Abstract
Shigella dysenteriae type 1 can cause devastating pandemics with high case fatality rates; a vaccine for Shigella is unavailable currently. Because of the risks associated with performing challenge studies with wild-type S. dysenteriae 1 in human clinical trials to advance vaccine development, an improved nonhuman primate model is needed urgently. In the present study, cynomolgus macaques (Macaca fascicularis) were challenged with various doses of S. dysenteriae 1 strain 1617 to establish a dose that would produce shigellosis. Further, different routes of delivery of S. dysenteriae 1 were compared to establish the most appropriate route for infection. Animals receiving 1011 cfu S. dysenteriae 1 intragastrically consistently developed signs of shigellosis characterized by the onset of diarrhea and dysentery within 2 to 3 d. Administration of as many as 109 cfu S. dysenteriae 1 intraduodenally did not elicit signs characteristic of infection in macaques despite fecal shedding of bacteria for as long as 10 d. S. dysenteriae 1 administered intraduodenally at 109 cfu or intragastrically at 1011 cfu elicited robust IgG and IgA antibody responses to LPS. We have developed a reliable challenge model of infection with wild-type S. dysenteriae 1 in cynomolgus macaques that reproducibly induces disease and elicits robust immune responses. We believe that this animal model may provide unique insights into the immunologic mechanisms of protection to S. dysenteriae 1 infection and in advancing development of a vaccine against shigellosis.
Shigella has been classified by the National Institute of Allergy and Infectious Disease as a category B priority pathogen. Shigella has numerous features that characterize an effective biological weapon, including the potential to cause high morbidity and mortality, a low infectious dose (approximately 10 cfu) in humans, the ability to produce large outbreaks even in industrialized countries, ease of direct person-to-person transmission, the ability to contaminate food and water supplies, and the potential to be weaponized.15
Currently a vaccine for shigellosis is unavailable, and antibiotic therapy remains the only means of treatment.13,15 Continually emerging new and multiple-antibiotic-resistant strains pose a serious problem to treat this disease. The present studies were designed to establish an S. dysenteriae 1 challenge model for investigation of pathogenesis, immunogenicity, and protection from infection in cynomolgus macaques. Previous studies successfully established shigellosis models after challenge with wild-type strains of Shigella spp. in rhesus macaques,3,5–8,12,19,22 but a similar S. dysenteriae 1 challenge model in cynomolgus macaques has not yet been reported. This model has the potential to advance the design of novel Shigella vaccines. In the current study, we established and characterized an S. dysenteriae 1 challenge model in cynomolgus macaques. In addition, we determined the minimal challenge dose required to induce clinical signs of shigellosis, measured the duration of shedding of the organism from the macaques, and contrasted the effects (as evaluated by endoscopy) of intragastric versus intraduodenal inoculation on disease induction and immunogenicity.
Materials and Methods
Animals.
Male and female Chinese-origin cynomolgus macaques (Macaca fascicularis; age, 2 to 5 y) were purchased from an approved vendor (Sierra Biomedical Research, Reno, NV). Animals were negative for circopithecine herpesvirus 1, SIV, simian retrovirus, and simian T-lymphotrophic virus. Prior to study onset, all animals were quarantined for 3 mo and tested negative for intestinal parasites and Campylobacter, Salmonella, Shigella, and Yersinia spp. In addition, only animals that were negative for IgG and IgA antibodies to S. dysenteriae 1 LPS or had that titers lower than 50 were used in this study. The study took place in the animal facility of the Program of Comparative Medicine (University of Maryland School of Medicine). The facility is AAALAC-accredited, and all procedures in the study conformed to the policies and guidelines of the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine. Animals were housed in a Biosafety Level 2 containment facility, and appropriate measures were taken to ensure safe handling practices while working with this pathogen. Animals were fed a commercial primate diet (Teklad 2050, Harlan Laboratories, Indianapolis, IN) supplemented daily with fresh fruits and vegetables. In addition, animals were given supplemental food enrichment (fruit and nut mix, popcorn, peanuts, granola bars) once to twice weekly. All food items historically have been free of any bacterial contamination. Municipal drinking water (regularly tested in our facility and found free of any bacterial growth) was provided through an automatic watering system. In addition, a water bottle containing municipal drinking water was placed on each cage, to prevent interruption of water supply in the event of failure of the automatic watering system. All animals were housed individually (to enable individual collection of feces) in stainless steel primate caging for the duration of the study. All procedures conformed to guidelines in the Guide for the Care and Use of Laboratory Animals10 and the Animal Welfare Act1 and were fully compliant with recommendations in the Biosafety in Microbiological and Biomedical Laboratories Guide.2
Study design.
We first established the optimal dose that would induce shigellosis in cynomolgus macaques (dose optimization). The 6 macaques used for this purpose were fasted for 12 h prior to bacterial challenge. Water was available ad libitum to all animals throughout the study. Animals were challenged intraduodenally with increasing doses (105 to 109 cfu) of wild-type S. dysenteriae 1 strain 1617 (1 dose per animal). In an attempt to increase the virulence of the inoculated bacteria, the sixth macaque received 109 cfu of S. dysenteriae 1 that had been passaged in another macaque (that is, bacteria isolated from the feces of the passage macaque was grown in vitro and used for challenge at this particular dose).
After bacterial challenge, each animal was monitored twice daily for signs of illness. To monitor fecal shedding of the bacteria, stool specimens were collected from each animal twice daily until 4 d after inoculation and once daily thereafter until 14 d after infection. Blood samples were obtained on days 0, 7, 14, 28, and 56 after challenge to measure antiShigella LPS antibody levels.
