Abstract
We have previously demonstrated a neuroprotective mechanism of FMN (facial motoneuron) survival after facial nerve axotomy that is dependent on CD4+ Th2 cell interaction with peripheral antigen-presenting cells, as well as CNS (central nervous system)-resident microglia. PACAP (pituitary adenylate cyclase-activating polypeptide) is expressed by injured FMN and increases Th2-associated chemokine expression in cultured murine microglia. Collectively, these results suggest a model involving CD4+ Th2 cell migration to the facial motor nucleus after injury via microglial expression of Th2-associated chemokines. However, to respond to Th2-associated chemokines, Th2 cells must express the appropriate Th2-associated chemokine receptors. In the present study, we tested the hypothesis that Th2-associated chemokine receptors increase in the facial motor nucleus after facial nerve axotomy at timepoints consistent with significant T-cell infiltration. Microarray analysis of Th2-associated chemokine receptors was followed up with real-time PCR for CCR3, which indicated that facial nerve injury increases CCR3 mRNA levels in mouse facial motor nucleus. Unexpectedly, quantitative- and co-immunofluorescence revealed increased CCR3 expression localizing to FMN in the facial motor nucleus after facial nerve axotomy. Compared with WT (wild-type), a significant decrease in FMN survival 4 weeks after axotomy was observed in CCR3−/− mice. Additionally, compared with WT, a significant decrease in FMN survival 4 weeks after axotomy was observed in Rag2−/− (recombination activating gene-2-deficient) mice adoptively transferred CD4+ T-cells isolated from CCR3−/− mice, but not in CCR3−/− mice adoptively transferred CD4+ T-cells derived from WT mice. These results provide a basis for further investigation into the co-operation between CD4+ T-cell- and CCR3-mediated neuroprotection after FMN injury.
Keywords: CCR3, chemokine receptor, facial nerve axotomy, neuroprotection
Abbreviations: ALS, amyotrophic lateral sclerosis; CNS, central nervous system; DEPC, diethyl pyrocarbonate; DPA, days post-axotomy; FMN, facial motoneuron(s); GFAP, glial fibrillary acidic protein; GPCR, G-protein-coupled receptor; PACAP, pituitary adenylate cyclase-activating polypeptide; PNS, peripheral nervous system; Rag2, recombination activating gene-2; WT, wild-type
INTRODUCTION
Our laboratory has previously demonstrated a positive role for CD4+ T-cells in FMN (facial motoneuron) survival after peripheral facial nerve axotomy at the stylomastoid foramen in the mouse (Serpe et al., 1999, 2003). Raivich et al. (1998) have found a maximum increase in T-cell infiltration of the axotomized mouse facial motor nucleus between 7 and 21 days after injury. Furthermore, Ha et al. (2007) demonstrated a significant increase of CD4+ T-cells in the facial motor nucleus after facial nerve injury. In support of the findings by Raivich et al. (1998) and Ha et al. (2007), our laboratory has shown that CNS (central nervous system)-resident microglia are necessary to reactivate CD4+ T-cells centrally (Byram et al., 2004), suggesting the mechanism of peripheral CD4+ T-cell recruitment to the injured facial motor nucleus from the draining cervical lymph nodes.
Once activated, naïve CD4+ T-cells differentiate into IL-4 (interleukin-4)-positive (IL-4+) Th2 or IFNγ (interferon γ)-positive (IFN-γ+) Th1 effector cells (Stout and Bottomly, 1989). Previous work in our laboratory has found that both Th2 and Th1 subsets develop in draining lymph nodes after facial nerve axotomy (Xin et al., 2008), but that the Th2 effector subset alone is necessary for immune-mediated neuroprotection (Deboy et al., 2006). Additionally, Armstrong et al. (2003) demonstrated increased mRNA levels for PACAP (pituitary adenylate cyclase-activating polypeptide) in FMN after facial nerve axotomy. Since cultured murine microglia exposed to PACAP increase Th2-associated chemokine expression (Wainwright et al., 2008), the results suggest that, following a peripheral nerve injury outside the BBB (blood–brain barrier), microglia, which are capable of antigen presentation centrally (Carson et al., 1999), recruit Th2 cells to the facial motor nucleus. However, to respond to Th2-associated chemokines, Th2 effector cells must express the cognate Th2-associated chemokine receptors.
The recruitment of lymphocytes to different bodily compartments is a highly regulated process that involves chemokine receptors (Rand et al., 1996). Chemokine receptors are GPCRs (G-protein-coupled receptors) that, upon activation, induce chemotaxis and calcium mobilization. Different subsets of lymphocytes express different chemokine receptors, reflecting their unique migration patterns. For example, naïve CD4+ T-cells that develop into the Th2 subset express the Th2-associated chemokine receptor CCR3 (Sallusto et al., 1997). Furthermore, while several Th2-associated chemokine receptors have been demonstrated to be up-regulated by Th2 effector cells (Sallusto et al., 1998; Zingoni et al., 1998), experiments with receptor antagonists have demonstrated a critical requirement of CCR3 for Th2 cell accumulation after antigenic challenge (Mori et al., 2007).
In summary, CD4+ T-cells are present in the facial motor nucleus after facial nerve injury (Ha et al., 2007), interact with microglia (Byram et al., 2004) and protect FMN through a Th2 cell-dependent mechanism (Deboy et al., 2006). Since Th2 cells are recruited by means of Th2-associated chemokine receptors, we hypothesized that Th2-associated chemokine receptors increase in the facial motor nucleus after peripheral facial nerve injury. To investigate this hypothesis, we initially used microarray analysis to analyse Th2-associated chemokine receptor mRNA levels in the facial motor nucleus at 7 DPA (days post-axotomy), a time point consistent with significant T-cell infiltration (Raivich et al., 1998). Based on microarray analysis, we focused our investigation on the Th2-associated chemokine receptor CCR3. In addition, co-immunofluorescence revealed that CCR3 localizes to FMN and is increased after injury. Furthermore, we used the well-established facial nerve axotomy paradigm in conjunction with the CCR3-deficient (CCR3−/−) mouse model to show that CCR3 plays a role in rescuing FMN survival after injury to the facial nerve. We followed this analysis up with adoptive transfer mouse models including CD4+ T-cells derived from WT (wild-type) mice injected into CCR3−/− mice, and CD4+ T-cells isolated from CCR3−/− mice injected into Rag2 (recombination activating gene-2)-deficient (Rag2−/−; immunodeficient) mice.
