Abstract
In ischemic hearts, venous retroperfusion is a potential myocardial revascularization strategy. This study aimed to refine the technical and functional aspects of a pig model of acute myocardial infarction and retroperfusion with respect to the azygos connection. Global retroperfusion after ligation of the ramus interventricularis paraconalis (equivalent to the left anterior descending artery in humans) was performed in 16 Landrace pigs (Sus scrofa domestica). Coronary sinus perfusion was performed in 8 pigs (P+) but not in the other 8 (P–), and the azygos vein was ligated (L+) 4 of the 8 pigs in each of these groups but left open (L–) in the remaining animals. Hemodynamic performance (for example, cardiac output, stroke volume) was significantly better in P+L+ pigs that underwent coronary sinus perfusion with ligation of the azygos vein compared with all other animals. In addition, troponin I release was significant lower in P+L+ pigs (1.7 ± 1.3 ng/mL) than in P–L– (5.47 ± 2.1 ng/mL), P–L+ (6.63 ± 2.4 ng/mL), and P+L– (4.81 ± 2.3 ng/mL) pigs. Effective retrograde flow and thus hemodynamic stability was achieved by ligation of the azygos vein. Therefore, experiments focusing on global retroperfusion will benefit from effective inhibition of the blood flow through the azygos vein.
Abbreviations: ACS, aorta-to-coronary–sinus shunt; CS, coronary sinus; L, ligation; LAD, left anterior descending artery; P, perfusion
Animal models are used frequently to investigate myocardial revascularization techniques, and researchers have studied global or selective venous retroperfusion in dogs,22 pigs,9 and sheep.33 The goal underlying retrograde coronary sinus (CS) perfusion is perfusion of the ischemic myocardium proximal to the occlusion or stenosis. This method frequently is used for delivering cardioplegic solutions during cardiac surgery. In addition, both clinical2,3,6,16,28,30 and experimental 11,21,25,34,37,42 studies have validated the efficiency of CS retroperfusion.
Interpreting the results from experimental animal models and follow-up examinations of patients who have undergone venous revascularization has led to controversy.9,37 In particular, technical problems with some studies have been identified. Previous animal studies on interspecies anatomic differences in mammals4,7,19 have concentrated on the venous connections of the vessels draining the myocardium and have demonstrated a need for further feasibility studies of the pig model (German Landrace pigs, Sus scrofa domestica) that focus on hemodynamic performance.
We wanted to characterize in detail the contribution of the azygos vein connection in swine during retroperfusion after myocardial infarction and hypothesized that ligation of the azygos vein would preserve hemodynamic function after ligation of the left anterior descending artery (LAD) in a global retroperfusion model in pigs.
Materials and Methods
Animals.
This study used 16 German Landrace pigs (Schmidt's Farm, Langensendelbach, Germany), which received humane care in strict compliance with the Principles of Laboratory Animal Care formulated by the National Society for Medical Research and the Guide for the Care and Use of Laboratory Animals.20, 32 All procedures were performed in accordance with the European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes12 and the German Animal Protection Law of 1998,17 and permission was obtained from the local Veterinary Office (Regierungspräsidium Mittelfranken, Ansbach, Germany; permission no. 54-2531.31-30/06).
The animals were housed at Friedrich–Alexander University's Animal Research Center (Franz Penzoldt Center of Animal Sciences, Erlangen, Germany). The pigs had ad libitum access to water and were fed a commercial diet (V4133-000 SSNIFF 4 mm, Ssniff Spezialdiaeten, Soest, Germany). A room temperature of 18.5 to 21.5 °C and humidity of 50% to 70% were maintained. The animals were acclimated for at least 2 wk and carefully checked for preexisting diseases before undergoing the surgical procedures. Food was withheld on the morning of surgery.
Anesthesia and monitoring.