To explore whether cynomolgus macaques are susceptible to S. dysenteriae 1 infection, we used a procedure similar to that used in rhesus macaques.3,5-8,12,19,22 To this end, 5 cynomolgus macaques were divided into 2 groups: 2 animals were inoculated intraduodenally with 109 cfu S. dysenteriae 1, and 3 animals were given 1011 cfu of S. dysenteriae 1 intragastrically. To prevent killing of the inoculated bacteria by gastric acid, each macaque received 15 mEq sodium bicarbonate intragastrically immediately prior to inoculation.
For immunologic assays, blood samples were collected before and at multiple times after challenge.
Bacteria and preparation of the challenge inocula.
Challenge inocula were prepared from frozen master stocks of S. dysenteriae 1 strain 1617,28 which were plated onto trypticase soy agar (Becton Dickinson, Franklin Lakes, NJ) containing 0.01% Congo red dye (Sigma Chemical, St Louis, MO). After incubation for 18 to 24 h at 37 °C, the identity of single-well isolated Congo-red–positive colonies that exhibited Shigella morphologic characteristics was confirmed by use of Shigella dysenteriae antiserum (Denka Seiken, Tokyo, Japan). Several isolated Congo-red–positive colonies were picked and suspended in sterile saline. These colonies were used to inoculate, for heavy growth, several trypticase soy agar plates which then were incubated overnight at 37 °C. Overnight growth from these plates was harvested, washed, and standardized turbidmetrically, and appropriate dilutions were made in PBS for inoculation. The inocula were transported on wet ice and used within 4 h of preparation. A small aliquot of the inoculum was diluted appropriately in PBS and colony counts performed in 2 to 3 replicate plates after an overnight incubation to establish the actual inoculum that was administered to the cynomolgus macaques.
Intraduodenal inoculation.
Each macaque was challenged intraduodenally with wild-type Shiga-toxin–positive S. dysenteriae 1 strain 1617 by using an endoscope. After sedation with ketamine (10 mg/kg IM; Vedco, St Joseph, MO), animals underwent mask-induced anesthesia with 2% to 3% isoflurane (Aerrane, Baxter Healthcare, Deerfield, IL), were orotracheally intubated, and were maintained on 1.5% to 2.5% isoflurane in 100% oxygen. A pediatric gastroscope (GIF P140, Olympus America, Center Valley, PA) was passed orogastrically until the pylorus was visualized directly. At this point, an endoscopic retrograde cholangiopancreatography catheter (Cook Medical, Bloomington, IN) was passed down the biopsy channel of the scope and into the duodenum by direct visualization. After intraduodenal placement of the catheter was confirmed on the video screen, the inoculum was injected through the catheter into the duodenum and flushed with a minimum of 10 mL physiologic saline to ensure delivery of the entire inoculum.
Intragastric inoculation.
Macaques were sedated (10 mg/kg ketamine IM) for manipulation. After onset of sedation, an 8- to 14-French orogastric tube (Tyco Healthcare Group LP, Mansfield, MA) was passed through the mouth into the stomach of the animal. Location was verified by gently injecting 5 to 20 mL air while auscultating the upper abdomen with a stethoscope to confirm gas sounds in the stomach. Thereafter, 15 mEq sodium bicarbonate (Neogen, Lexington, KY) was administered intragastrically through the tube, after which animals were inoculated intragastrically with approximately 1011 cfu S. dysenteriae 1. After delivery of the inoculum, the orogastric tube was flushed with 10 to 20 mL of sterile saline to ensure delivery of the entire inoculum.
Blood collection.
To monitor immune responses elicited by inoculation of the bacteria, blood samples were collected from the femoral veins of ketamine-sedated infected animals at 0, 7, 14, 28, and 56 after inoculation. Prior to venipuncture, the area was aseptically prepared by washing with a povidone iodine scrub followed by rinsing with 70% isopropyl alcohol.
Fecal microbiology.
Shigella excretion was detected by culturing monkey feces that were passed during the first week of challenge or longer, depending on each animal's duration of bacterial shedding. The fecal specimens were collected into sterile containers twice daily for the first 4 d and then once daily until 14 d after inoculation. Whole stools were plated directly onto Salmonella–Shigella or MacConkey agar (or both) and inoculated into gram-negative enrichment broth. The plates and tubes were incubated overnight at 35 °C. After incubation, the broth cultures were subcultured into Salmonella–Shigella agar, and the plates incubated overnight at 35 °C. Single, pure, lactose-negative colonies isolated from Salmonella–Shigella or MacConkey agar were inoculated into triple-sugar–iron agar slants and incubated overnight at 35 °C. The typical slant was an alkaline slant with acid but without gas, and organisms were confirmed as S. dysenteriae 1 by slide agglutination with specific antiserum.
Clinical monitoring and treatment.
Challenged animals were monitored daily throughout the study for diarrhea, dysentery, fever, signs of respiratory illness, changes in food intake, and any other abnormal behavior. Rectal temperature (Sure Temp Plus Thermometer, Welch Allyn, New York, NY) was monitored daily (after ketamine sedation) for the first 7 d and then on days 14, 28, and 56 after inoculation. Monkeys that become ill due to Shigella infection (as manifested by diarrhea, fever in excess of 103 °F, dysentery, or any other signs) were treated with antibiotics (enrofloxacin at 5 mg/kg PO or IM twice daily for 5 d, Bayer Animal Health, Shawnee Mission, KS, or ceftriaxone at 50 mg/kg IM once daily for 5 d, Apotex, Weston, FL) within 24 h of the time at which diarrhea or dysentery was first observed. In addition to antibiotic therapy, ill animals received intravenous fluid therapy (10 to 20 mL/kg Ringers Lactate Solution once or twice daily) as determined by a facility veterinarian.
