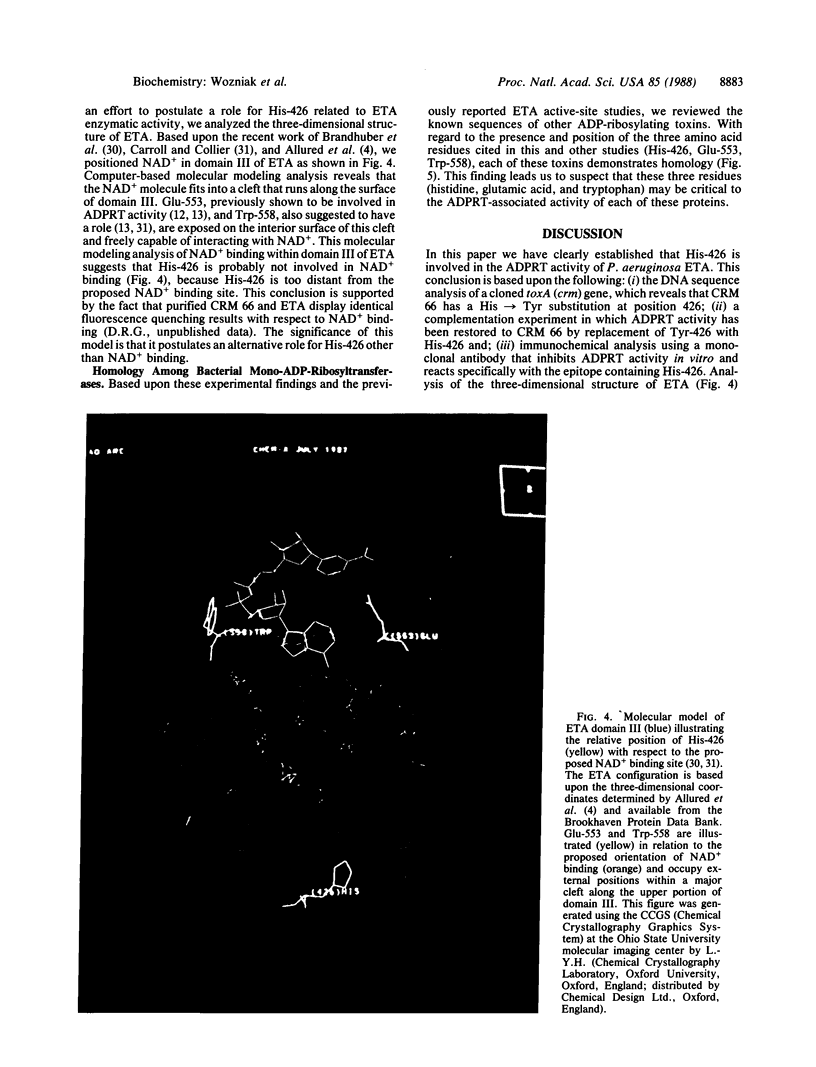
Abstract
Exotoxin A (ETA) is recognized as the most toxic product associated with the opportunistic pathogen Pseudomonas aeruginosa. Identification of the amino acids in the polypeptide sequence that are required for toxin activity is critical for vaccine development. By defining the nucleotide sequence of the structural gene of a mutant that encodes an enzymatically inactive ETA (CRM 66), we identified an essential amino acid (His-426), which is involved in the ADP-ribosyltransferase activity associated with functional ETA. A monoclonal antibody that inhibits ETA enzymatic activity in vitro fails to react with ETA variants that have a His 426----Tyr substitution. Several mono-ADP-ribosylating toxins, including diphtheria and pertussis toxins, within the primary amino acid sequences carry a histidine residue that is conserved in spacing and in location with respect to other critical residues. Analysis of the three-dimensional structure of ETA revealed that His-426 is not associated with the proposed NAD+ binding site. These findings should be useful for the design and construction of toxin vaccines.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allured V. S., Collier R. J., Carroll S. F., McKay D. B. Structure of exotoxin A of Pseudomonas aeruginosa at 3.0-Angstrom resolution. Proc Natl Acad Sci U S A. 1986 Mar;83(5):1320–1324. doi: 10.1073/pnas.83.5.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandhuber B. J., Allured V. S., Falbel T. G., McKay D. B. Mapping the enzymatic active site of Pseudomonas aeruginosa exotoxin A. Proteins. 1988;3(3):146–154. doi: 10.1002/prot.340030303. [DOI] [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. Active site of Pseudomonas aeruginosa exotoxin A. Glutamic acid 553 is photolabeled by NAD and shows functional homology with glutamic acid 148 of diphtheria toxin. J Biol Chem. 1987 Jun 25;262(18):8707–8711. [PubMed] [Google Scholar]
- Carroll S. F., Collier R. J. Amino acid sequence homology between the enzymic domains of diphtheria toxin and Pseudomonas aeruginosa exotoxin A. Mol Microbiol. 1988 Mar;2(2):293–296. doi: 10.1111/j.1365-2958.1988.tb00031.x. [DOI] [PubMed] [Google Scholar]
- Chaudhary V. K., FitzGerald D. J., Adhya S., Pastan I. Activity of a recombinant fusion protein between transforming growth factor type alpha and Pseudomonas toxin. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4538–4542. doi: 10.1073/pnas.84.13.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S. T., Jordan E. M., Wilson R. B., Draper R. K., Clowes R. C. Transcription and expression of the exotoxin A gene of Pseudomonas aeruginosa. J Gen Microbiol. 1987 Nov;133(11):3081–3091. doi: 10.1099/00221287-133-11-3081. [DOI] [PubMed] [Google Scholar]
- Cross A. S., Sadoff J. C., Iglewski B. H., Sokol P. A. Evidence for the role of toxin A in the pathogenesis of infection with Pseudomonas aeruginosa in humans. J Infect Dis. 1980 Oct;142(4):538–546. doi: 10.1093/infdis/142.4.538. [DOI] [PubMed] [Google Scholar]
- Cryz S. J., Jr, Friedman R. L., Iglewski B. H. Isolation and characterization of a Pseudomonas aeruginosa mutant producing a nontoxic, immunologically crossreactive toxin A protein. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7199–7203. doi: 10.1073/pnas.77.12.7199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. M., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: substitution of glutamic acid 553 with aspartic acid drastically reduces toxicity and enzymatic activity. J Bacteriol. 