice with AC-RTA resulted in transient virus replication in the lung that was cleared with kinetics similar to that of wild-type γHV68 (Fig. 9A). Unlike wild-type virus, however, AC-RTA does not readily reach the spleen to establish a latent infection and thus did not go through latency amplification (Fig. 9B) or induce splenomegaly (data not shown). In addition, there was no expansion of CD8 T cells expressing Vβ4 TCR after AC-RTA infection (Fig. 9C), consistent with a failure of AC-RTA to establish latency in C57BL/6 mice. We then examined how the lack of viral latency affects the kinetics of decline of virus-specific CD8 T-cell numbers (Fig. 9D). There was no difference in T-cell numbers of the pattern 2 responses in the presence or absence of latency. However, both pattern 1 responses showed a trend toward lower T-cell numbers 21 and 35 days after AC-RTA infection, and these reductions were statistically significant for the ORF61524-specific response. These data are consistent with pattern 1 cells requiring antigen expression during latency amplification for their prolonged proliferation and survival.
FIG. 9.
Pattern 1 cells require viral latency for optimal responses. Viral titers (±SD; n = 3/group) in the lungs (A) and infective centers in the spleen (B) following wild-type (WT) γHV68 infection or AC-RTA infection. (C) Forty-one days after infection spleens were harvested and stained with antibodies to CD8 and TCR Vβ4. Numbers indicate percentages of CD8 T cells expressing Vβ4 (n = 3/group). (D) Number (±SD) of tetramer+ CD8 T cells over time (n = 3 to 6/group; data representative of 3 experiments). *, P < 0.05; **, P < 0.01 (Student's t test).
DISCUSSION
The generation and maintenance of functional antiviral CD8 T cells are important for control of acute γHV68 infection and prevention of reactivation from latency (8, 37). Infection by the gammaherpesviruses involves at least three stages—acute lytic infection, latency establishment and amplification, and stable long-term latency—but how the cellular immune system responds to different viral epitopes during each stage of infection is poorly understood. Previous studies have shown that the kinetics of the CD8 T-cell responses to two immunodominant epitopes, ORF6487/Db and ORF61524/Kb, followed the expression of the two epitopes: ORF6487 is expressed exclusively during the acute phase and ORF61524 is expressed during the acute and early latent phases (22, 28, 33). However, it is unknown if these two patterns are representative of the overall response. We also do not know if some CD8 T-cell responses inflate during the latent phase (26) or if prolonged exposure to antigen during long-term latency drives some responses to functional exhaustion (2). To address these questions, we have identified a large number of new γHV68 epitopes and have examined the early and late CD8 T-cell responses to these and previously identified epitopes. We failed to find evidence for inflation or functional exhaustion. Rather, our analysis revealed that the T-cell numbers of all responses declined over time, with two distinct patterns defined by delayed (pattern 1) or rapid (pattern 2) decline. Interestingly these patterns corresponded precisely to the previously defined kinetics of the CD8 T-cell responses to ORF6487 and ORF61524, suggesting that we have identified a common feature of the early CD8 T-cell response to infection.
Differential antigen availability during distinct phases of infection has been shown to influence the kinetics of expression of epitope-specific T cells. Although the lytic and latent phases of infection are established concurrently, the lytic infection of epithelial cells is cleared rapidly, resulting in the elimination of antigens expressed exclusively during the acute phase. For example, it has been shown that the ORF6487 epitope is presented transiently by epithelial cells during the acute phase of infection in the lung and is not presented by spleen cells, and ORF6487-specific cells decline steeply after clearance of the lytic infection. On the other hand, latency is established in macrophages, dendritic cells, and B cells, all of which can serve as potent antigen-presenting cells (APCs) (11, 22). Latently infected cells undergo an amplification phase in the spleen before being controlled by cellular immunity, leading to the prolonged presence of antigens that are expressed during latency amplification (12, 27, 39). The ORF61524 epitope is presented both during the acute phase in the lung and in the spleen during latency amplification. Consistent with this pattern of epitope expression, ORF61524-specific CD8 T cells are sustained throughout latency amplification (22, 28). In the current study we have identified 4 epitopes that elicit T cells with a rapid decline and 9 epitopes that drive T cells with a slow kinetic decline. Consistent with a role for antigen, those cells with sustained responses underwent sustained proliferation and exhibited functional avidity maturation between 12 and 60 days p.i. In contrast, cells that declined more rapidly did not show sustained proliferation and did not undergo functional avidity maturation. To directly test the role of sustained antigen presence in driving pattern 1 T-cell kinetics, we analyzed the T-cell response following infection with either wild-type virus or a mutant virus, AC-RTA, which fails to establish a latent infection (18a). Importantly, CD8 T-cell responses to epitopes that elicited pattern 1 kinetics in mice infected with WT virus exhibited pattern 2 kinetics in mice infected with the latency-null virus. These data strongly support the idea that antigen expression during the establishment and amplification of latency is an important factor in driving pattern 1 T-cell response kinetics.