MATERIALS AND METHODS
Animals and surgical procedures
Seven-week old female C57BL/6 (stock # 000664) and Balb/c (stock # 000651) mice were obtained from Jackson Laboratories and Taconic respectively. Seven-week old mice deficient for CCR3 (CCR3−/−; Balb/c background) were a gift from Dr Marc E. Rothenberg at Cincinnati Children's Hospital (Cincinnati, OH, U.S.A.). For adoptive transfer experiments, 7-week-old female CCR3−/− (stock # 005440) mice were purchased from Jackson Laboratories and Rag2−/− (stock # 000601) mice were purchased from Taconic. All mice were provided autoclaved food pellets and water ad libitum. Mice were permitted 1 week to acclimatize to their environment before being manipulated and used at 8 weeks of age in all experiments. All experimental manipulations were performed ∼4 h into the light cycle under aseptic conditions. All surgical procedures were completed in accordance with NIH (National Institutes of Health) guidelines on the care and use of laboratory animals for research purposes. Mice were anaesthetized with 3% isoflurane for all surgical procedures. Using aseptic techniques, the right facial nerve of each animal was exposed and transected at its exit from the stylomastoid foramen (Jones and LaVelle, 1985). The distal nerve stump was pushed away from the proximal nerve stump, thereby preventing reconnection of the facial nerve. Behavioural observations were used to assess whether reconnection of the facial nerve was prevented, i.e. did the animals recover from unilateral facial paralysis. None of the animals in the present study showed any signs of recovering from unilateral facial paralysis after complete transection of the facial nerve.
Chemokine receptor gene array
At 7 DPA, C57BL/6 mice (n = 4) were killed with CO2 and the brains rapidly removed. Coronal sections of brain stem, including both facial motor nuclei, were collected and tissue punches of the left (uninjured control) and right (axotomized) facial motor nuclei obtained from these sections and pooled (right axotomy compared with left control). The tissue punches were placed into 0.65 ml microfuge tubes with 20 1.4 mm Lysing Matrix D (Q-Biogene) and 80 μL of working solution D (1 ml of solution D and 7.2 μl of 2-mercaptoethanol). RNA was isolated from tissue punches and harvested cells by guanidinium thiocyanate extraction (Chomczynski and Sacchi, 1987). Samples were processed at the Superarray Bioscience Corporation using the Mouse Chemokines and Receptors Microarray (OMM-022). Fold-changes in gene expression (right axotomy/left control) were calculated for pair-wise comparison using the GEArray Expression Analysis suite.
Laser microdissection
C57BL/6 mouse brains were removed at 7, 14 and 30 DPA (n = 3–6/timepoint) and flash-frozen [in 62.5% n-butyl bromide (Fisher Scientific) and 37.5% 2-methylbutane (Fisher Scientific) surrounded by crushed dry ice]. Frozen brains were sectioned on a Leica CM3000 cryostat (Leica) with a temperature of −24°C, immersed into Tissue Teck OCT Compound (Sakura Finetek USA) at 25 μm intervals and thaw-mounted on to membrane-coated glass slides (Leica Microsystems). Slides were stained for Nissl substance to visualize the facial motor nucleus and dehydrated sequentially as follows: 100% ethanol (30 s), 2×DEPC (diethyl pyrocarbonate)-treated water (30 s each), working thionin (30 s), 2×DEPC-treated water (30 s each), 70% ethanol (30 s), 95% ethanol (30 s) and 100% ethanol (30 s). Slides were dried in a closed container for 3 min. Laser microdissection of left (control) and right (axotomized) facial motor nuclei using the Leica AS LMD (Leica Microsystems) was accomplished, capturing tissue into 0.5 ml microcentrifuge tube caps with 65 μl of extraction buffer (PicoPure RNA Isolation kit; Arcturus). After microdissection for each animal, microfuge tubes were spun at 800 g for 2 min to collect the cell extract and were stored at −80°C until use.
RNA isolation and real-time PCR
A PicoPure RNA Isolation kit (Arcturus) was used to extract RNA following the manufacturer's instructions. cDNA was generated and used in real-time PCRs and amplification was detected with SYBR green fluorescent dye (Applied Biosystems). The following primers were obtained from Superarray Bioscience Corporation: CCR3 (GenBank® accession number NM 009914; PPM03173A-200) and GAPDH (glyceraldehyde-3-phosphate dehydrogenase; GenBank® accession number NM 008084; PPM02946E-200). GAPDH served as the reference gene. Amplification was performed using the iCycler iQ Detection System (Bio-Rad Laboratories) under the following conditions: 10 min at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at 54°C and 30 s at 65°C. For each sample, the percentage change in CCR3 mRNA levels was calculated using the formula: [(axotomy/control)×100]−100%.
Preparation of CD4+ T lymphocyte adoptive transfers
Spleens were aseptically removed from Balb/c (WT) or CCR3−/− mice and placed in HBSS (Hanks balanced salt solution; Gibco Invitrogen) and 5% FCS (fetal calf serum; Gibco Invitrogen). Spleens were pressed through a 100 μm cell strainer (Fisher Scientific) to remove splenic capsules. Whole splenocytes were prepared as previously described (Serpe et al., 1999, 2003; Byram et al., 2003; Deboy et al., 2006) to obtain a single cell suspension. Briefly, red blood cells were lysed with 0.8% ammonium chloride. Cells were washed in complete media (cRPMI; Gibco Invitrogen) and centrifuged in Lympholyte M (Cedarlane) at 1510 g for 20 min. The resulting cell interface containing lymphocytes was collected and washed in cRPMI. To select for CD4+ T-cells, lymphocytes were washed with running buffer [PBS (Gibco Invitrogen), 0.5% BSA (Sigma–Aldrich) and 2 mM EDTA (Ambion)]. As previously described (Byram et al., 2003; Serpe et al., 2003; Deboy et al., 2006), resuspension of 107 lymphocytes per 90 μl of running buffer and 10 μl of MACS (magnetic cell sorting) CD4 (L3T4) microbeads (Miltenyi Biotec) were incubated for 15 min at 6–12°C. Lymphocytes were washed in running buffer by centrifugation at 300 g for 10 min, resuspended in 1–10 ml of running buffer and passed through a 30 μM filter (Miltenyi Biotec) prior to magnetic cell separation with an autoMACS automated cell sorter (Miltenyi Biotec). CD4+ T-cells were resuspended in PBS to 0.5×107 cells/ml, and 0.2 ml of cell suspension or vehicle alone was injected into the lateral tail vein of recipient mice 1 week prior to axotomy. Adoptively transferred mouse groups included CD4+ T-cells derived from WT mice injected into CCR3−/− mice, and CD4+ T-cells isolated from CCR3−/− mice injected into Rag2−/− mice.
Cell-counting procedure for FMN survival
At 4 weeks after facial nerve axotomy, mouse brains were removed and flash-frozen from WT (n = 5), CCR3−/− (n = 6), CCR3−/− adoptively transferred CD4+CCR3+ T-cells (n = 6) and Rag2−/− adoptively transferred CD4+CCR3−/− T-cells (n = 6). Cryostat sections (25 μm) were collected throughout the rostral-caudal extent of the facial motor nucleus. Sections were matched so that the rostral end of the control and axotomized facial motor nucleus with the facial nerve from each side were aligned (Serpe et al., 1999). Surviving FMNs were counted based on the presence of a clear nucleolus. Sections were coded by an investigator and cell counting was performed by a different blinded investigator, where the percentage change between the left and right sides was calculated and compared between groups. The Abercrombie correction factor {N = n×[T/(T+D)]}, where N is the actual number of cells, n is the number of nuclear profiles, T is the section thickness (25 μm) and D is the average diameter of nuclei (5 μm) (Coggeshall, 1992), was used to compensate for double counting in adjacent sections (Serpe et al., 1999).