Blood chemistry, enzymes, and differentials.
Blood samples collected from animals at various time points of the study underwent clinical chemistry and hematology analysis (Antech Diagnostics, Lake Success, NY). Blood chemistry measurements included total protein, globulin, albumin, albumin:globulin ratio, creatinine, urea, BUN, alkaline phosphatase, ALT, AST, glucose, phosphorus, calcium, chloride, triglycerides, and amylase. Hematology included hemoglobin, hematocrit, WBC count, RBC count, MCV, MCHC, and platelet counts. Differentials included lymphoctyes, eosinophils, monocytes, neutrophils, and basophil counts.
Shigella antibody measurements.
Plasma levels of IgG and IgA antibody titers to S. dysenteriae 1 LPS were measured by ELISA before and at several times after challenge. In brief, ELISA plates (Immulon 2, Thermo, Milford, MA) were coated for 3 h at 37 °C with S. dysenteriae 1 LPS at 5 μg/mL. Plates were blocked overnight at 4 °C by using 10% dried milk (Nestle, Vevey, Switzerland) in PBS. After each incubation, plates were washed 6 times with PBS containing 0.05% Tween 20. Pre- and postchallenge plasma and serum samples were evaluated in 2-fold dilutions in 10% dried milk in PBS containing 0.05% Tween 20. Specific IgG and IgA antibodies were detected by use of peroxidase-labeled goat antimonkey Fcγ- (1:5000) and α- (1:2000) chains (KPL, Gaithersburg, MD) in 10% dried milk in PBS containing 0.05% Tween 20. Both plasma samples and secondary antibodies were incubated for 1 h at 37 ºC. Next, the substrate solution (TMB Microwell Peroxidase, KPL) was added for 15 min. The reaction was stopped by the addition of 100 μL of 1 mol/L H3PO4, and the optical density at 450 nm was measured in an ELISA microplate reader (Multiskan Ascent, Labsystems, Helsinki, Finland). Plasma and serum samples were run in duplicate; negative and positive control plasma or serum samples were included in each assay. Because the IgG and IgA levels against S. dysenteriae 1 LPS did not differ between measurements performed in plasma or serum (data not shown), plasma or serum samples were used in the individual studies depending on specimen availability as indicated in the corresponding experiments. Linear regression curves were plotted for each plasma or serum sample, and titers were calculated as the inverse of the serum dilution that produced an OD of 0.2 above the blank. Data was reported as ELISA units per milliliter.
Results
Development of a S. dysenteriae 1 challenge model in cynomolgus macaques inoculated intraduodenally.
One of the main goals of these studies was to develop a challenge model of infection with wild-type S. dysenteriae 1 in cynomolgus macaques that reproducibly induced disease from a minimal inoculum. To this end, we intraduodenally (by using an endoscope) administered S. dysenteriae 1 at 5 different doses (105, 106, 107, 108, and 109 cfu), as well as 109 cfu of the organism recovered from a challenged cynomolgus macaque (that is, in vivo passaged bacteria). However none of the animals developed any clinical disease despite having persistent fecal shedding of organisms for up to 10 d in animals inoculated with 108 and 109 organisms (Table 1). Of note, 1 macaque (28110M) developed persistent infection with fecal shedding for 21 d (data not shown).
Table 1.
Fecal shedding after intraduodenal inoculation of macaques with S. dysenteriae 1
| Days after challenge |
|||||||||||||||||
| Inoculum (cfu) |
1 |
2 |
3 |
6 |
|||||||||||||
| Target | Actual | Monkey | am | pm | am | pm | am | pm | 4 | 5 | am | pm | 7 | 8 | 9 | 10 | 12 |
| 1 × 105 | 1.7 × 105 | 2631M | – | – | – | – | – | – | – | – | |||||||
| 1 × 106 | 1.1 × 106 | 2542M | – | – | – | – | + | + | – | + | – | ||||||
| 1 × 107 | 8.4 × 106 | 2604M | + | – | – | – | – | – | – | ||||||||
| 1 × 108 | 9.0 × 107 | 28110M | – | – | + | + | + | + | + | + | + | – | – | + | – | ||
| 1 × 109 | 8.6 × 109 | 2618M | – | – | + | + | + | + | + | – | + | + | + | + | – | + | |
| 1 × 109 | 2.8 × 109 | 15175a | + | + | + | + | + | + | + | – | |||||||
+, positive culture; –, no growth on culture; empty cell, data not available
Challenge strain used for monkey 15175 was isolated from the feces of monkey 2618M
Kinetics of induction of antiLPS IgG and IgA in cynomolgus macaques challenged intraduodenally with wild-type S. dysenteriae 1.