It is unclear how epitope expression is differentially regulated during infection, but interestingly, in two separate cases two epitopes from the same protein drove T-cell responses in different kinetic classes. Thus, ORF75c176 drove pattern 1 responses whereas ORF75c940 drove pattern 2 responses. Similarly, ORF39209 drove pattern 1 responses whereas ORF39167 drove pattern 2 responses. These data suggest that epitope-specific response patterns are not directly linked to the kinetics of viral protein expression during infection. Rather, the expression of these epitopes is likely controlled by differences in antigen processing, presentation, or infected cell type during the lytic or latency amplification stage of infection in the lung, mesenteric lymph nodes (MLN), or spleen. This is currently being examined.
Importantly, although we did not directly examine the role of sustained antigen in the long-term functionality of epitope-specific responses in the current study, we saw no evidence that γHV68-specific CD8 T cells became functionally impaired. Indeed, both pattern 1 and pattern 2 responses maintained their abilities to synthesize cytokines and release lytic granules ex vivo and lyse peptide-loaded target cells in vivo (Fig. 4). These results suggest that, although prolonged antigen stimulation during latency amplification is sufficient to affect T-cell response kinetics, levels of antigen expression during long-term latency are apparently insufficient to drive CD8 T cells to exhaustion. Whether long-term latency is required for the prolonged maintenance of the number of virus-specific CD8 T cells over time is currently under investigation.
Here we carried out a genome-wide screen and used ELISpot assays to identify 33 γHV68-specific CD8 T-cell epitopes, confirming 14 previously published epitopes and identifying 21 new epitopes. It is instructive to compare the results of our epitope screen with those of another screen recently reported (14). Consistent with the previous screen, the epitopes we identified came predominantly from early and late genes (24), with a bias toward H-2Kb restriction. However, our screen identified 21 additional epitopes, encompassing a broader range of MHC binding affinities. One possible explanation for why our screen identified so many new epitopes is the breadth of the peptide library we used: unlike previous screens, our screen included H-2Db-restricted 9-mers and 10-mers and H-2Kb-restricted 8-mers and 9-mers, totaling 1,383 peptides. Thus, of the 21 new responses we detected, 10 epitopes (4 Db-restricted 10-mers and 6 Kb-restricted 9-mers) had not been tested in other assays (14, 22, 33). A second reason is that our screen may be more sensitive than the caged MHC-I tetramer approach (14), in that we identified peptides with significantly lower affinity for binding to MHC-I (Fig. 2).
Taken together, the results of the current analysis of a large number of γHV68-specific CD8 T-cell responses show that the virus-specific CD8 T-cell numbers can be categorized into two general kinetic classes, those that decline slowly (pattern 1) and those that decline rapidly (pattern 2). The slow decliners show sustained rates of proliferation and undergo functional avidity maturation, and their slow rate of decline is dependent on continued antigen expression during latency amplification. These data advance our understanding of the complexities of CD8 T-cell immune control of this latent gammaherpesvirus, which is essential for the development of effective vaccines and therapeutics.
Acknowledgments
We thank Scottie Adams for help with tetramer generation, Claire Burkum for excellent technical assistance, and Jacob Kohlmeier for critically reading the manuscript.