Immunohistochemistry
At 14 DPA, C57BL/6 mice were killed with CO2 and the brains flash frozen as previously described. Mouse brains were sectioned at a thickness of 8 μm and thaw-mounted (37°C for 5–10 min) on to pre-cleaned SuperFrost slides (Fisher Scientific). Sections were pre-incubated with 4% (w/v) paraformaldehyde, blocked for endogenous biotin for 5 min (1% H2O2 in PBS), and blocked for non-specific staining with 10% BSA (A4503, Sigma–Aldrich) in PBS for 1 h. Sections were incubated overnight with a rabbit anti-CCR3 antibody (AHP1010, AbD Serotec) in PBS at 4°C. Sections were washed extensively (in PBS) and incubated with a biotinylated donkey anti-rabbit antibody (Santa Cruz Biotechnology) at room temperature (25°C) for 1 h, followed by an avidin–biotin–HRP (horseradish peroxidase) complex (Standard Ultra-Sensitive ABC Staining Kit), and chromogenic reaction with 3,3′-diaminobenzidine (Peroxidase Substrate Kit; Vector Laboratories). Sections were covered with Permount (Fisher Scientific), coverslipped and viewed using an Olympus IX70 Fluoview microscope. As a negative control, adjacent sections were processed with primary antibody omission (secondary antibody only). Additionally, preliminary experiments were performed with an anti-CCR3 antibody dilution series of 1:50, 1:100, 1:500, 1:1000 and 1:10 000. While the 1:500 dilution resulted in the highest signal-to-noise ratio, no signal was detected at 1:10 000.
Co-immunofluorescence
At 14 DPA, C57BL/6 mouse brains were flash-frozen, sectioned, and blocked for endogenous biotin and non-specific binding as described above. Primary rabbit anti-CCR3 antibody (AbD Serotec) was incubated with biotinylated mouse anti-NeuN antibody (A60, Millipore), mouse anti-GFAP (glial fibrillary acidic protein)–Alexa Fluor® 488 antibody (131-17719, Invitrogen) or rat-anti-CD68-Alexa Fluor® 488 antibody (FA-11, AbD Serotec) and were incubated in PBS at 4°C overnight. Sections were washed and incubated with donkey anti-rabbit–Alexa Fluor® 350 antibody (Invitrogen) and streptavidin–Alexa Fluor®-488 antibody (for NeuN; Invitrogen), or donkey anti-rat-Alexa Fluor®-488 (for CD68; Invitrogen) for 1 h. Following extensive washing, sections were covered with ProLong Gold Antifade reagent (Invitrogen) and immediately visualized on an Olympus IX70 Fluoview microscope.
Quantitative immunoreactivity
At 14 DPA, C57BL/6 mouse brains were flash-frozen, sectioned, and blocked for endogenous biotin and non-specific binding as described above. Seven 14 DPA systematically random sampled sections of brain stem, throughout the extent of the facial motor nucleus, with a minimum of 80 μm between each section, were analysed (n = 3). Sections were incubated overnight with a rabbit anti-CCR3 antibody (AbD Serotec) in PBS at 4°C. Sections were washed extensively (in PBS) and incubated with a biotinylated donkey anti-rabbit antibody (Santa Cruz Biotechnology) at room temperature for 1 h, washed and incubated with streptavidin-conjugated Alexa Fluor®-488 for 30 min. Following extensive washing, sections were covered with ProLong Gold Antifade reagent (Invitrogen). Additionally, directly adjacent sections were thionin-stained as described above. Images of antibody-stained sections were captured using the Olympus IX70 Fluoview fluorescent microscope attached to a Retiga 2000R (QImaging) CCD (charge-coupled-device) camera and image-capturing system using Image Pro Plus software (version 6.3; Media Cybernetics). Fluorescent images of control and 14 DPA facial motor nucleus were captured using a 10× objective and 100× magnification, with identical camera settings. CCR3+ and thionin+ FMN were counted by an independent investigator blinded to the experimental conditions. For each sample, the percentage of CCR3+ FMN was calculated using the formula [(CCR3+ FMN/thionin+ FMN)×100]. Additionally, using the pixel histogram generated for each original image, the product of the pixel number and pixel intensity value (0–255) was computed and summed for the entire facial motor nucleus. This provided a composite measurement of changes in both the immunoreactive area and fluorescence intensity.
Statistical analysis
All results are presented as means±S.E.M. The results from the experiments were analysed using GB-STAT School Pak (Dynamic Microsystems). FMN survival data were analysed using ANOVA, followed by post-hoc comparisons using the Fisher's LSD (least significant difference) test. All other data were analysed using ANOVA, followed by post-hoc comparisons using the Newman–Keuls test.
RESULTS
CCR3 mRNA levels increase in the facial motor nucleus after axotomy
To determine whether Th2-associated chemokine receptor mRNA levels are increased in the facial motor nucleus after facial nerve injury, a preliminary microarray analysis was performed. At 7 DPA, mRNA levels were increased for the three examined Th2-associated chemokine receptors, CCR3, CCR4 and CCR8 (87.2-, 11.3- and 9.6-fold respectively), in WT mice (Figure 1). Thus facial nerve axotomy appears to increase mRNA levels for Th2-associated chemokine receptors in WT mouse facial motor nucleus.
Figure 1. Th2-associated chemokine receptor mRNA levels in mouse facial motor nucleus 7 DPA.
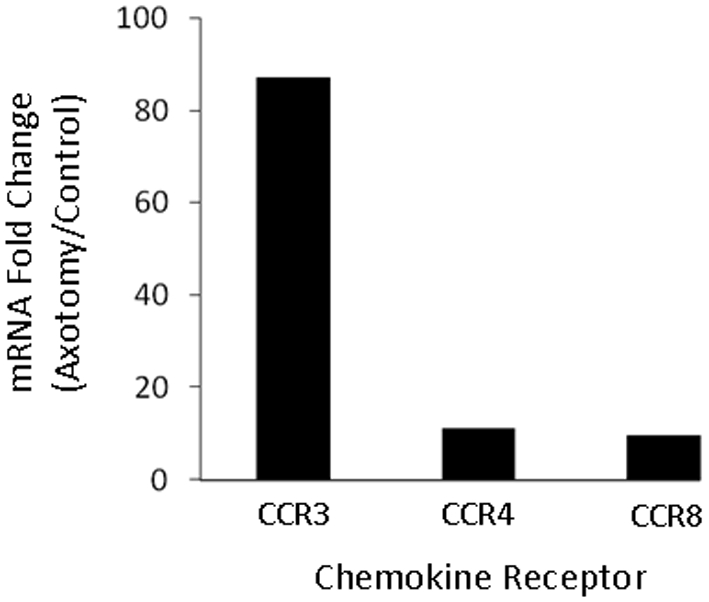
Chemokine receptor mRNA levels are displayed as the fold-change between the axotomized side and the uninjured control side.