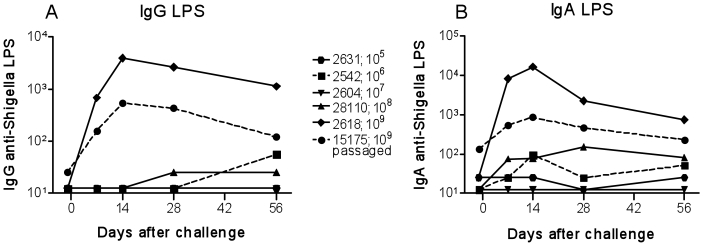
In these experiments, healthy cynomolgus macaques were prescreened for IgG and IgA serum antibodies against S. dysenteriae 1 LPS to exclude animals with preexisting immunity that might have affected the immunologic responses elicited by exposure to the bacterium. Preliminary experiments were directed to compare IgG and IgA antibody titers to S. dysenteriae 1 LPS antigen in serum and plasma from 2 monkeys challenged intraduodenally with 109 cfu wild-type S. dysenteriae 1 strain 1617. Little or no differences were observed between plasma and serum IgG or IgA levels in these monkeys at different time points (data not shown). We then measured IgG and IgA antibody responses specific for S. dysenteriae 1 LPS antigen in plasma samples collected before and at several times after challenge with different inocula, ranging from 105 to 109 cfu intraduodenally. We also evaluated the plasma of a cynomolgus macaque (15175) that was challenged with 109 cfu wild-type S. dysenteriae 1 strain isolated from the feces of one of the previously challenged monkeys. Despite the lack of clinical shigellosis, cynomolgus macaques immunized intraduodenally with 109 cfu wild-type S. dysenteriae 1 mounted robust serologic IgG and IgA S. dysenteriae 1 LPS antibody responses (Figure 1). In contrast, monkeys immunized with low doses (105 to 108 cfu) of wild-type S. dysenteriae 1 exhibited only background antibody levels. Responses were present as early as 7 d after immunization and persisted, albeit at lower levels, for as long as 56 d after challenge.
Figure 1.
Antibody responses to S. dysenteriae 1 LPS in cynomolgus macaques challenged intraduodenally with increasing doses of S. dysenteriae 1. Blood was collected 1 d before and at several times after challenge with the indicated inocula of S. dysenteriae 1, and (A) IgG and (B) IgA antibodies to S. dysenteriae 1 LPS were measured. The challenge strain used in monkey 15175 was isolated from the feces of monkey 2618M.
Development of a S. dysenteriae 1 challenge model in cynomolgus macaques inoculated intragastrically.
Because of the lack of clinical disease in cynomolgus inoculated intraduodenally despite colonization of the animals by S. dysenteriae 1 and induction of antibody responses, we next studied whether S. dysenteriae 1 was able to induce shigellosis in cynomolgus macaques exposed through another route. To this end, 3 macaques were inoculated intragastrically with 15 mEq sodium bicarbonate followed by 1011 cfu wild-type S. dysenteriae 1 strain 1617. A similar dose of S. dysenteriae 1 was used successfully to challenge rhesus macaques.9,14,17 We also included 2 macaques inoculated intraduodenally with 109 cfu, both as a control of the inoculum and to confirm that a 109 cfu administered intraduodenally failed to induce shigellosis. Although all animals in the intraduodenal 109 cfu and intragastric 1011 cfu groups shed S. dysenteriae 1 for several days, only animals inoculated intragastrically with 1011 cfu S. dysenteriae 1 developed clinical shigellosis (Table 2). Diarrhea or dysentery were evident as early as the day after inoculation and lasted for several days (Table 2). These animals were treated successfully with antibiotics, were initiated within 24 h of evidence of clinical signs. Dehydrated animals were administered supportive fluid therapy. Symptoms resolved within 2 d of initiation of antibiotic therapy. These macaques showed no other signs of illness (fever, weight loss, and so on; data not shown). Clinical hematology analysis, liver enzymes, creatinine levels, comprehensive chemistry profiles, and WBC and differential counts of these animals did not reveal any noteworthy abnormalities.
Table 2.
Fecal shedding (+ or –) and clinical signs (score, 0 through 4) in macaques inoculated with S. dysenteriae 1
| Days after challenge |
||||||||||||||
| Inoculum (cfu) |
1 |
2 |
3 |
4 |
||||||||||
| Target | Actual | Monkey | am | pm | am | pm | am | pm | am | pm | 5 | 6 | 7 | 8 |
| 1 × 109 ID | 4.4 × 109 | 19238M | –/0 | /0 | –/0 | –/0 | –/0 | –/0 | –/0 | +/0 | +/0 | –/0 | –/0 | –/ |
| 1 × 109 ID | 4.4 × 109 | 32216F | –/0 | –/0 | +/1 | +/0 | +/0 | /0 | +/0 | –/0 | +/0 | +/0 | –/0 | +/ |
| 1 × 1011 IG | 7.8 × 1010 | 22341F | +/2 | +/2 | +/2 | +/2 | –/0 | –/0 | –/0 | –/0 | –/0 | /0 | –/0 | –/ |
| 1 × 1011 IG | 7.8 × 1010 | 11206M | +/0 | +/ | +/4 | +/4 | –/2 | –/2 | +/0 | –/3 | +/2 | –/0 | –/0 | –/ |
| 1 × 1011 IG | 7.8 × 1010 | 21382F | +/0 | –/0 | +/3 | +/4 | +/2 | +/2 | –/0 | –/0 | –/0 | /0 | –/0 | –/ |
Empty cell, data unavailable; ID, intraduodenally; IG, intragastrically; +, positive culture; –, no growth on culture; 0, no diarrhea; 1, soft formed stool; 2, diarrhea without blood (sometimes with mucus); 3, diarrhea with blood and mucus; 4, watery diarrhea with blood and mucus
Kinetics of induction of antiLPS IgG and IgA antibodies in cynomolgus macaques challenged intragastrically with wild-type S. dysenteriae 1.
