Abstract
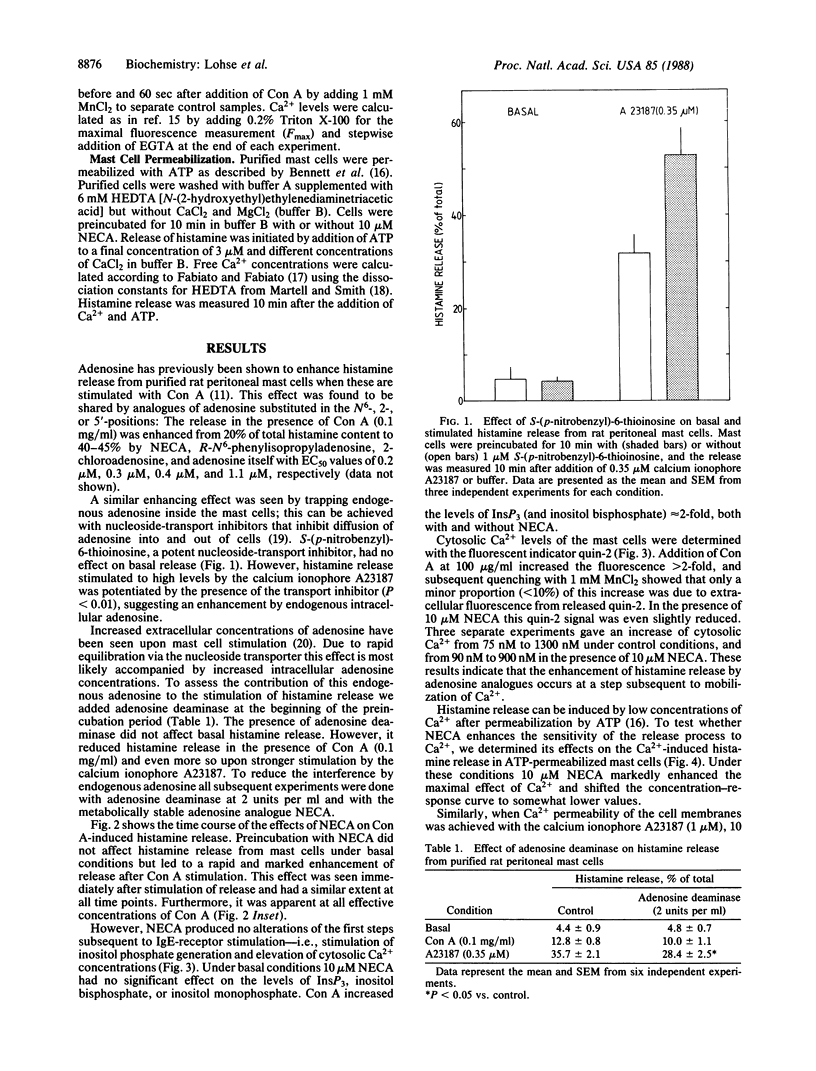
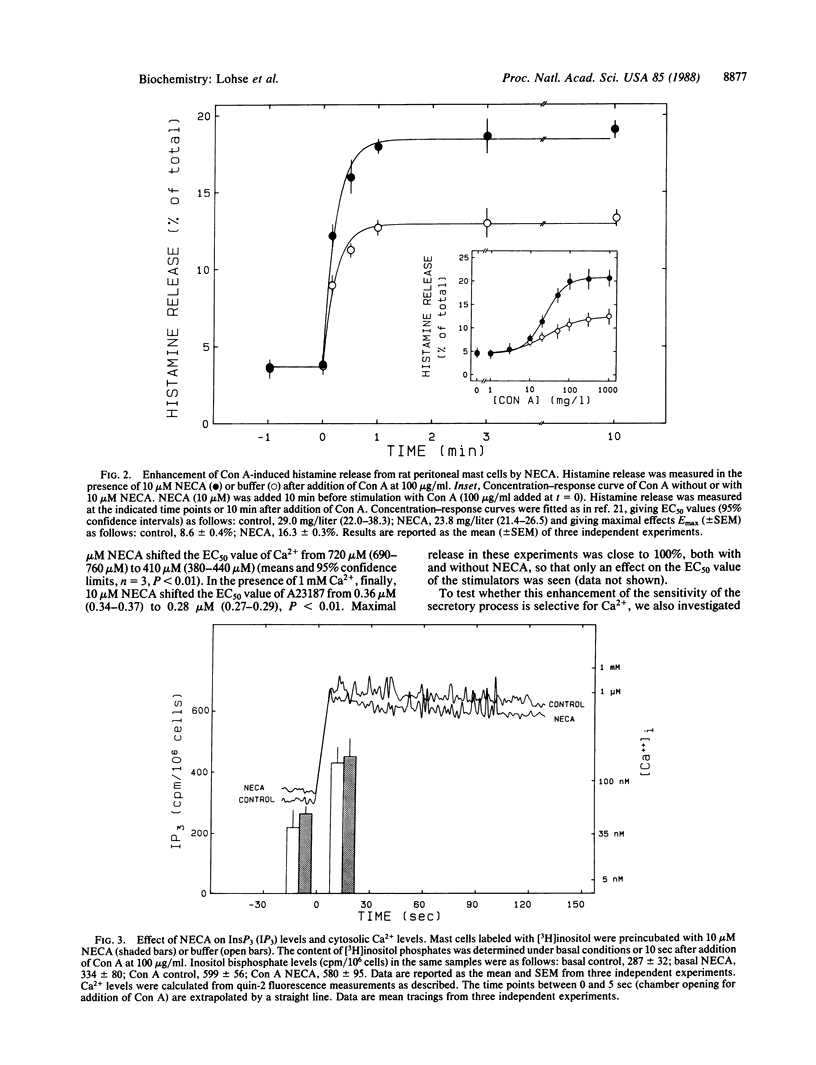
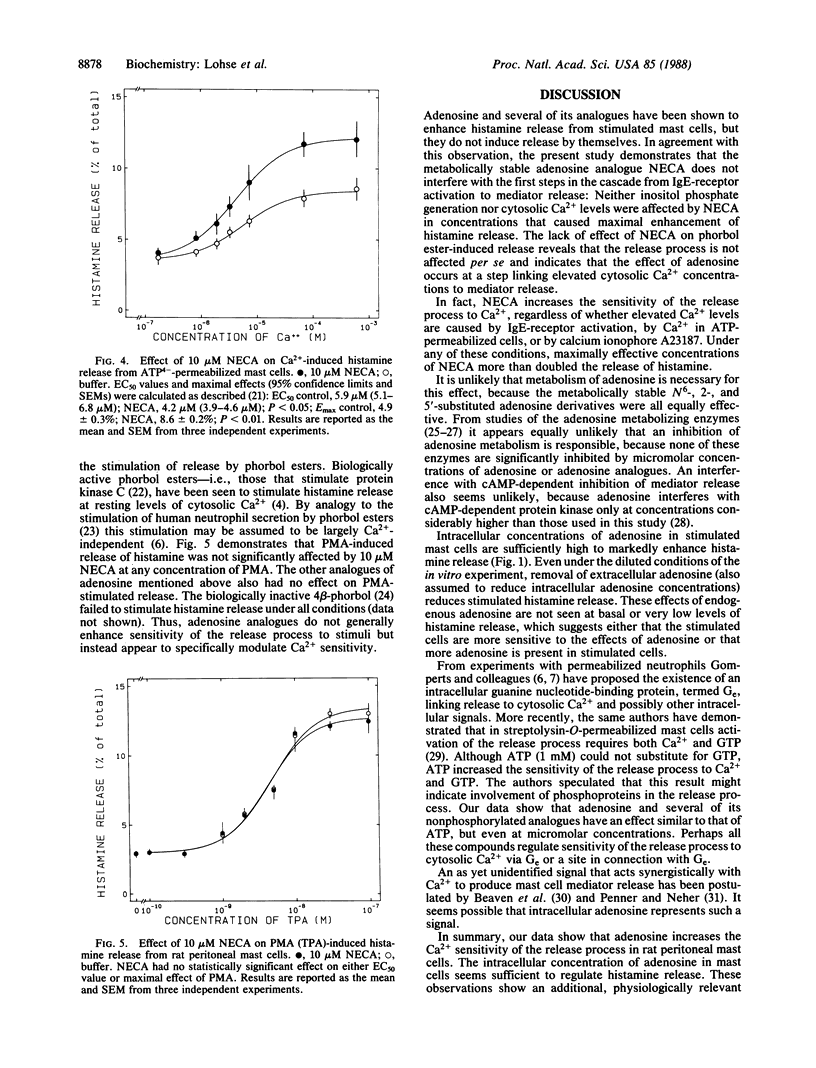
Mast cells release histamine and other mediators of allergy in response to stimulation of their IgE receptors. This release is generally thought to be mediated by an elevation of cytosolic Ca2+. Recent evidence suggests that there might be factors that modulate the coupling between Ca2+ levels and mediator release. The present report identifies adenosine as one such modulator. Adenosine and several of its metabolically stable analogues were shown to enhance histamine release from rat peritoneal mast cells in response to stimuli such as concanavalin A. Metabolizing endogenous adenosine with adenosine deaminase dampened the response to stimuli, whereas trapping endogenous adenosine inside mast cells with nucleoside-transport inhibitors markedly enhanced stimulated histamine release. The metabolically stable adenosine analogue 5'-(N-ethylcarboxamido)adenosine (NECA) did not affect the initial steps in the sequence from IgE-receptor activation to mediator release, which are generation of inositol trisphosphate and increase of cytosolic Ca2+. However, NECA did enhance the release induced in ATP-permeabilized cells by exogenous Ca2+, but it had no effect on the release induced by phorbol esters. These data suggest that adenosine sensitizes mediator release by a mechanism regulating stimulus-secretion coupling at a step distal to receptor activation and second-messenger generation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almers W., Neher E. The Ca signal from fura-2 loaded mast cells depends strongly on the method of dye-loading. FEBS Lett. 1985 Nov 11;192(1):13–18. doi: 10.1016/0014-5793(85)80033-8. [DOI] [PubMed] [Google Scholar]