Because CCR3 mRNA changes were so much greater, CCR3 mRNA expression was further analysed with real-time PCR (Figure 2). At 7 DPA, WT mouse CCR3 mRNA levels were 1403±199% above control values, significantly above 14 DPA and 30 DPA values [459±127% and 115±126% respectively (P<0.01)]. Thus facial nerve axotomy increases CCR3 mRNA levels in mouse facial motor nucleus within the first week after injury, before significantly decreasing over the next several weeks.
Figure 2. CCR3 mRNA levels in axotomized mouse facial motor nucleus 7, 14 and 30 DPA.
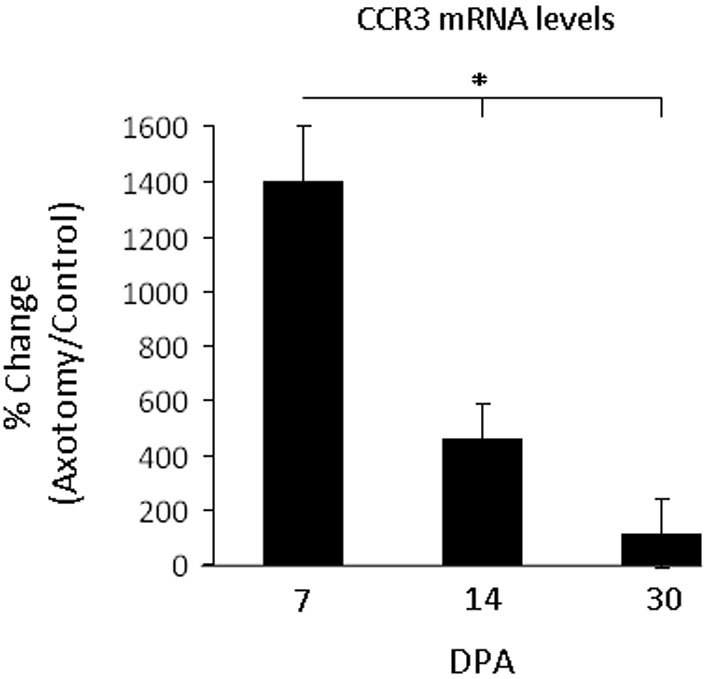
CCR3 mRNA levels in the facial motor nucleus at 7, 14 and 30 DPA (values are means± S.E.M.). CCR3 levels are displayed as the percentage change in facial motor nuclei in the axotomized relative to control facial motor nucleus within each animal. *P≤0.01.
CCR3 is involved in CD4+ T-cell-mediated FMN survival following axotomy
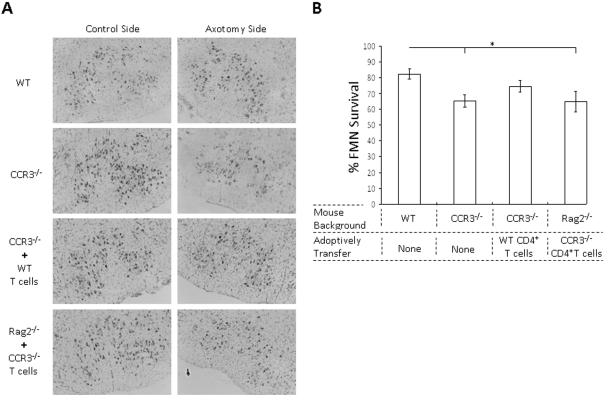
To determine whether the CCR3 receptor plays a role in FMN survival after injury, a right facial nerve axotomy was performed on WT and CCR3−/− mice. In WT mice, FMN survival levels were 82±3.0%, relative to control values at 4 weeks post-axotomy (Figures 3A and 3B). In contrast, in CCR3−/− mice, FMN survival levels were significantly decreased to 65±4.0%, relative to control values (P<0.05), a ∼17% decrease when compared with WT. To determine whether the CCR3 receptor on CD4+ T-cells plays a role in FMN survival, a right facial nerve axotomy was performed on CCR3−/− mice that were adoptively transferred CD4+ T-cells from WT mice. In CCR3−/− mice adoptively transferred WT T-cells, FMN survival levels were slightly decreased to 75±4.0%, relative to control values, and were not significantly different compared with WT. To confirm whether the CCR3 receptor on CD4+ T-cells plays a role in FMN survival, a right facial nerve axotomy was performed on Rag2−/− mice that were adoptively transferred CD4+ T cells from CCR3−/− mice. In Rag2−/− mice adoptively transferred CCR3−/− T-cells, FMN survival levels were significantly decreased to 65±7.0%, relative to control values (P<0.05), a ∼17% decrease when compared with WT. Thus CCR3 expression by CD4+ T-cells appears to be required for maintaining FMN survival levels after injury.
Figure 3. FMN survival levels 4 weeks after facial nerve axotomy in mice that are WT, CCR3−/−, CCR3−/− adoptively transferred WT mouse CD4+ T-cells or Rag2−/− adoptively transferred CCR3−/− mouse CD4+ T-cells.
(A) Thionin-stained FMNs in control or axotomized facial motor nuclei of mice that are WT, CCR3−/−, CCR3−/− adoptively transferred WT mouse CD4+ T-cells or Rag2−/− adoptively transferred CCR3−/− mouse CD4+ T-cells (original magnification, ×10). (B) Average percentage of FMN survival after facial nerve injury (values are means±S.E.M.). *P≤0.05.
Constitutive expression of CCR3 on normal FMN
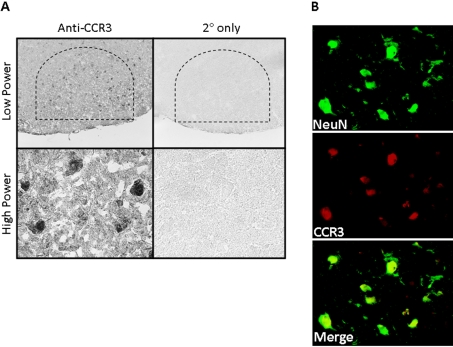
To determine CCR3 localization in the control (uninjured) facial motor nucleus, CCR3 immunohistochemistry was performed on uninjured brainstem sections (Figure 4). CCR3 was observed in FMN nuclei in the facial motor nucleus as shown at low and high power. In control experiments in which the primary antibody was omitted, CCR3 immunoreactivity was abolished. To verify that CCR3 localized to FMN nuclei, co-immunofluorescence for CCR3 and NeuN (a neuronal nuclei marker) was accomplished in the control facial motor nucleus. Co-localization of CCR3 with NeuN demonstrated labelling overlap and confirmed that CCR3 localizes to the nucleus of FMN in the uninjured facial motor nucleus. Thus there is expression of CCR3 in normal FMN nuclei.
Figure 4. CCR3 immunoreactivity in control mouse facial motor nucleus.
(A) Low- (original magnification, ×100) and high- (original magnification, ×600) power photomicrographs of mouse facial motor nucleus. Sections were processed for CCR3 immunohistochemistry. Control experiments include omission of CCR3 primary antibody. (B) High-power immunofluorescence photomicrographs of mouse facial motor nucleus immunoreactive for anti-NeuN (green) and anti-CCR3 (red) antibodies. Photomicrographs are based on the typical result of at least four independent experiments.