1987 Nov;169(11):4967–4971. doi: 10.1128/jb.169.11.4967-4971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas C. M., Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: active, cloned toxin is secreted into the periplasmic space of Escherichia coli. J Bacteriol. 1987 Nov;169(11):4962–4966. doi: 10.1128/jb.169.11.4962-4966.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. R., Hedstrom R. C., Pavlovskis O. R. Production and characterization of monoclonal antibodies to exotoxin A from Pseudomonas aeruginosa. Infect Immun. 1984 May;44(2):262–267. doi: 10.1128/iai.44.2.262-267.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg J. B., Ohman D. E. Cloning and expression in Pseudomonas aeruginosa of a gene involved in the production of alginate. J Bacteriol. 1984 Jun;158(3):1115–1121. doi: 10.1128/jb.158.3.1115-1121.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray G. L., Smith D. H., Baldridge J. S., Harkins R. N., Vasil M. L., Chen E. Y., Heyneker H. L. Cloning, nucleotide sequence, and expression in Escherichia coli of the exotoxin A structural gene of Pseudomonas aeruginosa. Proc Natl Acad Sci U S A. 1984 May;81(9):2645–2649. doi: 10.1073/pnas.81.9.2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield L., Bjorn M. J., Horn G., Fong D., Buck G. A., Collier R. J., Kaplan D. A. Nucleotide sequence of the structural gene for diphtheria toxin carried by corynebacteriophage beta. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6853–6857. doi: 10.1073/pnas.80.22.6853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guidi-Rontani C., Collier R. J. Exotoxin A of Pseudomonas aeruginosa: evidence that domain I functions in receptor binding. Mol Microbiol. 1987 Jul;1(1):67–72. doi: 10.1111/j.1365-2958.1987.tb00528.x. [DOI] [PubMed] [Google Scholar]
- Hedstrom R. C., Funk C. R., Kaper J. B., Pavlovskis O. R., Galloway D. R. Cloning of a gene involved in regulation of exotoxin A expression in Pseudomonas aeruginosa. Infect Immun. 1986 Jan;51(1):37–42. doi: 10.1128/iai.51.1.37-42.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Hwang J., Fitzgerald D. J., Adhya S., Pastan I. Functional domains of Pseudomonas exotoxin identified by deletion analysis of the gene expressed in E. coli. Cell. 1987 Jan 16;48(1):129–136. doi: 10.1016/0092-8674(87)90363-1. [DOI] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Sadoff J. C. Toxin inhibitors of protein synthesis: production, purification, and assay of Pseudomonas aeruginosa toxin A. Methods Enzymol. 1979;60:780–793. doi: 10.1016/s0076-6879(79)60071-x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorberboum-Galski H., FitzGerald D., Chaudhary V., Adhya S., Pastan I. Cytotoxic activity of an interleukin 2-Pseudomonas exotoxin chimeric protein produced in Escherichia coli. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1922–1926. doi: 10.1073/pnas.85.6.1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicosia A., Perugini M., Franzini C., Casagli M. C., Borri M. G., Antoni G., Almoni M., Neri P., Ratti G., Rappuoli R. Cloning and sequencing of the pertussis toxin genes: operon structure and gene duplication. Proc Natl Acad Sci U S A. 1986 Jul;83(13):4631–4635. doi: 10.1073/pnas.83.13.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W., Moffatt B. A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986 May 5;189(1):113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Nakazawa T., Miyata T., Kaji A., Yokota T. Evolution and structure of two ADP-ribosylation enterotoxins, Escherichia coli heat-labile toxin and cholera toxin. FEBS Lett. 1984 Apr 24;169(2):241–246. doi: 10.1016/0014-5793(84)80326-9. [DOI] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]