Healthy male pigs (weight, 30.8 to 50.7 kg; median, 38.5 kg; age, 86.2 ± 8.5 d) were premedicated intramuscularly with ketamine hydrochloride (20 mg/kg; Ketavet, Pfizer, Karlsruhe, Germany), azaperone (4 mg/kg; Stresnil, Janssen-Cilag, Neuss, Germany), midazolam hydrochloride (1 mg/kg; Midazolam, Ratiopharm, Ulm, Germany), and atropine (0.01 mg/kg; Atropine, Braun, Melsungen, Germany). After loss of consciousness, the spontaneously breathing pigs were transferred to the operating theater. A marginal vein in 1 ear was cannulated with a 32-mm 20-gauge intravenous catheter (Venflon 2, Braun) for fluid and drug administration. After 3 min of preoxygenation with 15 L oxygen flow by face mask, anesthesia was supplemented by rapid intravenous infusion of ketamine (10 mg/kg; Ketavet, Pfizer) and bolus infusion of propofol (1 to 2 mg/kg; Disoprivan, Pfizer) and rocuronium (1 mg/kg; Esmeron, Essex Pharma, Munich, Germany) or cis-atracurium (0.2 mg/kg; Nimbex, Glaxo Smith Klein, Munich, Germany). Sixty seconds later, the animals were endotracheally intubated with a cuffed tube (inner diameter, 6.5 or 7.5 mm; Woodbridge endotracheal tube, Mallinckrodt, Gosport, Hampshire, UK).
After induction of unconsciousness and sufficient anesthesia as determined by hemodynamic and clinical observation (lack of reaction to noxious somatic and autonomic stimuli34); neuromuscular blockade (rocuronium or cis-atracurium) was given only to achieve good intubation conditions. Depth of anesthesia was maintained by inhaled isoflurane (1% to 3%; Forene, Abbott, Wiesbaden, Germany) and intravenous fentanyl (10 µg/kg; Fentanyl, Janssen-Cilag).
Ventilation was performed by using a respirator in volume-control mode (Fabius, Dräger, Lübeck, Germany) at a frequency of 20 to 25 breaths per min, tidal volume of 10 to 12 mL/kg, positive end-expiratory pressure of 5 mbar, and inspired oxygen fraction of 0.5. A 3-lead electrocardiogram was attached. Body temperature was monitored by using a rectal probe thermometer and maintained at 37 ± 1 °C by warm infusions, a heating pillow, and a room temperature of 25 °C. Central venous catheterization of the right jugular vein was accomplished by using a 7-French, 30-cm multilumen catheter (Arrow, Reading, PA) for further infusions and blood sampling. Hemodynamic monitoring was performed by using PiCCO technology (Pulsion Medical Systems, Munich, Germany), a pulse-pressure method that measures the pressure in an artery over time to derive a waveform and uses this information to calculate cardiac performance for continuous monitoring of cardiac output.5,13,14,18,26,29,39,41 To this end, an arterial catheter (5 French, 20 cm; Pulsiocath Thermodilution Catheter PV2015L20, Pulsion Medical Systems) was inserted into the right inguinal artery, threaded through the femoral artery until it rested entirely within the abdominal aorta, and connected to the PiCCO Monitor (PULSION Medical Systems); normal saline (15 mL, 4 to 8 °C) then was injected.
The heart was exposed a median sternotomy, and the pulmonary artery was catheterized directly (under echocardiographic control) with a multiple-lumen central venous catheter (7 French, 30 cm; Arrow) just to the bifurcation to monitor pulmonary artery pressure and to obtain blood for analyses of oxygen tension, acid–base balance, and electrolyte levels (ABL800 Flex and Hemoximeter OSM3, Radiometer, Copenhagen, Denmark).
A 32-mm, 20-gauge intravenous catheter (Venflon 2; Braun) was placed in the left atrial appendage for measurement of mean left atrial pressure, which correlates directly with the left ventricular end-diastolic pressure in the absence of preexisting mitral valvular disease (as in our pigs).27,38 A transducer system (Becton Dickinson Critical Care Systems, Salt Lake City, UT) was connected, placed at the level of the heart, and calibrated. The transducer was zeroed to atmospheric pressure and not calibrated against a known standard.
Experimental design.
We allocated the 16 pigs into 4 groups of 4 pigs each. In each group, acute ischemia was induced by ligation of the ramus interventricularis paraconalis (equivalent to the LAD) in the middle of the sulcus interventricularis by using a beating heart technique. The hemodynamic effect of CS perfusion was studied, either with the azygos vein open or ligated. To this end, the azygos vein was identified angiographically. Intraoperative venograms were obtained by using mobile X-ray imaging (Arcadis Avantic, Siemens, Medical Solutions, Erlangen, Germany) and 50 to 70 mL iomeprol (Imeron 350, Altana Pharma, Konstanz, Germany) as the contrast medium.
Surgical procedures.
A median incision and sternotomy were performed in anesthetized pigs, and the heart was exposed by using pericardial stay sutures. Heparin (200 IU/kg) was administered, and the activated clotting time (Hemochron Jr Whole Blood Coagulation System, International Technidyne Corporation, Edison, NJ) was kept above 200 s.