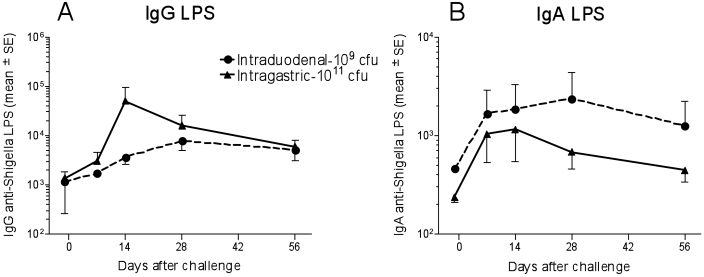
Blood samples were collected from each animal before and at several times after challenge to study immune responses elicited by exposure to S. dysenteriae 1. These macaques mounted robust antibody responses to S. dysenteriae 1 LPS (Figure 2). Increases in IgG and IgA titers against S. dysenteriae 1 LPS in monkeys challenged intraduodenally with 109 cfu or intragastrically with 1011 cfu wild-type S. dysenteriae 1 occurred as early as 7 d after challenge, peaking at day 14 and then steadily declining, reaching a plateau after day 28 (Figure 2). No significant differences in antibody levels were noted between these 2 groups.
Figure 2.
Antibody responses to S. dysenteriae 1 LPS in cynomolgus macaques challenged intraduodenally or intragastrically with S. dysenteriae 1. Blood was collected 1 d before and at several times after challenge with the indicated inocula of S. dysenteriae 1, and (A) IgG and (B) IgA antibodies to S. dysenteriae 1 LPS were measured. Results are presented as the mean titer (± SE) of 2 or 3 individual animals at each time point for cynomolgus challenged intraduodenally or intragastrically, respectively.
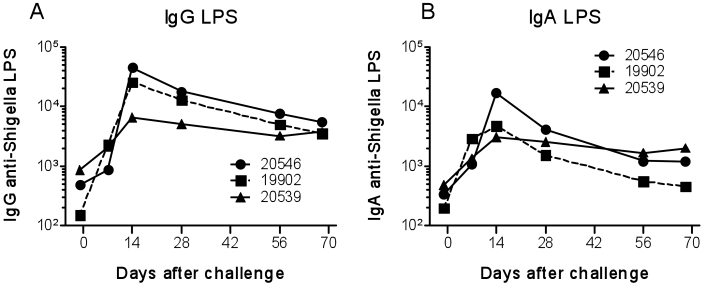
To confirm these findings, a second group of 3 monkeys was challenged intragastrically with 1011 cfu wild-type S. dysenteriae 1 strain 1617. The results were similar to those from the previous experiment and showed little variability among animals. Shedding started as early as the day after challenge and persisted for as long as a week (Table 3). In addition, diarrhea occurred from 1 to 3 d after challenge (Table 3). As in the previous experiment, these macaques were treated successfully with antibiotic therapy, which was initiated within 24 h of evidence of clinical signs. Dehydrated animals received supportive fluid therapy. Symptoms resolved within 2 d of initiation of antibiotic therapy. These macaques showed no other signs of illness (fever, weight loss, and so on; data not shown). IgG and IgA responses against S. dysenteriae 1 LPS were first present 7 d after challenge and persisted for as long as 70 d (Figure 3). Of note, these antibody responses were similar, both in magnitude and persistence, among these animals (Figure 3). No other signs of illness (fever, weight loss, and so on) or changes in blood cell counts and differentials or in blood chemistry occurred in these animals (data not shown).
Table 3.
Fecal shedding (+ or –) and clinical signs (score, 0 through 4) in macaques inoculated intragastrically with S. dysenteriae 1 (target inoculum, 1 × 1011cfu; actual inoculum, 1.05 × 1011cfu)
| Days after challenge |
|||||||||||||||||||
| 1 |
2 |
3 |
4 |
5 |
6 |
7 |
12 |
||||||||||||
| Monkey | am | pm | am | pm | am | pm | am | pm | |||||||||||
| 20546 | + | + | 2 | + | – | 0 | + | – | 2 | – | – | 2 | – | 0 | + | 0 | + | 0 | – |
| 19902 | – | – | 1 | + | – | 0 | + | + | 2 | + | – | 0 | + | 0 | + | 0 | – | 0 | – |
| 20539 | – | – | 1 | + | + | 0 | + | – | 2 | + | + | 0 | + | 0 | + | 0 | – | 0 | – |
Empty cell, data unavailable; ID, intraduodenally; IG, intragastrically; +, positive culture; –, no growth on culture; 0, no diarrhea; 1, soft formed stool; 2, diarrhea without blood (sometimes with mucus); 3, diarrhea with blood and mucus; 4, watery diarrhea with blood and mucus
Figure 3.
Antibody responses to S. dysenteriae 1 LPS in cynomolgus macaques challenged intragastrically with S. dysenteriae 1. Blood was collected 1 d before and at several times after challenge with the indicated inocula of S. dysenteriae 1, and (A) IgG and (B) IgA antibodies to S. dysenteriae 1 LPS were measured. Results represent kinetic data from individual animals.