This project was funded by NIH grants AI42927 and CA148250 (to M.A.B.); T32 AI49823 (to D.L.W.); F32 AI084327 (to M.L.F.); DE18337 (to T.-T.W.); and CA91791, DE14153, and DE15752 (to R.S.). Funding was also provided by NIH contracts N01-AI400023C and N01-AI400024C (to A.S.), the Stop Cancer Foundation (to R.S.), and the Trudeau Institute.
Footnotes
Published ahead of print on 6 January 2010.
REFERENCES
- 1.Alter, G., J. M. Malenfant, and M. Altfeld. 2004. CD107a as a functional marker for the identification of natural killer cell activity. J. Immunol. Methods 294:15-22. [DOI] [PubMed] [Google Scholar]
- 2.Blattman, J. N., E. J. Wherry, S. J. Ha, R. G. van der Most, and R. Ahmed. 2009. Impact of epitope escape on PD-1 expression and CD8 T-cell exhaustion during chronic infection. J. Virol. 83:4386-4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cheng, Y., and W. H. Prusoff. 1973. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22:3099-3108. [DOI] [PubMed] [Google Scholar]
- 4.Coppola, M. A., E. Flano, P. Nguyen, C. L. Hardy, R. D. Cardin, N. Shastri, D. L. Woodland, and M. A. Blackman. 1999. Apparent MHC-independent stimulation of CD8+ T cells in vivo during latent murine gammaherpesvirus infection. J. Immunol. 163:1481-1489. [PubMed] [Google Scholar]
- 5.Cush, S. S., K. M. Anderson, D. H. Ravneberg, J. L. Weslow-Schmidt, and E. Flano. 2007. Memory generation and maintenance of CD8+ T cell function during viral persistence. J. Immunol. 179:141-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cush, S. S., and E. Flano. 2009. Protective antigen-independent CD8 T cell memory is maintained during {gamma}-herpesvirus persistence. J. Immunol. 182:3995-4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darrah, P. A., D. T. Patel, P. M. De Luca, R. W. Lindsay, D. F. Davey, B. J. Flynn, S. T. Hoff, P. Andersen, S. G. Reed, S. L. Morris, M. Roederer, and R. A. Seder. 2007. Multifunctional TH1 cells define a correlate of vaccine-mediated protection against Leishmania major. Nat. Med. 13:843-850. [DOI] [PubMed] [Google Scholar]
- 8.Ehtisham, S., N. P. Sunil-Chandra, and A. A. Nash. 1993. Pathogenesis of murine gammaherpesvirus infection in mice deficient in CD4 and CD8 T cells. J. Virol. 67:5247-5252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Evans, A. G., J. M. Moser, L. T. Krug, V. Pozharskaya, A. L. Mora, and S. H. Speck. 2008. A gammaherpesvirus-secreted activator of Vbeta4+ CD8+ T cells regulates chronic infection and immunopathology. J. Exp. Med. 205:669-684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Flano, E., C. L. Hardy, I. J. Kim, C. Frankling, M. A. Coppola, P. Nguyen, D. L. Woodland, and M. A. Blackman. 2004. T cell reactivity during infectious mononucleosis and persistent gammaherpesvirus infection in mice. J. Immunol. 172:3078-3085. [DOI] [PubMed] [Google Scholar]
- 11.Flano, E., S. M. Husain, J. T. Sample, D. L. Woodland, and M. A. Blackman. 2000. Latent murine gamma-herpesvirus infection is established in activated B cells, dendritic cells, and macrophages. J. Immunol. 165:1074-1081. [DOI] [PubMed] [Google Scholar]
- 12.Flano, E., Q. Jia, J. Moore, D. L. Woodland, R. Sun, and M. A. Blackman. 2005. Early establishment of {gamma}-herpesvirus latency: implications for immune control. J. Immunol. 174:4972-4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuse, S., J. J. Obar, S. Bellfy, E. K. Leung, W. Zhang, and E. J. Usherwood. 2006. CD80 and CD86 control antiviral CD8+ T-cell function and immune surveillance of murine gammaherpesvirus 68. J. Virol. 80:9159-9170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gredmark-Russ, S., E. J. Cheung, M. K. Isaacson, H. L. Ploegh, and G. M. Grotenbreg. 