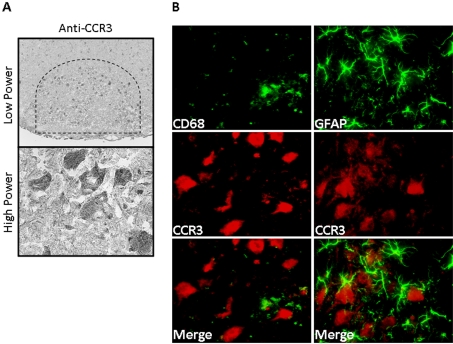
To determine CCR3 localization in the injured facial motor nucleus, CCR3 immunohistochemistry was performed on brainstem sections from animals 14 DPA (Figure 5). CCR3 was observed in FMN cytoplasm throughout the facial motor nucleus, as shown at low and high power. Additional immunofluorescent experiments with a microglial marker, CD68, and an astrocytic marker, GFAP, demonstrated no overlap of signal. Thus injured FMN continue to express CCR3, but there is de novo localization to the cytoplasm. Additionally, there is no injury-induced expression of CCR3 on microglia or astrocytes.
Figure 5. CCR3 immunoreactivity in 14 DPA mouse facial motor nucleus.
(A) Low- (original magnification, ×100) and high- (original magnification, ×600) power photomicrographs of mouse facial motor nucleus. Sections were processed for CCR3 immunohistochemistry. (B) High-power immunofluorescence photomicrographs of mouse facial motor nucleus immunoreactive for anti-CD68 (green) and anti-CCR3 [red; left-hand panel)] or anti-GFAP (green) and anti-CCR3 [red; right-hand panel)] antibodies. Photomicrographs are based on the typical result of at least four independent experiments.
CCR3 immunoreactivity increases in the facial motor nucleus after axotomy
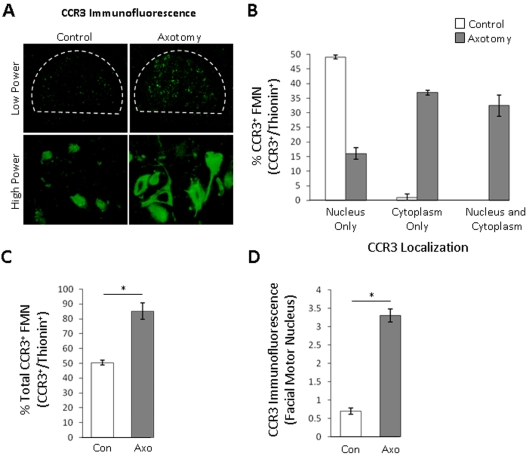
To quantify CCR3 immunoreactivity, CCR3 immunofluorescence was performed on brainstem sections from animals at 14 DPA (Figure 6A). The percentage of CCR3+ FMN with nucleus-only CCR3 immunoreactivity in control and 14 DPA facial motor nuclei were 49±0.6% and 16±2.0% respectively (Figure 6B). The percentage of CCR3+ FMN with cytoplasm-only CCR3 immunoreactivity in control and 14 DPA facial motor nuclei were 1.1±1.1% and 37±0.8% respectively. The percentage of CCR3+ FMN with both nuclei and cytoplasm CCR3 immunoreactivity in control and 14 DPA facial motor nuclei were 0% and 33±3.6% respectively. Therefore the percentage total of CCR3+ FMN with nuclear immunoreactivity in control and 14 DPA facial motor nuclei was equivalent at 49%. In contrast, the percentage total of CCR3+ FMN with cytoplasmic immunoreactivity in control and 14 DPA facial motor nuclei were 1.1% and 70% respectively. Collectively, the percentage total CCR3+ FMN in control and 14 DPA facial motor nuclei were 50±1.6% and 86±5.6% respectively (Figure 6C). Overall, the CCR3 mean fluorescence intensities in the control and 14 DPA facial motor nuclei were 0.7±0.09 and 3.3±0.18 (P<0.01) (Figure 6D). This represented a significant increase of 371% of CCR3 expression in the facial motor nucleus after facial nerve axotomy. Thus facial nerve axotomy increases the number of CCR3-expressing FMN, the expression of CCR3 in the cytoplasm of FMN, as well as total CCR3 immunoreactivity.
Figure 6. Quantitative immunoreactivity for CCR3 in control and 14 DPA mouse facial motor nucleus.
(A) Low- (original magnification, ×100) and high- (original magnification, ×600) photomicrographs of control (left-hand panels) and axotomized (right-hand panels) mouse facial motor nucleus. (B) Average percentage of CCR3+ FMN with nucleus only, cytoplasm only or both nucleus and cytoplasm immunoreactivity in the control (open bars) and axotomized (grey bars) mouse facial motor nucleus (values are means±S.E.M.). (C) Average percentage total of CCR3+ FMN in the control (open bars) and axotomized (grey bars) mouse facial motor nucleus (values are means±S.E.M.). (D) Mean fluorescence intensity of the control (open bars) and axotomized (grey bars) mouse facial motor nucleus (values are means±S.E.M.). *P≤0.01. Con, control; Axo, axotomized.
DISCUSSION
The present study was initiated based on previous work from our laboratory demonstrating that mouse FMN survival after facial nerve axotomy depends on both peripheral antigen-presenting cells for initial CD4+ T-cell activation, and centrally located microglia for CD4+ T-cell reactivation (Byram et al., 2004). Furthermore, we discovered that, after facial nerve axotomy, CD4+ Th2 cells develop (Xin et al., 2008) and are responsible for mediating FMN survival (Deboy et al., 2006). These results, combined with evidence that PACAP mRNA is expressed by injured mouse FMN after facial nerve axotomy (Armstrong et al., 2003), and that PACAP increases Th2-associated chemokine expression in cultured murine microglia (Wainwright et al., 2008), strongly encouraged us to undertake the present study. Using microarray analysis, we initially identified an increase in Th2-associated chemokine receptor CCR3 mRNA levels in the mouse facial motor nucleus after facial nerve axotomy. Based on this preliminary analysis, combined with previous data suggesting that Th2 cells migrate to the facial motor nucleus after facial nerve axotomy (Byram et al., 2004; Deboy et al., 2006), and that Th2 cell-expressed CCR3 was required for efficient Th2 cell migration (Mori et al., 2007), we chose to focus on the role of CCR3 in the facial motor nucleus after facial nerve axotomy.
Gao et al. (1996) first identified and characterized mouse CCR3 with respect to the high level of mRNA expression in eosinophils, as well as the calcium-mobilization response to the Th2-associated chemokine, CCL11, in HEK (human embryonic kidney)-293 cells. Since then, the majority of research investigating CCR3 has focused on Th2 cell and eosinophil recruitment to the lungs and resulting pathogenesis of asthma and allergies (Pope et al., 2005; Fulkerson et al., 2006; Munitz et al., 2008). However, it is now clear that CCR3 is expressed by additional cell types.