The CS was catheterized by introducing an occlusive cardioplegia catheter (RC2014MIB, Edwards, Unterschleißheim, Germany) transatrially after a 5-0 polypropylene pursestring suture was placed on the lateral wall of the right atrium. The presence of an azygos connection was checked angiographically through the CS (Figure 1). The azygos vein was identified, slung with a tourniquet, and held open until the start of the experiment. The ascending aorta was cannulated with an antegrade cardioplegia cannula (CA21VK, Maquet, Rastadt, Germany), and global retrograde perfusion was established by creating an aorta-to-CS shunt (ACS) connecting the 2 conventional cannulae. Intraoperative transesophageal echocardiography was performed by means of an ultrasound machine (Acuson CV70, Siemens, Erlangen, Germany).
Figure 1.
The operating field seen from the perspective of the surgeon; cranial is to the left. (A) A balloon-tipped catheter (1) is placed transatrially into the coronary sinus and connected to an aortic canula, creating an aorta-to-coronary–sinus shunt (3). The ramus interventricularis paraconalis (2) and its concomitant vein (vena cordis magna) also are shown. (B) The facies diaphragmatica of the heart is shown, with a view of the coronary sinus (1) and the vena cava caudalis (3). (C) Preparation of the vena azygos sinistra after creation of a pericardial window (3). The azygos vein (2) is shown before ligation, for which a suture is placed by means of a clamp (1). (D) The vena cava cranialis (1), azygos vein (ligated with a tourniquet; 2), and the pericardial window (3).
In all 16 pigs, the LAD was ligated in the middle of the sulcus interventricularis for 20 min by using 5-0 polypropylene sutures, and hemodynamic performance was observed for 1 h with (P+) or without (P–) global retrograde perfusion via the ACS, during which time the azygos vein was either open (L–) or ligated (L+) by closing the tourniquet. Efficiency of the ACS was evaluated by coronary angiography and online pressure measurement. The following parameters were monitored: electrocardiographic changes, cardiac output, mean arterial pressure, mean pulmonary artery pressure, heart rate, stroke volume, and ejection fraction. After the end of the observation period, the animals were euthanized (80 mg/kg pentobarbital sodium; Eutha 77, Essex Pharma) during deep anesthesia. Cardiac arrest was induced by infusion of Bretschneider cardioplegic solution (Custodiol, Köhler Chemie, Alsbach-Hähnlein, Germany). The hearts were explanted and histopathologically examined.
Laboratory measurements.
The concentration of cardiac troponin I was determined automatically (Access 2, Beckman Coulter, Krefeld, Germany). The cutoff point was 0.07 ng/mL. The analytic specificity for swine cardiac troponins has been published elsewhere.15
Statistical analysis.
Data were analyzed by using SigmaStat statistical software (Systat Software, San Jose, CA). All data are presented as mean ± 1 SD. Two-way ANOVA for repeated measurements was used to compare variables within and between groups. A P value of less than 0.05 was considered statistically significant.
Results
All pigs survived surgery and remained alive without signs of nociception throughout the 1-h observation period.
Angiographic results of the examination of the azygos connections.
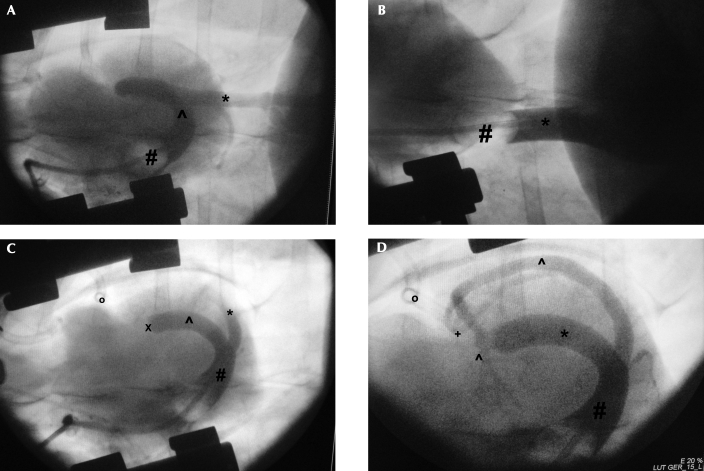
Intraoperative venograms confirmed the presence of an azygos connection (left, 14; right, 2) in all 16 pigs (Figure 2). After ACS catheterization, effective retrograde flow could be verified angiographically only when the azygos vein was ligated (Figure 1).
Figure 2.