- Barrowman M. M., Cockcroft S., Gomperts B. D. Two roles for guanine nucleotides in the stimulus-secretion sequence of neutrophils. Nature. 1986 Feb 6;319(6053):504–507. doi: 10.1038/319504a0. [DOI] [PubMed] [Google Scholar]
- Beaven M. A., Guthrie D. F., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. Synergistic signals in the mechanism of antigen-induced exocytosis in 2H3 cells: evidence for an unidentified signal required for histamine release. J Cell Biol. 1987 Sep;105(3):1129–1136. doi: 10.1083/jcb.105.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaven M. A., Moore J. P., Smith G. A., Hesketh T. R., Metcalfe J. C. The calcium signal and phosphatidylinositol breakdown in 2H3 cells. J Biol Chem. 1984 Jun 10;259(11):7137–7142. [PubMed] [Google Scholar]
- Bennett J. P., Cockcroft S., Gomperts B. D. Rat mast cells permeabilized with ATP secrete histamine in response to calcium ions buffered in the micromolar range. J Physiol. 1981 Aug;317:335–345. doi: 10.1113/jphysiol.1981.sp013828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang D. M. Carbachol-induced accumulation of inositol-1-phosphate in neurohybridoma NCB-20 cells: effects of lithium and phorbol esters. Biochem Biophys Res Commun. 1986 Apr 29;136(2):622–629. doi: 10.1016/0006-291x(86)90486-9. [DOI] [PubMed] [Google Scholar]
- Di Virgilio F., Lew D. P., Pozzan T. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984 Aug 23;310(5979):691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Fisher M. N., Newsholme E. A. Properties of rat heart adenosine kinase. Biochem J. 1984 Jul 15;221(2):521–528. doi: 10.1042/bj2210521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesketh T. R., Smith G. A., Moore J. P., Taylor M. V., Metcalfe J. C. Free cytoplasmic calcium concentration and the mitogenic stimulation of lymphocytes. J Biol Chem. 1983 Apr 25;258(8):4876–4882. [PubMed] [Google Scholar]