Although it has previously been reported that CCR3 localizes to neurons in the CNS (Zhang et al., 1998), no other studies have demonstrated the effect of injury on neuron-expressed CCR3. Previous reports have suggested that the chemokine receptors CXCR2, CCR5 and CX3CR1 have the potential to modulate neuronal survival after an injury (Meucci et al., 2000; Cardona et al., 2006; Vallès et al., 2006; Gamo et al., 2008). Prior to this investigation, it was unknown whether CCR3 was required to mediate neuronal survival after an injury. It was also unknown whether CCR3 was required by FMN, CD4+ T-cells or both cell types, to mediate FMN survival after facial nerve injury.
In the present study, the preliminary microarray analysis led us to focus on the chemokine receptor CCR3. The results indicate a transient, but large, increase in CCR3 mRNA levels in WT mice coincident with peripheral immune activation. Unexpectedly, normal FMN express CCR3 in the nucleus, whereas axotomy-injured FMN express CCR3 in both the nucleus and/or cytoplasm of FMN, and not in microglia or astrocytes. Additionally, the percentage of CCR3+ FMN and quantitative immunofluorescence indicate a large increase in the number of FMN that express CCR3, as well as an increase in total CCR3 expression throughout the facial motor nucleus respectively.
Our results show that CCR3 is critical to maintaining WT levels of FMN survival, since a facial nerve axotomy in CCR3−/− mice showed decreased FMN survival. Furthermore, our results indicate that CCR3 expression by CD4+ T-cells is required to rescue FMN survival from axotomy-induced FMN loss, since only WT, but not CD4+ T-cells deficient for CCR3, could rescue FMN survival after a facial nerve axotomy. Noticeably, FMN survival levels after axotomy in CCR3−/− mice adoptively transferred with WT CD4+ T-cells are not statistically different from CCR3−/− mice with no adoptive transfer. We hypothesize that this may be due to the already fully populated CD4+ T-cell compartment in CCR3−/− mice, resulting in an inefficient engraftment of the WT CD4+ T-cell population that is adoptively transferred. Regardless of the requirement of CCR3 by CD4+ T-cells to mediate FMN survival after axotomy, the question still remains as to the role of CCR3 expressed by FMN.
Since CCR3 is a GPCR, localization to the nucleus of normal FMN was unexpected. However, immunohistochemistry for CCR3 has previously demonstrated nuclear localization in neurons (Zhang et al., 1998), as well as cells of the nasal mucosa (Salib et al., 2004) and renal cell carcinoma (Jöhrer et al., 2005). Furthermore, numerous GPCRs have been localized to the nucleus of cells in diverse tissues and have been extensively reviewed (Goetzl, 2007; Boivin et al., 2008). Although the significance of nuclear CCR3 localization has not yet been investigated, the nuclear compartmentalization may be the result of a transcription-factor-like function, involved with maintenance and growth under normal conditions. Possibly, CCR3 undergoes post-translational modifications that target it toward the nucleus normally. Alternatively, CCR3 may be trafficked to the nucleus from the plasma membrane as a result of chemokine interaction. Recently, CCR2 was shown to translocate from the cytoplasm to the nucleus after activation by the cognate chemokine, CCL2, in cultured HeLa cells (Favre et al., 2008). It is also possible that CCR3 is alternatively spliced in FMN relative to peripherally circulating leucocytes. Although not yet shown for chemokine receptors, it has been demonstrated that some chemokines are alternatively spliced in the CNS relative to the PNS (peripheral nervous system), giving rise to a nuclear relative to a secreted isoform respectively (Baird et al., 1999). Regardless of the mechanism by which CCR3 is transported to the nucleus, clues to the functional significance may be found in injured FMN.
After facial nerve axotomy, the percentage of FMN with nuclear CCR3 immunoreactivity was maintained at constant levels. In contrast, the percentage of FMN with cytoplasmic immunoreactivity increased significantly. This translated into a large increase in the percentage of total FMNs immunoreactive for CCR3. Accordingly, total CCR3 immunoreactivity was increased in the facial motor nucleus after facial nerve axotomy. Thus one of the effects of facial nerve axotomy in FMN could be de novo synthesis of CCR3. Whether new CCR3 is inserted into the plasma membrane, translocated to the nucleus and/or transported down FMN axons is an interesting question. In accordance with our findings of changes in CCR3 expression in axotomized FMN, other immunohistochemical experiments with the leukaemia inhibitor factor receptor have demonstrated axotomy-induced changes in the intracellular localization of immunoreactivity in DRG (dorsal root ganglia) neurons (Gardiner et al., 2002). However, the significance of the translocation was not determined.
The unexpected normal and/or injury-induced anatomical location of CCR3 expression by FMN may be due, in part, to the normal and/or injury-induced anatomical location of chemokines that interact with CCR3. CCL5, CCL7, CCL8, CCL11, CCL13, CCL15, CCL18, CCL24, CCL26 and CCL27 can all bind CCR3 with variable affinities (Ponath et al., 1996; Coulin et al., 1997; Heath et al., 1997; Stellato et al., 1997; White et al., 1997; Shinkai et al., 1999; Nibbs et al., 2000; Pan et al., 2000). Of those chemokines, only CCL11, CCL24 and CCL26 are reported to be specific to CCR3. The remaining chemokines, CCL5, CCL7, CCL8, CCL13, CCL15, CCL18 and CCL27, can interact with CCR3, in addition to other select chemokine receptors. Given the plethora of chemokines that interact with CCR3, as well as those that interact with additional chemokine receptors, determining which CCR3-interacting chemokines are required for FMN survival after facial nerve injury provides a challenging opportunity.
In summary, in the present study it was determined that, after facial nerve axotomy, CCR3 mRNA and protein levels increase in the facial motor nucleus, CCR3 localizes to FMNs and cytoplasmic immunoreactivity is elevated. Also, the present study demonstrates, for the first time, a neuroprotective role for the chemokine receptor CCR3. It was determined that CD4+ T-cells require CCR3 to maintain WT levels of FMN survival after a facial nerve injury. Co-incidentally, it was previously demonstrated that CCR3 is expressed by CD4+ Th2 cells (Sallusto et al., 1997) and that CD4+ Th2 cells mediate WT levels of FMN survival after a facial nerve injury through interaction with PNS antigen-presenting cells, as well as CNS microglia (Byram et al., 2004; Deboy et al., 2006). Taken together, these results indicate that CCR3 is involved in the recruitment of CD4+ Th2 cells to the facial motor nucleus after facial nerve axotomy.
To maintain a healthy CNS, the immune system must balance the destructive effects that develop for defence against infection, disease or malignancy, with the neuroprotective effects that develop after a peripheral nerve injury (Byram et al., 2004). In mouse models that recapitulate motoneuron diseases such as ALS (amyotrophic lateral sclerosis), data indicate that this balance is disturbed, resulting in impaired chemokine receptor signalling (Luo et al., 2007). Whether CCR3-expressing CD4+ T-cells prevent disease onset and/or slows disease progression during ALS pathogenesis is an interesting hypothesis. Therefore future studies will investigate the combined role of CCR3 and CD4+ T-cells in mice that recapitulate ALS pathology, as well as delineate the function of CCR3 expression by FMN after injury.