Discussion
Shigella is a global infection that is notorious for disseminating rapidly in settings where there is overcrowding, inadequate sanitation, and insufficient clean water.15 The spectrum of symptoms ranges from mild watery diarrhea to fulminant bacillary dysentery, characterized by bloody stools, high fever, prostration, cramps, tenesmus, and an array of severe intestinal and extraintestinal complications.13,15 One serotype, S. dysenteriae 1, can cause devastating pandemics with high fatality rates in all age groups.15 S. dysenteriae 1 is unique among the Shigella spp. in its ability to produce Shiga toxin and consequently causes the most serious forms of disease. Widespread use of a safe and effective Shigella vaccine has long been considered a desirable strategy for combating this naturally occurring infection, and developing such a vaccine is a high public health priority.13,15
The ability of Shigella to cause diarrheal illness is restricted to human and nonhuman primate hosts. The lack of a small animal model that fully replicates human bacillary dysentery is a major hurdle to the development of successful Shigella vaccines.13,15 Small animal models including mice, guinea pigs, and rabbits have been evaluated. In mice, the pulmonary pneumonia model, which involves intranasal inoculation with Shigella, has been used to study degree of attenuation, immunogenicity, and even protection from challenge.16 However, the lack of clinical relevance of the target organ is a drawback to this model. Although a model of intragastric infection in newborn mice using a high dose of Shigella (109 cfu)4 led to inflammatory destruction of the mucosa and significant infiltration of neutrophils into the intestines, this model cannot be used to evaluate protective immunity after immunization because it has to be initiated within a narrow time window (4 to 5 d after birth). In a human intestine xenograft model using severe combined immunodeficient mice,10 direct injection of bacteria into the lumen of the intestinal xenograft resulted in high levels of IL1β and IL8 production as well as increased infiltration of neutrophils.30 Although valuable for studying Shigella pathogenesis in the human intestine, this model is too artificial for assessing protective efficacy.30
In guinea pigs, the keratoconjunctivitis assay (known as the Sereny test) is considered the ‘gold standard’ to assess the degree of attenuation of novel attenuated Shigella vaccine strains because the assay measures mucosal invasiveness and reactogenicity.24 Although not an intestinal model, the guinea pig model also is useful for testing the efficacy of vaccine candidates and correlating efficacy with antibody responses. In a recent guinea pig model, typical bacillary dysentery occurred in animals challenged intrarectally, rather than by the natural oral route, with high doses (109 cfu) of virulent S. flexneri 2a or 5a strains,25 S. dysenteriae 1 was not evaluated in those studies. Studies also have been performed in rabbit ileal-ligated loops and in rabbits which developed shigellosis after challenge with 108 cfu S. flexneri 2a by colonic intubation after ligation of the distal cecum.21 Although these models are useful to study pathogenesis and inflammatory responses, the artificial nature of these systems might hamper their ability to advance vaccine development in humans. To date, macaques remain the only animals in which typical bacillary dysentery can be induced by oral administration of S. flexneri without extreme fasting or pretreatment with antibiotics.
Shigella is a commonly isolated pathogen from laboratory-housed nonhuman primates, a fact that makes these animals a particularly well-suited model for Shigella infection in humans.23 Shigellosis occurs in a variety of species of captive Old World primates including macaques, chimpanzees, and baboons. It has also been reported to occur in New World primates including marmosets, tamarins, and black spider monkeys. The Shigella species isolated most commonly from captive monkeys are S. flexneri and S. sonnei, although S. dysenteriae 2 and S. bodydii have also been isolated, and mixed infections have been reported.26 In contrast, natural infections of the human pathogen S. dysenteriae 1 have not been reported in nonhuman primates. Of importance, inoculation of S. flexneri directly into the cecum causes dysentery but not diarrhea. In contrast, administration of the bacteria by the oral route results in net fluid secretion in the small intestine in animals with diarrhea alone or in combination with dysentery.15 As in humans, infection is spread by the oral–fecal route among animals, and the duration of infection in monkeys has been reported to be 1 to 5 wk.29 In sum, naturally occurring shigellosis in monkeys resembles the disease in humans clinically and pathologically, whereas small animal models are largely resistant to shigellosis.
In contrast to the 10 to 100 organisms that can cause disease in humans, a large inoculum of S. dysenteriae 1 (1010 to 1011 cfu) administered orogastrically with sodium bicarbonate is required to induce diarrhea or dysentery in 40% to 60% of rhesus macaques.9,14,17 However, the similarities in symptomatology, shedding of the organism and histopathologic findings between nonhuman primates and humans infected with Shigella spp.3,5–9,11,12,14,17,19,22,26,27 makes the nonhuman primate model the best currently available for studying pathogenesis, infection-derived immunity and, likely, vaccine efficacy.
A multitude of factors may be responsible for the increased susceptibility of humans to S. dysenteriae 1 and other Shigella spp. when compared with that of monkeys. These factors might include differences in the ability of Shigella to attach to specific colonic epithelial cells (host–receptor specificity) and other host–pathogen interactions, as well as differences in immune responses elicited in humans and monkeys. These differences might affect one or more steps in the complex multistage process involved in pathogenesis and immunity elicited by Shigella. After entering the cell, Shigella organisms replicate in phagosomes, lyse phagocytic vacuoles, multiply freely in the cytoplasm, and spread.20 Intra- and intercellular spread requires many host cell and bacterial interactions. Shigella spp. also produce toxins that further contribute to disease pathogenesis.18 Although extensive literature has been published about the molecular pathogenesis and immunology of Shigella infections, the precise differences that could explain the marked differences observed between the inocula that cause shigellosis in humans and nonhuman primates remain largely unknown.