2008. The CD8 T-cell response against murine gammaherpesvirus 68 is directed toward a broad repertoire of epitopes from both early and late antigens. J. Virol. 82:12205-12212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gruener, N. H., F. Lechner, M. C. Jung, H. Diepolder, T. Gerlach, G. Lauer, B. Walker, J. Sullivan, R. Phillips, G. R. Pape, and P. Klenerman. 2001. Sustained dysfunction of antiviral CD8+ T lymphocytes after infection with hepatitis C virus. J. Virol. 75:5550-5558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gulukota, K., J. Sidney, A. Sette, and C. DeLisi. 1997. Two complementary methods for predicting peptides binding major histocompatibility complex molecules. J. Mol. Biol. 267:1258-1267. [DOI] [PubMed] [Google Scholar]
- 17.Hardy, C. L., E. Flano, R. D. Cardin, I. J. Kim, P. Nguyen, S. King, D. L. Woodland, and M. A. Blackman. 2001. Factors controlling levels of CD8+ T-cell lymphocytosis associated with murine gamma-herpesvirus infection. Viral Immunol. 14:391-402. [DOI] [PubMed] [Google Scholar]
- 18.Hikono, H., J. E. Kohlmeier, S. Takamura, S. T. Wittmer, A. D. Roberts, and D. L. Woodland. 2007. Activation phenotype, rather than central- or effector-memory phenotype, predicts the recall efficacy of memory CD8+ T cells. J. Exp. Med. 204:1625-1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18a.Jia, Q., M. L. Freeman, E. J. Yager, I. McHardy, L. Tong, D. Martinez-Guzman, T. Rickabaugh, S. Hwang, M. A. Blackman, R. Sun, and T.-T. Wu. 2010. Induction of protective immunity against murine gammaherpesvirus 68 infection in the absence of viral latency. J. Virol. 84:2453-2465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaech, S. M., J. T. Tan, E. J. Wherry, B. T. Konieczny, C. D. Surh, and R. Ahmed. 2003. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 4:1191-1198. [DOI] [PubMed] [Google Scholar]
- 20.Karrer, U., S. Sierro, M. Wagner, A. Oxenius, H. Hengel, U. H. Koszinowski, R. E. Phillips, and P. Klenerman. 2003. Memory inflation: continuous accumulation of antiviral CD8+ T cells over time. J. Immunol. 170:2022-2029. [DOI] [PubMed] [Google Scholar]
- 21.Kotturi, M. F., B. Peters, F. Buendia-Laysa, Jr., J. Sidney, C. Oseroff, J. Botten, H. Grey, M. J. Buchmeier, and A. Sette. 2007. The CD8+ T-cell response to lymphocytic choriomeningitis virus involves the L antigen: uncovering new tricks for an old virus. J. Virol. 81:4928-4940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu, L., E. Flano, E. J. Usherwood, S. Surman, M. A. Blackman, and D. L. Woodland. 1999. Lytic cycle T cell epitopes are expressed in two distinct phases during MHV-68 infection. J. Immunol. 163:868-874. [PubMed] [Google Scholar]
- 23.Liu, L., E. J. Usherwood, M. A. Blackman, and D. L. Woodland. 1999. T-cell vaccination alters the course of murine herpesvirus 68 infection and the establishment of viral latency in mice. J. Virol. 73:9849-9857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinez-Guzman, D., T. Rickabaugh, T. T. Wu, H. Brown, S. Cole, M. J. Song, L. Tong, and R. Sun. 2003. Transcription program of murine gammaherpesvirus 68. J. Virol. 77:10488-10503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moutaftsi, M., B. Peters, V. Pasquetto, D. C. Tscharke, J. Sidney, H. H. Bui, H. Grey, and A. Sette. 2006. A consensus epitope prediction approach identifies the breadth of murine T(CD8+)-cell responses to vaccinia virus. Nat. Biotechnol. 24:817-819. [DOI] [PubMed] [Google Scholar]
- 26.Munks, M. W., K. S. Cho, A. K. Pinto, S. Sierro, P. Klenerman, and A. B. Hill. 2006. Four distinct patterns of memory CD8 T cell responses to chronic murine cytomegalovirus infection. J. Immunol. 177:450-458. [DOI] [PubMed] [Google Scholar]