ACKNOWLEDGEMENTS
We thank Dr Marc E. Rothenberg (Cincinatti Children's Hospital, Cincinnati, OH, U.S.A.) and Regeneron Pharmaceuticals (Tarrytown, NY, U.S.A.) for CCR3-deficient mice.
Footnotes
This work was supported by the National Institutes of Health [grant number NS40433 (to K.J.J. and V.M.S.)]; and the Les Turner ALS Foundation (to D.A.W. and K.J.J.).
REFERENCES
- Armstrong BD, Hu Z, Abad C, Yamamoto M, Rodriguez WI, Cheng J, Tam J, Gomariz RP, Patterson PH, Waschek JA. Lymphocyte regulation of neuropeptide gene expression after neuronal injury. J Neurosci Res. 2003;74:240–247. doi: 10.1002/jnr.10750. [DOI] [PubMed] [Google Scholar]
- Baird JW, Nibbs RJ, Komai-Koma M, Connolly JA, Ottersbach K, Clark-Lewis I, Liew FY, Graham GJ. ESkine, a novel β-chemokine, is differentially spliced to produce secretable and nuclear targeted isoforms. J Biol Chem. 1999;274:33496–33503. doi: 10.1074/jbc.274.47.33496. [DOI] [PubMed] [Google Scholar]
- Boivin B, Vaniotis G, Allen BG, Hebert TE. G protein-coupled receptors in and on the cell nucleus: a new signaling paradigm? J Recept Signal Transduct Res. 2008;28:15–28. doi: 10.1080/10799890801941889. [DOI] [PubMed] [Google Scholar]
- Byram SC, Serpe CJ, Pruett SB, Sanders VM, Jones KJ. Natural killer cells do not mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:417–425. doi: 10.1016/s0889-1591(03)00089-8. [DOI] [PubMed] [Google Scholar]
- Byram SC, Carson MJ, DeBoy CA, Serpe CJ, Sanders VM, Jones KJ. CD4-positive T cell-mediated neuroprotection requires dual compartment antigen presentation. J Neurosci. 2004;24:4333–4339. doi: 10.1523/JNEUROSCI.5276-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- Carson MJ, Sutcliffe JG, Campbell IL. Microglia stimulate naïve T-cell differentiation without stimulating T-cell proliferation. J Neurosci Res. 1999;55:127–134. doi: 10.1002/(SICI)1097-4547(19990101)55:1<127::AID-JNR14>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. A consideration of neuronal counting methods. Trends Neurosci. 1992;15:9–12. doi: 10.1016/0166-2236(92)90339-a. [DOI] [PubMed] [Google Scholar]
- Coulin F, Power CA, Alouani S, Peitsch MC, Schroeder JM, Moshizuki M, Clark-Lewis I, Wells TN. Characterisation of macrophage inflammatory protein-5/human CC cytokine-2, a member of the macrophage-inflammatory-protein family of chemokines. Eur J Biochem. 1997;248:507–515. doi: 10.1111/j.1432-1033.1997.00507.x. [DOI] [PubMed] [Google Scholar]
- Deboy CA, Xin J, Byram SC, Serpe CJ, Sanders VM, Jones KJ. Immune-mediated neuroprotection of axotomized mouse facial motoneurons is dependent on the IL-4/STAT6 signaling pathway in CD4+ T cells. Exp Neurol. 2006;201:212–224. doi: 10.1016/j.expneurol.2006.04.028. [DOI] [PubMed] [Google Scholar]
- Favre N, Camps M, Arod C, Chabert C, Rommel C, Pasquali C. Chemokine receptor CCR2 undergoes transportin1-dependent nuclear translocation. Proteomics. 2008;8:4560–4576. doi: 10.1002/pmic.200800211. [DOI] [PubMed] [Google Scholar]
- Fulkerson PC, Fischetti CA, McBride ML, Hassman LM, Hogan SP, Rothenberg ME. A central regulatory role for eosinophils and the eotaxin/CCR3 axis in chronic experimental allergic airway inflammation. Proc Natl Acad Sci USA. 2006;103:16418–16423. doi: 10.1073/pnas.0607863103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamo K, Kiryu-Seo S, Konishi H, Aoki S, Matsushima K, Wada K, Kiyama H. G-protein-coupled receptor screen reveals a role for chemokine receptor CCR5 in suppressing microglial neurotoxicity. J Neurosci. 2008;28:11980–11988. doi: 10.1523/JNEUROSCI.2920-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JL, Sen AI, Kitaura M, Yoshie O, Rothenberg ME, Murphy PM, Luster AD. Identification of a mouse eosinophil receptor for the CC chemokine eotaxin. Biochem Biophys Res Commun. 1996;223:679–684. doi: 10.1006/bbrc.1996.0955. [DOI] [PubMed] [Google Scholar]
- Gardiner NJ, Cafferty WB, Slack SE, Thompson SW. Expression of gp130 and leukaemia inhibitory factor receptor subunits in adult rat sensory neurones: regulation by nerve injury. J Neurochem. 2002;83:100–109. doi: 10.1046/j.1471-4159.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- Goetzl EJ. Diverse pathways for nuclear signaling by G protein-coupled receptors and their ligands. FASEB J. 2007;21:638–642. doi: 10.1096/fj.06-6624hyp. [DOI] [PubMed] [Google Scholar]
- Ha GK, Huang Z, Parikh R, Pastrana M, Petitto JM. Immunodeficiency impairs re-injury induced reversal of neuronal atrophy: relation to T cell subsets and microglia. Exp Neurol. 2007;208:92–99. doi: 10.1016/j.expneurol.2007.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath H, Qin S, Rao P, Wu L, LaRosa G, Kassam N, Ponath PD, Mackay CR. Chemokine receptor usage by human eosinophils. The importance of CCR3 demonstrated using an antagonistic monoclonal antibody. J Clin Invest. 1997;99:178–184. doi: 10.1172/JCI119145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jöhrer K, Zelle-Rieser C, Perathoner A, Moser P, Hager M, Ramoner R, Gander H, Höltl L, Bartsch G, Greil R, Thurnher M. Up-regulation of functional chemokine receptor CCR3 in human renal cell carcinoma. Clin Cancer Res. 2005;11:2459–2465. doi: 10.1158/1078-0432.CCR-04-0405. [DOI] [PubMed] [Google Scholar]
- Jones KJ, LaVelle A. Changes in nuclear envelope invaginations in axotomized immature and mature hamster facial motoneurons. Brain Res. 1985;353:241–249. doi: 10.1016/0165-3806(85)90212-3. [DOI] [PubMed] [Google Scholar]
- Luo Y, Xue H, Pardo AC, Mattson MP, Rao MS, Maragakis NJ. Impaired SDF1/CXCR4 signaling in glial progenitors derived from SOD1(G93A) mice. J Neurosci Res. 2007;85:2422–2432. doi: 10.1002/jnr.21398. [DOI] [PubMed] [Google Scholar]
- Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc Natl Acad Sci USA. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori A, Ogawa K, Someya K, Kunori Y, Nagakubo D, Yoshie O, Kitamura F, Hiroi T, Kaminuma O. Selective suppression of Th2-mediated airway eosinophil infiltration by low-molecular weight CCR3 antagonists. Int Immunol. 2007;19:913–921. doi: 10.1093/intimm/dxm049. [DOI] [PubMed] [Google Scholar]