In the current study, we have presented evidence that cynomolgus macaques are a suitable species to be used as a challenge model with wild-type S. dysenteriae 1, exhibiting to date an attack rate of 100% (6 of 6 cynomolgus macaques challenged with 1011 cfu intragastrically developed diarrhea or dysentery). These results compare favorably with those of previous studies using S. dysenteriae 1 challenges in rhesus macaques. For example, in 2 separate studies, oral challenge with approximately 1010 S. dysenteriae 1 resulted in diarrhea or dysentery in 2 of 5 (40%) and 3 of 6 (50%) of the animals.14,17 In another study in rhesus macaques, 9 of the 15 monkeys each fed 5 × 1010 S. dysenteriae 1 strain 3818 exhibited evidence of disease ranging from diarrhea to dysentery.9 Of note, the use of other Shigella spp. in rhesus macaques was unable to achieve a 100% attack rate. For example, 13 of 24 (54%) rhesus macaques challenged orally with 2 × 1010 Shigella flexneri 2a showed clinical shigellosis (that is, diarrhea with or without mucus and blood).8 In the same study,8 8 of 12 (66%) rhesus monkeys challenged with 2 × 1010 Shigella sonnei became ill. In another study, 24 of 40 (60%) rhesus macaques were challenged with 1 to 2 × 1011 virulent wild-type S. flexneri 2a developed clinical signs of illness.5 These results are similar to those reported from other studies, in which 31 of 76 (40.8%)22 and 7 of 22 (32%)12 of rhesus macaques challenged with Shigella spp. developed signs of disease. In the context of these observations, our cynomolgus model compares very favorably with the rhesus macaque model, which consistently resulted in approximately 30% to 70% of the monkeys developing diarrhea or dysentery after intragastric challenge with Shigella spp.
To begin the identification of the immunologic mechanisms of protection in the cynomolgus model, we developed assays to evaluate the induction and persistence of IgG and IgA antibody responses Shigella LPS by using serum and plasma specimens obtained from these monkeys before and after challenge. The measurement of these responses was selected because antibodies to Shigella LPS O-polysaccharide are among the effector immune responses believed to play a key role in protection.15 We observed that exposure of cynomolgus macaques to S. dysenteriae 1 elicited the appearance of antibodies to S. dysenteriae 1 LPS as early as 7 d after immunization and persisted, albeit at lower levels, for as long as 70 d after exposure. Peak responses in monkeys challenged intragastrically consistently occurred 14 to 28 d after immunization, reaching antibody titers of approximately 6,500 to 45,000 and approximately 1,000 to 17,000 for IgG and IgA, respectively. Although IgA responses were similar in magnitude whether monkeys were challenged intraduodenally (no clinical disease) or intragastrically (resulted in shigellosis), antiLPS IgG responses appeared to be lower in magnitude in cynomolgus challenged intraduodenally than in those challenged intragastrically. The significance of these immunologic findings will become apparent in upcoming studies directed to correlate in-depth measurements of the immune responses in cynomolgus macaques challenged with S. dysenteriae 1 with protection from rechallenge.
To our knowledge, the current study is the first demonstration of clinical shigellosis resulting from S. dysenteriae 1 infection in cynomolgus macaques. We have developed a robust challenge model with 100% attack rate (all 6 monkeys tested developed diarrhea or dysentery). Given the close genetic proximity of cynomolgus macaques and humans and the high attack rate, we believe that our cynomolgus model will be useful to study protection from reinfection and disease pathogenesis and to identify correlates of protective immunity to S. dysenteriae 1 infection. In addition, cynomolgus macaques currently are more readily available in the United States than are rhesus macaques, thus contributing to the attractiveness of the cynomolgus macaque model for use in vaccine development and evaluation.
Acknowledgments
Authors AP, AQK, and STS contributed equally toward the completion of this study, and authors MBS and LJD contributed equally to the direction of this work.
We thank Rebecca Yerkey, Theresa Alexander, Dawn McKenna, Elisa Luna, and Leisha Alexander for excellent veterinary technical support. These studies were supported in part by grants U54-AI057168 (to MML), R01-AI057927 (to MBS), and U19 AI082655 (to MBS).
References
- 1.Animal Welfare Act as Amended 20077 USC §2131–2156 [Google Scholar]
- 2.Centers for Disease Control and Prevention and National Institutes of Health [Internet] 2007. Biosafety in microbiological and biomedical laboratories (BMBL), 5th ed [Cited Jan 2007]. Available at http://www.cdc.gov/OD/ohs/biosfty/bmbl5/bmbl5toc.htm. [Google Scholar]
- 3.Dinari G, Hale TL, Austin SW, Formal SB. 1987. Local and systemic antibody responses to Shigella infection in rhesus monkeys. J Infect Dis 155:1065–1069 [DOI] [PubMed] [Google Scholar]
- 4.Fernandez MI, Thuizat A, Pedron T, Neutra M, Phalipon A, Sansonetti PJ. 2003. A newborn mouse model for the study of intestinal pathogenesis of shigellosis. Cell Microbiol 5:481–491 [DOI] [PubMed] [Google Scholar]