- 27.Nash, A. A., B. M. Dutia, J. P. Stewart, and A. J. Davison. 2001. Natural history of murine gammaherpesvirus infection. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 356:569-579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Obar, J. J., S. Fuse, E. K. Leung, S. C. Bellfy, and E. J. Usherwood. 2006. Gammaherpesvirus persistence alters key CD8 T-cell memory characteristics and enhances antiviral protection. J. Virol. 80:8303-8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar, S., V. Kalia, W. N. Haining, B. T. Konieczny, S. Subramaniam, and R. Ahmed. 2008. Functional and genomic profiling of effector CD8 T cell subsets with distinct memory fates. J. Exp. Med. 205:625-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shankar, P., M. Russo, B. Harnisch, M. Patterson, P. Skolnik, and J. Lieberman. 2000. Impaired function of circulating HIV-specific CD8(+) T cells in chronic human immunodeficiency virus infection. Blood 96:3094-3101. [PubMed] [Google Scholar]
- 31.Sidney, J., S. Southwood, C. Oseroff, M. F. del Guercio, A. Sette, and H. M. Grey. 2001. Measurement of MHC/peptide interactions by gel filtration. Curr. Protoc. Immunol., chapter 18, unit 18.3. [DOI] [PubMed]
- 32.Snyder, C. M., K. S. Cho, E. L. Bonnett, S. van Dommelen, G. R. Shellam, and A. B. Hill. 2008. Memory inflation during chronic viral infection is maintained by continuous production of short-lived, functional T cells. Immunity 29:650-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1999. Changing patterns of dominance in the CD8+ T cell response during acute and persistent murine gamma-herpesvirus infection. Eur. J. Immunol. 29:1059-1067. [DOI] [PubMed] [Google Scholar]
- 34.Stevenson, P. G., G. T. Belz, J. D. Altman, and P. C. Doherty. 1998. Virus-specific CD8(+) T cell numbers are maintained during gamma-herpesvirus reactivation in CD4-deficient mice. Proc. Natl. Acad. Sci. U. S. A. 95:15565-15570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stevenson, P. G., and P. C. Doherty. 1998. Kinetic analysis of the specific host response to a murine gammaherpesvirus. J. Virol. 72:943-949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson, P. G., S. Efstathiou, P. C. Doherty, and P. J. Lehner. 2000. Inhibition of MHC class I-restricted antigen presentation by gamma 2-herpesviruses. Proc. Natl. Acad. Sci. U. S. A. 97:8455-8460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stewart, J. P., E. J. Usherwood, A. Ross, H. Dyson, and T. Nash. 1998. Lung epithelial cells are a major site of murine gammaherpesvirus persistence. J. Exp. Med. 187:1941-1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thimme, R., V. Appay, M. Koschella, E. Panther, E. Roth, A. D. Hislop, A. B. Rickinson, S. L. Rowland-Jones, H. E. Blum, and H. Pircher. 2005. Increased expression of the NK cell receptor KLRG1 by virus-specific CD8 T cells during persistent antigen stimulation. J. Virol. 79:12112-12116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Usherwood, E. J., D. J. Roy, K. Ward, S. L. Surman, B. M. Dutia, M. A. Blackman, J. P. Stewart, and D. L. Woodland. 2000. Control of gammaherpesvirus latency by latent antigen-specific CD8(+) T cells. J. Exp. Med. 192:943-952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Voehringer, D., C. Blaser, P. Brawand, D. H. Raulet, T. Hanke, and H. Pircher. 2001. Viral infections induce abundant numbers of senescent CD8 T cells. J. Immunol. 167:4838-4843. [DOI] [PubMed] [Google Scholar]
- 41.Wherry, E. J., J. N. Blattman, K. Murali-Krishna, R. van der Most, and R. Ahmed. 2003. Viral persistence alters CD8 T-cell immunodominance and tissue distribution and results in distinct stages of functional impairment. J. Virol. 77:4911-4927. [DOI] [PMC free article] [PubMed] [Google Scholar]