Intraoperative retrograde venograms of the connection of the venus azygos sinistra; cranial is to the left. (A) Retrograde venogram of the vena azygos sinistra (*) before ligation; a balloon-tipped catheter (#) is placed transatrially into the coronary sinus (∧). The cardiac venous system (for example, vena cordis magna) lacks contrast because of backflow of blood to the vena azygos sinistra. (B) Venogram of the vena cava caudalis (*) is shown to distinguish the vena cava caudalis from the vena azygos sinistra (#, balloon-tipped catheter). (C) Venogram of the cardiac venous system. The vena azygos sinistra (∧) contains contrast material just to the point of ligation (X). The tourniquet (o), vena cordis magna (*), and sinus coronarius (#) are shown. (D) After backflow of the contrast medium has been blocked by ligation of the azygos vein, the vena cordis magna (∧) is now visible. Visualization of the vena cordis magna indicates effective retrograde flow through the aorta-to-coronary– sinus catheter. In addition, the vena cordis media (+), sinus coronarius (#), and tourniquet (o) are shown.
Myocardial enzyme release.
Troponin I release was significantly (P < 0.05) lower in pigs with ACS perfusion and ligation of the azygos vein (P+L+ group; 1.7 ± 1.3 ng/mL) than in the P–L– (5.47 ± 2.1 ng/mL), P–L+ (6.63 ± 2.4 ng/mL), and P+L– (4.81 ± 2.3 ng/mL) groups.
Hemodynamic performance.
Parameters of hemodynamic performance declined after LAD ligation in the groups without ACS perfusion and that with ACS perfusion and an open azygos vein. Only in pigs with ACS perfusion and ligated azygos veins did intraoperative hemodynamic variables remain unchanged from preoperative values (Table 1). Left ventricular ejection fraction, measured planimetrically by using transesophageal echocardiography, declined (P < 0.05) from approximately 60% preoperatively to 29% in P–L– pigs, 28% in P–L+ animals, and 32% in the P+L– group. In contrast, the intraoperative left ventricular ejection fraction in the P+L+ group (53%) did not differ from that preoperatively but was significantly (P < 0.05) higher than the intraoperative values of the other groups. Similar changes occurred in the left ventricular end-diastolic pressure, which was significantly increased intraoperatively compared with preoperative values in the P+L–, P–L+, and P–L– groups but not in the P+L+ group (Table 1). In all groups, changes in cardiac output, the clinically most important parameter, reflected changes in stroke volume (Table 1). The effect of azygos vein ligation on cardiac output is shown in Figure 3.
Table 1.
Hemodynamic variables at before (preoperative) and 60 min after LAD ligation
| Preoperative |
After LAD ligation |
|||||||
| P–L– | P–L+ | P+L– | P+L+ | P–L– | P–L+ | P+L– | P+L+ | |
| Ejection fraction (%) | 61 ± 6.9 | 61.5 ± 7.1 | 59.5 ± 7.9 | 60.3 ± 7.5 | 29.4 ± 9.5a | 28.2 ± 11a | 32.3 ± 9.8a | 53.1 ± 13b |
| LVEDP (mm Hg) | 7 ± 1.6 | 8.3 ± 0.8 | 7.3 ± 2 | 7.4 ± 2 | 11.2 ± 4.3a | 13.3 ± 7.3a | 12.2 ± 6.7a | 8.8 ± 3.9b |
| Cardiac output (L/min) | 5.5 ± 1.3 | 6.1 ± 1.1 | 5.3 ± 1.3 | 5.7 ± 0.9 | 2.9 ± 1.2a | 3.2 ± 1.3a | 2.7 ± 1.8a | 4.9 ± 1.1b |
| Heart rate (bpm) | 77 ± 9 | 79 ± 11 | 79 ± 13 | 78 ± 10 | 87 ± 17 | 81 ± 19a | 89 ± 17 | 80 ± 11 |
| Stroke volume (mL) | 57.4 ± 7 | 53.5 ± 9 | 57 ± 6 | 54.3 ± 6 | 31.3 ± 13a | 29.2 ± 8.8a | 27.3 ± 9a | 48.2 ± 12b |
| ST segment elevation (mm) | 0.3 ± 0.2 | 0.3 ± 0.3 | 0.1 ± 0.1 | 0.3 ± 0.2 | 11.5 ± 6.2a | 12.1 ± 5.1a | 10.1 ± 3a | 2.1 ± 1.9ab |
| Ventricular extrasystole (bpm) | 1 ± 1.5 | 1 ± 1 | 1 ± 1 | 0 ± 1 | 4 ± 8a | 3 ± 4a | 3 ± 3a | 1 ± 2b |
| Mean arterial pressure (mm Hg) | 64 ± 8 | 66 ± 9 | 65 ± 9 | 68 ± 9 | 44 ± 14a | 42 ± 16a | 45 ± 11a | 60 ± 11b |
| MPAP (mm Hg) | 24 ± 3 | 21 ± 2 | 25 ± 2 | 20 ± 2 | 36 ± 4a | 39 ± 8a | 33 ± 11a | 26 ± 8b |
| Troponin I (ng/mL) | 0.55 ± 0.3 | 0.4 ± 0.5 | 0.43 ± 0.4 | 0.35 ± 0.4 | 5.47 ± 2.1a | 6.63 ± 2.4a | 4.81 ± 2.3a | 1.7 ± 1.3b |
LAD, left anterior descending artery; CS, coronary sinus; L, ligation of azygous vein (+, present; –, absent); LVEDP, left ventricular end-diastolic pressure; MPAP, mean pulmonary artery pressure; P, perfusion through ACS (+, present; –, absent).