- Howell T. W., Cockcroft S., Gomperts B. D. Essential synergy between Ca2+ and guanine nucleotides in exocytotic secretion from permeabilized rat mast cells. J Cell Biol. 1987 Jul;105(1):191–197. doi: 10.1083/jcb.105.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes P. J., Holgate S. T., Church M. K. Adenosine inhibits and potentiates IgE-dependent histamine release from human lung mast cells by an A2-purinoceptor mediated mechanism. Biochem Pharmacol. 1984 Dec 1;33(23):3847–3852. doi: 10.1016/0006-2952(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Iwai H., Inamasu M., Takeyama S. Inhibition of the protein kinase by adenine compounds: competitive inhibition with respect to ATP. Biochem Biophys Res Commun. 1972 Jan 31;46(2):824–830. doi: 10.1016/s0006-291x(72)80215-8. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Klotz K. N., Schwabe U. Agonist photoaffinity labeling of A1 adenosine receptors: persistent activation reveals spare receptors. Mol Pharmacol. 1986 Oct;30(4):403–409. [PubMed] [Google Scholar]
- Lohse M. J., Lenschow V., Schwabe U. Two affinity states of Ri adenosine receptors in brain membranes. Analysis of guanine nucleotide and temperature effects on radioligand binding. Mol Pharmacol. 1984 Jul;26(1):1–9. [PubMed] [Google Scholar]
- Lohse M. J., Maurer K., Gensheimer H. P., Schwabe U. Dual actions of adenosine on rat peritoneal mast cells. Naunyn Schmiedebergs Arch Pharmacol. 1987 May;335(5):555–560. doi: 10.1007/BF00169124. [DOI] [PubMed] [Google Scholar]
- Marquardt D. L., Gruber H. E., Wasserman S. I. Adenosine release from stimulated mast cells. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6192–6196. doi: 10.1073/pnas.81.19.6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquardt D. L., Parker C. W., Sullivan T. J. Potentiation of mast cell mediator release by adenosine. J Immunol. 1978 Mar;120(3):871–878. [PubMed] [Google Scholar]