- Munitz A, Brandt EB, Mingler M, Finkelman FD, Rothenberg ME. Distinct roles for IL-13 and IL-4 via IL-13 receptor α1 and the type II IL-4 receptor in asthma pathogenesis. Proc Natl Acad Sci USA. 2008;105:7240–7245. doi: 10.1073/pnas.0802465105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nibbs RJ, Salcedo TW, Campbell JD, Yao XT, Li Y, Nardelli B, Olsen HS, Morris TS, Proudfoot AE, Patel VP, Graham GJ. C-C chemokine receptor 3 antagonism by the beta-chemokine macrophage inflammatory protein 4, a property strongly enhanced by an amino-terminal alanine-methionine swap. J Immunol. 2000;164:1488–1497. doi: 10.4049/jimmunol.164.3.1488. [DOI] [PubMed] [Google Scholar]
- Pan J, Kunkel EJ, Gosslar U, Lazarus N, Langdon P, Broadwell K, Vierra MA, Genovese MC, Butcher EC, Soler D. A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol. 2000;165:2943–2949. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- Ponath PD, Qin S, Ringler DJ, Clark-Lewis I, Wang J, Kassam N, Smith H, Shi X, Gonzalo JA, Newman W, Gutierrez-Ramos JC, Mackay CR. Cloning of the human eosinophil chemoattractant, eotaxin. Expression, receptor binding, and functional properties suggest a mechanism for the selective recruitment of eosinophils. J Clin Invest. 1996;97:604–612. doi: 10.1172/JCI118456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol. 2005;175:5341–5350. doi: 10.4049/jimmunol.175.8.5341. [DOI] [PubMed] [Google Scholar]
- Raivich G, Jones LL, Kloss CU, Werner A, Neumann H, Kreutzberg GW. Immune surveillance in the injured nervous system: T-lymphocytes invade the axotomized mouse facial motor nucleus and aggregate around sites of neuronal degeneration. J Neurosci. 1998;18:5804–5816. doi: 10.1523/JNEUROSCI.18-15-05804.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand ML, Warren JS, Mansour MK, Newman W, Ringler DJ. Inhibition of T cell recruitment and cutaneous delayed-type hypersensitivity-induced inflammation with antibodies to monocyte chemoattractant protein-1. Am J Pathol. 1996;148:855–864. [PMC free article] [PubMed] [Google Scholar]
- Salib RJ, Kumar S, Wilson SJ, Howarth PH. Nasal mucosal immunoexpression of the mast cell chemoattractants TGF-β, eotaxin, and stem cell factor and their receptors in allergic rhinitis. J. Allergy Clin Immunol. 2004;114:799–806. doi: 10.1016/j.jaci.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998;187:875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe CJ, Kohm AP, Huppenbauer CB, Sanders VM, Jones KJ. Exacerbation of facial motoneuron loss after facial nerve transection in severe combined immunodeficient (scid) mice. J Neurosci. 1999;19:RC7. doi: 10.1523/JNEUROSCI.19-11-j0004.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serpe CJ, Coers S, Sanders VM, Jones KJ. CD4+ T, but not CD8+ or B, lymphocytes mediate facial motoneuron survival after facial nerve transection. Brain Behav Immun. 2003;17:393–402. doi: 10.1016/s0889-1591(03)00028-x. [DOI] [PubMed] [Google Scholar]
- Shinkai A, Yoshisue H, Koike M, Shoji E, Nakagawa S, Saito A, Takeda T, Imabeppu S, Kato Y, Hanai N, Anazawa H, Kuga T, Nishi T. A novel human CC chemokine, eotaxin-3, which is expressed in IL-4-stimulated vascular endothelial cells, exhibits potent activity toward eosinophils. J Immunol. 1999;163:1602–1610. [PubMed] [Google Scholar]
- Stellato C, Collins P, Ponath PD, Soler D, Newman W, La Rosa G, Li H, White J, Schwiebert LM, Bickel C, Liu M, Bochner BS, Williams T, Schleimer RP. Production of the novel C-C chemokine MCP-4 by airway cells and comparison of its biological activity to other C-C chemokines. J Clin Invest. 1997;99:926–936. doi: 10.1172/JCI119257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stout RD, Bottomly K. Antigen-specific activation of effector macrophages by IFN-γ producing (TH1) T cell clones. Failure of IL-4-producing (TH2) T cell clones to activate effector function in macrophages. J Immunol. 1989;142:760–765. [PubMed] [Google Scholar]
- Vallès A, Grijpink-Ongering L, de Bree FM, Tuinstra T, Ronken E. Differential regulation of the CXCR2 chemokine network in rat brain trauma: implications for neuroimmune interactions and neuronal survival. Neurobiol Dis. 2006;22:312–322. doi: 10.1016/j.nbd.2005.11.015. [DOI] [PubMed] [Google Scholar]
- Wainwright DA, Xin J, Sanders VM, Jones KJ. Differential actions of pituitary adenylyl cyclase-activating polypeptide and interferon γ on Th2- and Th1-associated chemokine expression in cultured murine microglia. J Neurodegen Regen. 2008;1:31–34. [PMC free article] [PubMed] [Google Scholar]
- White JR, Imburgia C, Dul E, Appelbaum E, O’Donnell K, O’Shannessy DJ, Brawner M, Fornwald J, Adamou J, Elshourbagy NA, Kaiser K, Foley JJ, Schmidt DB, Johanson K, Macphee C, Moores K, McNulty D, Scott GF, Schleimer RP, Sarau HM. Cloning and functional characterization of a novel human CC chemokine that binds to the CCR3 receptor and activates human eosinophils. J Leukoc Biol. 1997;62:667–675. doi: 10.1002/jlb.62.5.667. [DOI] [PubMed] [Google Scholar]
- Xin J, Wainwright DA, Serpe CJ, Sanders VM, Jones KJ. Phenotype of CD4+ T cell subsets that develop following mouse facial nerve axotomy. Brain Behav Immun. 2008;22:528–537. doi: 10.1016/j.bbi.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, He T, Talal A, Wang G, Frankel SS, Ho DD. In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5. J Virol. 1998;72:5035–5045. doi: 10.1128/jvi.72.6.5035-5045.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zingoni A, Soto H, Hedrick JA, Stoppacciaro A, Storlazzi CT, Sinigaglia F, D’Ambrosio D, O’Garra A, Robinson D, Rocchi M, Santoni A, Zlotnik A, Napolitano M. The chemokine receptor CCR8 is preferentially expressed in Th2 but not Th1 cells. J Immunol. 1998;161:547–551. [PubMed] [Google Scholar]