- 5.Formal SB, Hale TL, Kapfer C, Cogan JP, Snoy PJ, Chung R, Wingfield ME, Elisberg BL, Baron LS. 1984. Oral vaccination of monkeys with an invasive Escherichia coli K12 hybrid expressing Shigella flexneri 2a somatic antigen. Infect Immun 46:465–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Formal SB, Kent TH, May HC, Palmer A, Falkow S, LaBrec EH. 1966. Protection of monkeys against experimental shigellosis with a living attenuated oral polyvalent dysentery vaccine. J Bacteriol 92:17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Formal SB, LaBrec EH, Palmer A, Falkow S. 1965. Protection of monkeys against experimental shigellosis with attenuated vaccines. J Bacteriol 90:63–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Formal SB, Oaks EV, Olsen RE, Wingfield-Eggleston M, Snoy PJ, Cogan JP. 1991. Effect of prior infection with virulent Shigella flexneri 2a on the resistance of monkeys to subsequent infection with Shigella sonnei. J Infect Dis 164:533–537 [DOI] [PubMed] [Google Scholar]
- 9.Gemski P, Jr, Takeuchi A, Washington O, Formal SB. 1972. Shigellosis due to Shigella dysenteriae. 1. Relative importance of mucosal invasion versus toxin production in pathogenesis. J Infect Dis 126:523–530 [DOI] [PubMed] [Google Scholar]
- 10.Institute of Laboratory Animal Resources 1996. Guide for the care and use of laboratory animals. Washington (DC): National Academies Press; [DOI] [PubMed] [Google Scholar]
- 11.Karnell A, Reinholt FP, Katakura S, Lindberg AA. 1991. Shigella flexneri infection: a histopathologic study of colonic biopsies in monkeys infected with virulent and attenuated bacterial strains. APMIS 99:787–796 [DOI] [PubMed] [Google Scholar]
- 12.Katakura S, Reinholt FP, Karnell A, Huan PT, Trach DD, Lindberg AA. 1990. The pathology of Shigella flexneri infection in rhesus monkeys: an endoscopic and histopathological study of colonic lesions. APMIS 98:313–319 [DOI] [PubMed] [Google Scholar]
- 13.Kweon MN. 2008. Shigellosis: the current status of vaccine development. Curr Opin Infect Dis 21:313–318 [DOI] [PubMed] [Google Scholar]
- 14.Levine MM, DuPont HL, Formal SB, Hornick RB, Takeuchi A, Gangarosa EJ, Snyder MJ, Libonati JP. 1973. Pathogenesis of Shigella dysenteriae 1 (Shiga) dysentery. J Infect Dis 127:261–270 [DOI] [PubMed] [Google Scholar]
- 15.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. 2007. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol 5:540–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallett CP, Hale TL, Kaminski RW, Larsen T, Orr N, Cohen D, Lowell GH. 1995. Intransal or intragastric immunization with proteosome–Shigella lipopolysaccharide vaccines protects against lethal pneumonia in a murine model of Shigella infection. Infect Immun 63:2382–2386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McIver J, Grady GF, Formal SB. 1977. Immunization with Shigella dysenteriae type 1: evaluation of antitoxic immunity in prevention of experimental disease in rhesus monkeys (Macaca mulatta). J Infect Dis 136:416–421 [DOI] [PubMed] [Google Scholar]
- 18.O'Brien AD, Holmes RK. 1987. Shiga and Shiga-like toxins. Microbiol Rev 51:206–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oaks EV, Hale TL, Formal SB. 1986. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun 53:57–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ogawa M, Sasakawa C. 2006. Intracellular survival of Shigella. Cell Microbiol 8:177–184 [DOI] [PubMed] [Google Scholar]
- 21.Rabbani GH, Albert MJ, Rahman H, Islam M, Mahalanabis D, Kabir I, Alam K, Ansaruzzaman M. 1995. Development of an improved animal model of shigellosis in the adult rabbit by colonic infection with Shigella flexneri 2a. Infect Immun 63:4350–4357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rout WR, Formal SB, Giannella RA, Dammin GJ. 1975. Pathophysiology of Shigella diarrhea in the rhesus monkey: intestinal transport, morphological, and bacteriological studies. Gastroenterology 68:270–278 [PubMed] [Google Scholar]
- 23.Russell RG, DeTolla LJ. 1993. Shigellosis, p 46–53 : Jones TH, Mohr U, Hunt RD. Nonhuman primates II. Berlin (Germany): Springer–Verlag [Google Scholar]
- 24.Sereny B. 1955. Experimental Shigella keratoconjunctivitis; a preliminary report. Acta Microbiol Acad Sci Hung 2:293–296 [PubMed] [Google Scholar]
- 25.Shim DH, Suzuki T, Chang SY, Park SM, Sansonetti PJ, Sasakawa C, Kweon MN. 2007. New animal model of shigellosis in the guinea pig: its usefulness for protective efficacy studies. J Immunol 178:2476–2482 [DOI] [PubMed] [Google Scholar]
- 26.Takeuchi A. 1982. Early colonic lesions in experimental Shigella infection in rhesus monkeys: revisited. Vet Pathol Suppl 7: 1–8 [PubMed] [Google Scholar]
- 27.Takeuchi A, Jervis HR, Formal SB. 1975. Animal model of human disease. Bacillary dysentery, shigellosis, Shigella dysentery. Animal model: monkey shigellosis or dysentery. Am J Pathol 81:251–254 [PMC free article] [PubMed] [Google Scholar]
- 28.Venkatesan MM, Hartman AB, Newland JW, Ivanova VS, Hale TL, McDonough M, Butterton J. 2002. Construction, characterization, and animal testing of WRSd1, a Shigella dysenteriae 1 vaccine. Infect Immun 70:2950–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynne ES, Henrikson DM, Daye GT., Jr 1970. Persistence of an Escherichia coli-Shigella flexneri hybrid in the intestinal tract of Macaca mulatta. Appl Microbiol 19:731–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Z, Jin L, Champion G, Seydel KB, Stanley SL., Jr 2001. Shigella infection in a SCID mouse–human intestinal xenograft model: role for neutrophils in containing bacterial dissemination in human intestine. Infect Immun 69:3240–3247 [DOI] [PMC free article] [PubMed] [Google Scholar]