Values are presented as mean ± 1 SD (n = 4).
P < 0.05 versus baseline value for same group and parameter.
P < 0.05 versus postligation values for the same parameter for the P–L–, P–L+, and P+L– groups.
Figure 3.
Changes in cardiac output after LAD ligation: effect of azygos vein ligation. Data are presented as percentages of the preoperative (preop.) cardiac output. Each line represents an individual animal. The solid line represents the mean value (error bars, 1 SD). The 4 pigs in the P+L+ group comprise the upper cluster of data points, whereas those of the P+L–group comprise the lower cluster. 0 min, start of LAD ligation; 60 min, start of reperfusion. *, Significantly (P < 0.05) different from preoperative value.
Electrocardiographic changes and rhythm disorders.
All pigs showed signs of myocardial ischemia after LAD ligation (elevation of the ST segment, ventricular extrasystole as a sign of electrical instability due to ischemia), but the changes were nonsignificant in the P+L+ group only (Table 1). In addition, mean arterial pressure was maintained throughout the observation period in the P+L+ group but declined compared with preoperative values in the other 3 groups.
Discussion
Retroperfusion of the CS is used to improve myocardial perfusion and postischemic systolic and diastolic function during many surgical procedures, such as off-pump coronary artery bypass grafting9 and percutaneous intermittent coronary sinus occlusion.30,31 In routine clinical settings, retroperfusion of the CS is frequently used to deliver cardioplegic solutions.25 However, clinical use of retroperfusion is not widespread because findings from pig models have generated controversy.9,10 One problem is that pig studies have not always included structured analysis of the animals' anatomy, perhaps explaining some reports of poor outcomes. In the current study, we therefore aimed to refine the experimental techniques used in a short-term retroperfusion model in pigs.
In previous animal studies, the anatomy of the azygos vein has been of morphologic interest only,4,19 and various azygos connections have been observed in mammals. In one study, the left azygos vein in Landrace pigs did not always divide into a right azygos vein, whereas the right azygos vein of dogs did not divide into a left azygos vein; Landrace horses often had a branching left azygos vein arising from the right azygos vein, whereas cows often demonstrated a right azygos beside the left azygos vein.19 We used intraoperative angiography to confirm the presence of an azygos connection in all pigs in the present study. In 2 of the 16 pigs (12%), the right azygos vein branched off of the left azygos vein.
Previous studies using animal retroperfusion models in dogs,43 pigs,9,37 and sheep33,35 did not focus on the potential obstacle created by anatomical variation. For example, one retroperfusion study in pigs sought to improve systolic and diastolic dysfunction during off-pump cardiac surgery.9 The authors reported that retrograde CS perfusion during simulated off-pump coronary bypass revascularization in Yorkshire-Duroc pigs diminished systolic and diastolic dysfunction and concluded that retrograde perfusion was insufficient because ischemia-induced ventricular fibrillation occurred and contractile function did not return to normal after 60 min of reperfusion.9 However, the authors failed to establish whether an azygos connection was present in their animal model. Other investigators used a similar model to analyze the effect on infarct size of arterialization of the cardiac venous system by means of the CS in dogs42 and observed only limited myocardial protection.