- Nakamura T., Ui M. Simultaneous inhibitions of inositol phospholipid breakdown, arachidonic acid release, and histamine secretion in mast cells by islet-activating protein, pertussis toxin. A possible involvement of the toxin-specific substrate in the Ca2+-mobilizing receptor-mediated biosignaling system. J Biol Chem. 1985 Mar 25;260(6):3584–3593. [PubMed] [Google Scholar]
- Neher E., Almers W. Fast calcium transients in rat peritoneal mast cells are not sufficient to trigger exocytosis. EMBO J. 1986 Jan;5(1):51–53. doi: 10.1002/j.1460-2075.1986.tb04176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Penner R., Neher E. Secretory responses of rat peritoneal mast cells to high intracellular calcium. FEBS Lett. 1988 Jan 4;226(2):307–313. doi: 10.1016/0014-5793(88)81445-5. [DOI] [PubMed] [Google Scholar]
- Schrader J., Schütz W., Bardenheuer H. Role of S-adenosylhomocysteine hydrolase in adenosine metabolism in mammalian heart. Biochem J. 1981 Apr 15;196(1):65–70. doi: 10.1042/bj1960065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Pozzan T., Rink T. J. Calcium homeostasis in intact lymphocytes: cytoplasmic free calcium monitored with a new, intracellularly trapped fluorescent indicator. J Cell Biol. 1982 Aug;94(2):325–334. doi: 10.1083/jcb.94.2.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J. R., Ishizaka T., Ishizaka K., Sha'afi R. Direct demonstration of increased intracellular concentration of free calcium as measured by quin-2 in stimulated rat peritoneal mast cell. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3978–3982. doi: 10.1073/pnas.81.13.3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiginton D. A., Coleman M. S., Hutton J. J. Purification, characterization and radioimmunoassay of adenosine deaminase from human leukaemic granulocytes. Biochem J. 1981 May 1;195(2):389–397. doi: 10.1042/bj1950389. [DOI] [PMC free article] [PubMed] [Google Scholar]