Our study assessed the clinical importance of the azygos connection in a pig model of retroperfusion. We studied the effect of simultaneous global retrograde perfusion of the CS by means of an aorta-to-coronary–sinus shunt by analyzing cardiocirculatory parameters during acute ischemia due to LAD ligation. As expected, we found that mid-LAD occlusion reduced cardiac output and worsened cardiac and circulatory parameters, including ejection fraction, end-diastolic pressure, stroke volume, and mean arterial pressure, as well as created electrocardiographic changes (ST elevation) that were consistent with myocardial ischemia. In addition, all pigs manifested rhythm disturbances during the 20-min LAD occlusion period. We interpret these combined data as an indirect sign of sufficient myocardial oxygen supply, a conclusion that is supported by the serum levels of cardiac troponin I. In short, global retroperfusion prevented hemodynamic deterioration only if it was used in combination with azygos ligation. By combining global retroperfusion with azygos ligation, arterialized blood was prevented from flowing into the abdomen (bypassing the heart) and was redirected to the CS and ischemic myocardium. In this acute infarction model, global retroperfusion led to improved hemodynamic stability. Here we have shown for the first time the importance of identifying whether an azygos connection is present in pig models and the clinical benefit of ligating it.
A potential limitation of the current study is that the anesthetic regimen we used may have caused both negative and positive effects on hemodynamic function. We used a combination of different classes of anesthetics, including sedatives, analgesics, and hypnotics, consistent with the practice of ‘balanced anesthesia.’ The strategy of balanced anesthesia is to achieve well-tolerated anesthesia by using the additive effects of synergistic agents, thus enabling reduction in the dose required for each single agent and minimizing negative side effects (for example, on hemodynamic function parameters).8,23,24,36,40 In terms of the methodology of the current study, the greatest difference from the human clinical setting is that the juvenile pig hearts we used lacked any coronary artery disease. Therefore, our experimental model differs from patients with extensive coronary artery disease, in whom collateral flow may develop and limit the extent of ischemia after sudden LAD occlusion. Nevertheless, our findings may be directly relevant for patients with acute single-vessel disease without sufficient collateral flow.
In this acute pig model of myocardial infarction, the negative hemodynamic effects of LAD ligation were prevented by global retroperfusion by means of the coronary sinus only when the azygos vein was ligated. It is, therefore, crucial to identify and eliminate an azygos vein connection to the coronary sinus when using a porcine model of global retroperfusion. Experiments focusing on global retroperfusion through the coronary sinus have to accommodate the particular anatomy of pigs. Our finding that retroperfusion with identification and ligation of the azygos vein maintains stable hemodynamic function may allow this technique to become a useful adjunct in experimental settings involving retroperfusion in pigs.
Acknowledgments
This study was supported mainly by the ELAN Fund of the Friedrich–Alexander University of Erlangen–Nuernberg (HC 06.11.01.08). We thank the staff of the Franz Penzoldt Center of Animal Research for the excellent scientific and clinical work (Drs Dirk Labahn and Edyta Adamek) and for taking care of the animals (Franziska Klein and Harald Keck).
References
- 1.Agnew NM, Pennefather SH, Russell GN. 2002. Isoflurane and coronary heart disease. Anaesthesia 57:338–347 [DOI] [PubMed] [Google Scholar]
- 2.Aldea GS, Zhang X, Rivers S, Shemin RJ. 1996. Salvage of ischemic myocardium with simplified and even delayed coronary sinus retroperfusion. Ann Thorac Surg 62:9–15 [DOI] [PubMed] [Google Scholar]
- 3.Arealis EG, Volder JGR, Kolff WJ. 1973. Arterialization of the coronary vein coming from an ischemic area. Chest 63:462–463 [DOI] [PubMed] [Google Scholar]
- 4.Beddard F. 1907. The azygos veins in the Mammalia. Proc Zool Soc Lond 2:10–20 [Google Scholar]
- 5.Bein B, Worthmann F, Tonner PH, Paris A, Steinfath M, Hedderich J, Scholz J. 2004. Comparison of esophageal Doppler, pulse contour analysis, and real-time pulmonary artery thermodilution for the continuous measurement of cardiac output. J Cardiothorac Vasc Anesth 18:185–189 [DOI] [PubMed] [Google Scholar]
- 6.Benedict JS, Buhl TL, Henney RP. 1975. Cardiac vein myocardial revascularization: an experimental study and report of 3 clinical cases. Ann Thorac Surg 20:550–557 [DOI] [PubMed] [Google Scholar]
- 7.Bozkus H, Crawford NR, Chamberlain RH, Valenzuela TD, Espinoza A, Yüksel Z, Dickman CA. 2005. Comparative anatomy of the porcine and human thoracic spines with reference to thoracoscopic surgical techniques. Surg Endosc 19:1652–1665 [DOI] [PubMed] [Google Scholar]
- 8.Buffington CW, Romson JL, Levine A, Duttlinger NC, Huang AH. 1987. Isoflurane induces coronary steal in a canine model of chronic coronary occlusion. Anesthesiology 66:280–292 [DOI] [PubMed] [Google Scholar]
- 9.Castellá M, Buckberg GD. 2004. Reduction of systolic and diastolic dysfunction by retrograde coronary sinus perfusion during off-pump coronary surgery. J Thorac Cardiovasc Surg 127:1018–1025 [DOI] [PubMed] [Google Scholar]
- 10.Chowdhry MF, Davies J, McCance A, Galiñanes M. 2005. Lack of durability of surgical arterialization of coronary veins for the treatment of ischemic heart disease. J Card Surg 20:326–328 [DOI] [PubMed] [Google Scholar]
- 11.Choy JS, Kassab GS. 2006. A novel strategy for increasing wall thickness of coronary venules prior to retroperfusion. Am J Physiol Heart Circ Physiol 291:H972–H978 [DOI] [PubMed] [Google Scholar]
- 12.Council of Europe. 1986. [Internet]. European Convention for the Protection of Vertebrate Animals Used for Experimental and Other Scientific Purposes. Strasbourg, 18.III.1986. [Cited 14 Aug 2008]. Available at: http://conventions.coe.int/Treaty/en/Treaties/Html/123.htm.
- 13.Dahl MK, Vistisen ST, Koefoed-Nielsen J, Larsson A. 2009. Using an expiratory resistor, arterial pulse pressure variations predict fluid responsiveness during spontaneous breathing: an experimental porcine study. Crit Care 13:R39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Felbinger TW, Reuter DA, Eltzschig HK, Bayerlein J, Goetz AE. 2005. Cardiac index measurements during rapid preload changes: a comparison of pulmonary artery thermodilution with arterial pulse contour analysis. J Clin Anesth 17:241–248 [DOI] [PubMed] [Google Scholar]
- 15.Fredericks S, Merton GK, Lerena MJ, Heinig P, Carter ND, Holt DW. 2001. Cardiac troponins and creatine kinase content of striated muscle in common laboratory animals. Clin Chim Acta 304:65–74 [DOI] [PubMed] [Google Scholar]
- 16.Gardner RS, Magovern GJ, Park SB, Dixon CM. 1974. Arterialization of coronary veins in the treatment of myocardial ischemia. J Thorac Cardiovasc Surg 68:273–282 [PubMed] [Google Scholar]
- 17.German Animal Protection Act. 1998. BGBL. I [Federal Gazette] p 1105.
- 18.Godje O, Hoke K, Goetz AE, Felbinger TW, Reuter DA, Reichart B, Friedl R, Hannekum A, Pfeiffer UJ. 2002. Reliability of a new algorithm for continuous cardiac output determination by pulse-contour analysis during hemodynamic instability. Crit Care Med 30:52–58 [DOI] [PubMed] [Google Scholar]
- 19.Grau H. 1933. Contributions to the comparative anatomy of azygos veins in our domestic animals. PhD thesis, University of Leipzig, Germany [Google Scholar]
- 20.Institute for Laboratory Animal Research 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [PubMed] [Google Scholar]
- 21.Kassab GS, Navia JA, March K, Choy JS. 2008. Coronary venous retroperfusion: an old concept, a new approach. J Appl Physiol 104:1266–1272 [Review] [DOI] [PubMed] [Google Scholar]
- 22.Katircioglu SF, Yücel D, Saritas Z, Yamak B, Elsheikh AE, Köse K. 1998. Simplified retroperfusion system preserves the myocardial function during acute coronary artery occlusion. Thorac Cardiovasc Surg 46:1–6 [DOI] [PubMed] [Google Scholar]
- 23.Kato R, Foex P. 2002. Myocardial protection by anesthetic agents against ischemia–reperfusion injury: an update for anesthesiologists. Can J Anaesth 49:777–791 [DOI] [PubMed] [Google Scholar]
- 24.Kevin LG, Novalija E, Stowe DF. 2005. Reactive oxygen species as mediators of cardiac injury and protection: the relevance to anesthesia practice. Anesth Analg 101:1275–1287 [DOI] [PubMed] [Google Scholar]
- 25.Lazar HL. 1988. Coronary sinus interventions during cardiac surgery. Ann Thorac Surg 46:475–482 [DOI] [PubMed] [Google Scholar]
- 26.Lemson J, Backx AP, van Oort AM, Bouw TP, van der Hoeven JG. 2009. Clinical investigations—extravascular lung water measurement using transpulmonary thermodilution in children. Pediatr Crit Care Med 10:227–233 [DOI] [PubMed] [Google Scholar]
- 27.López-Herce J, Rupérez M, Sánchez C, García C, García E. 2006. Estimation of the parameters of cardiac function and of blood volume by arterial thermodilution in an infant animal model. Paediatr Anaesth 16:635–640 [DOI] [PubMed] [Google Scholar]
- 28.Meerbaum S, Lang TW, Osher JV, Hashimoto K, Lewis GW, Feldstein C, Corday E. 1976. Diastolic retroperfusion of acutely ischemic myocardium. Am J Cardiol 37:588–598 [DOI] [PubMed] [Google Scholar]
- 29.Michard F. 2007. Bedside assessment of extravascular lung water by dilution methods: Temptations and pitfalls. Crit Care Med 35:1186–1192 [DOI] [PubMed] [Google Scholar]
- 30.Mohl W, Glogar DH, Mayr H, Losert U, Sochor H, Pachinger O, Kaindl F, Wolner E. 1984. Reduction of infarct size induced by pressure-controlled intermittent coronary sinus occlusion. Am J Cardiol 53:923–928 [DOI] [PubMed] [Google Scholar]
- 31.Mohl W, Kajgana I, Bergmeister H, Rattay F. 2005. Intermittent pressure elevation of the coronary venous system as a method to protect ischemic myocardium. Interact Cardiovasc Thorac Surg 4:66–69 [DOI] [PubMed] [Google Scholar]
- 32.National Society for Medical Research. 1956. AIBS Bulletin, vol 6, No. 3, p 26. Washington (DC): American Institute of Biological Sciences. Available at: http://www.jstor.org/stable/1292434.
- 33.Pocar M, Rossi V, Addis A, Monaco A, Sichel S, Addis F, Grossi A, Donatelli F. 2007. Spinal cord retrograde perfusion: review of the literature and experimental observations. J Card Surg 22:124–128 [DOI] [PubMed] [Google Scholar]
- 34.Prys-Roberts C. 1987. Anaesthesia: a practical or impractical construct? Br J Anaesth 59:1341–1345 [DOI] [PubMed] [Google Scholar]
- 35.Resetar ME, Ullmann C, Broeske P, Ludwig-Schindler K, Doll NK, Salameh A, Dhein S, Mohr FW. 2007. Selective arterialization of a cardiac vein in a model of cardiac microangiopathy and microangiopathy in sheep. J Thorac Cardiovasc Surg 133:1252–1256 [DOI] [PubMed] [Google Scholar]
- 36.Riess ML, Camara AK, Rhodes SS, McCormick J, Jiang MT, Stowe DF. 2005. Increasing heart size and age attenuate anesthetic preconditioning in guinea pig isolated heart. Anesth Analg 101:1572–1576 [DOI] [PubMed] [Google Scholar]
- 37.Ropchan GV, Feindel CM, Wilson GJ, Boylen P, Sandhu R. 1992. Salvage of ischemic myocardium by nonsynchronized retroperfusion in the pig. J Thorac Cardiovasc Surg 104:619–625 [PubMed] [Google Scholar]
- 38.Rupérez M, López-Herce J, García C, Sánchez C, García E, Vigil D. 2004. Comparison between cardiac output measured by the pulmonary arterial thermodilution technique and that measured by the femoral arterial thermodilution technique in a pediatric animal model. Pediatr Cardiol 25:119–123 [DOI] [PubMed] [Google Scholar]
- 39.Segal E, Katzenelson R, Berkenstadt H, Perel A. 2002. Transpulmonary thermodilution cardiac output measurement using the axillary artery in critically ill patients. J Clin Anesth 14:210–213 [DOI] [PubMed] [Google Scholar]
- 40.Suleiman MS, Zacharowski K, Angelini GD. 2008. Inflammatory response and cardioprotection during open-heart surgery: the importance of anaesthetics. Br J Pharmacol 153:21–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wesseling KH, Jansen JRC, Settels JJ, Schreuder JJ. 1993. Computation of aortic flow pressure in humans by using a nonlinear, three element model. J Appl Physiol 74:2566–2573 [DOI] [PubMed] [Google Scholar]
- 42.Zalewski A, Goldberg S, Slysh S, Maroko PR. 1985. Myocardial protection via coronary sinus interventions: superior effects of arterialization compared with intermittent occlusion. Circulation 71:1215–1223 [DOI] [PubMed] [Google Scholar]