Abstract
The social brain hypothesis proposes that large neocortex size in Homonoids evolved to cope with the increasing demands of complex group living and greater numbers of interindividual relationships. Group living requires that individuals communicate effectively about environmental and internal events. Recent data have highlighted the complexity of chimpanzee communication, including graded facial expressions and referential vocalizations. Among Hominoids, elaborate facial communication is accompanied by specializations in brain areas controlling facial movement. Finally, the evolution of empathy, or emotional awareness, might have a neural basis in specialized cells in the neocortex, that is, spindle cells that have been associated with self-conscious emotions, and mirror neurons that have recently been shown to activate in response to communicative facial gestures.
Introduction
Emotion has long been viewed as an important phenomenon underlying animal behavior. Emotion helps to organize physiological, motivational and cognitive systems that facilitate adaptive responses to aid the survival of the organism. As such, emotion can be thought of as a process that facilitates appropriate responding to a wide range of situations, including predator avoidance, intra- and inter-specific aggression, reproduction, child care, and maintaining stability within social groups. Some of the most recent advances in our understanding of emotion in animals, particularly nonhuman primates, have come from communication research [1]. This includes significant advances in our understanding of the signals used by nonhuman primates to communicate about emotion, how this information is perceived by group members, and, ultimately, its influence on subsequent behavior and group cohesion. Only recently, however, has enough evidence been accumulated to suggest that the neural and emotional systems of nonhuman primates have become specialized to cope with the increasing demands of complex social organizations and more elaborate inter-individual relationships. Here, we provide a brief review of the most recent and important contributions to the areas of expression production, expression perception, and the evolution of specialized neural substrates for understanding emotion within a social environment.
Emotional communication in nonhuman primates
Facial expressions and vocalizations are the primary means for communicating about emotion among primates. Several independent lines of evidence suggest that the facial expression repertoire of related primate species, despite highly varied patterns of social organization, is very similar. First, the mimetic facial musculature that forms the structure of facial expressions is highly conserved across primates [2]. A recent dissection of chimpanzee facial muscles revealed an almost identical anatomy to that of humans [3]. This infers that, with few exceptions, the facial behaviors of related species are directly comparable, and this similarity reinforces evolutionary continuity. This approach is important because the most widely recognized coding system for classifying human facial behavior is based on identifying the actions of the underlying mimetic musculature. Therefore, to directly compare emotional expressions across species, and increase our understanding of their evolutionary similarities, it is important to use a common metric (see Figure 1) [1].
Figure 1.
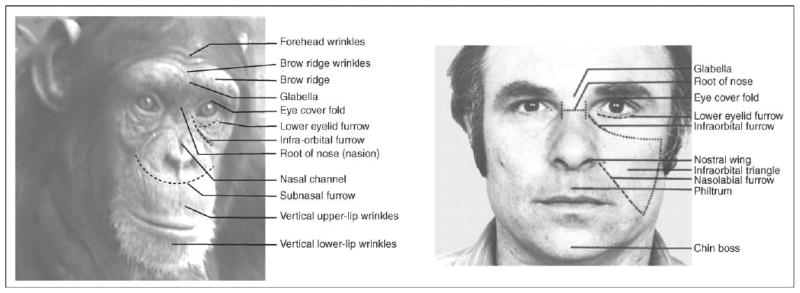
A comparative illustration of facial landmarks in a chimpanzee and a human. These differences in facial morphology affect the function of facial expressions in changing the appearance of the face.
Second, similar facial expressions have been described in many different species of nonhuman primates, and some of these expressions are elicited in similar social contexts, which is suggestive of, although not evidence for, a common function or meaning that was important in our evolutionary history. Parr et al. [4••] recently described the facial repertoire of the chimpanzee with particular attention to displays that are highly graded in their intensity and show features that appear blended across expression categories. The ability of a species to produce similar expressions and presumably decode their meaning in such a diverse range of social contexts highlights their advanced socio-cognitive skills. Moreover, Waller and Dunbar [5••] have demonstrated that two seemingly different facial displays in chimpanzees, the silent bared-teeth face (i.e. fear grin) and relaxed open mouth face (i.e. play face), have similar social functions in increasing rates of affinitive behavior between individuals. Authors have long argued that these signals might be homologous with smiling and laughing, respectively, which have similar social bonding functions in humans (Figure 2; [6,7]). Thus, the use and comprehension of facial signals, such as those described above, might have played a crucial role in the formation of long-lasting and complex social relationships in our early Homonoid ancestors, and perhaps among far more distant relatives [8•]. The fact that their muscular basis is also similar strongly supports the argument that many facial expressions among closely related species are indeed homologous, including important expressions in chimpanzees and humans.
Figure 2.

Two chimpanzee facial expressions believed to be homologous with human expressions. The left figure shows the relaxed open mouth face, or play face, believed homologous with laughter and the right figure shows the silent bared-teeth display, believed to be homologous with the human smile. These expressions are shown in the same young female chimpanzee.
Perception of emotion expressions
If communicative signaling is additionally influenced by emotional state, it is a reasonable assumption that species living in complex social groups must also possess well-developed emotion processing skills, as they must be able to interpret the different meanings associated with similar facial displays used in different emotional contexts [4••]. This assumption need not imply that these signals are predictive of specific basic emotions, as has been argued for human facial expressions [9], but at a minimum they should reflect a general motivation and/or tendency of the organism to engage in a series of actions given a set of social and environment conditions [10]. Additionally, the signals must be easy to recognize and discriminate from other signals and/or noise in the environment, or their information content and predictive value will be lost. Historically, some researchers have suggested that the communicative repertoire of chimpanzees is too graded to provide unambiguous, or even referential, information as has been described for some monkey species [11–13].
However, others have argued that if the signals could be deciphered reliably based on structure and context, or if they were to occur in context-specific combination, the result would be that graded signals could provide additional information resulting, ultimately, in a richer and more diverse communication system than is possible from referential communication alone [4••,14,15]. In fact, several recent studies have described acoustic differences between calls made by wild chimpanzees depending on their social role and/or context of use, suggesting not only a referential nature but a rich source of information about emotion [16,17••]. Moreover, the multimodal components of chimpanzee facial signals, such as the auditory and visual components of expressions, do not necessarily provide additive or redundant information [18•]. Thus, the context in which the signals are produced, the identity of the caller and the specific features of the visual and auditory components appear to interact in nonredundant ways, which is suggestive of a complex communication system [19].
Neural specializations for emotional communication?
Dunbar [20,21] proposed a provocative, but testable, theory ‘The Social Brain Hypothesis’ (see glossary) to explain the relationship between large brain size and complex societies among Homonoids. The hypothesis proposes that large neocortex volume and superior socio-cognitive skills co-evolved to deal effectively with the increasing demands stemming from large and complex social groups, and, more specifically, the increased number of individual relationships that one can sustain [21]. A large brain to body ratio, such as that present in chimpanzees and humans, equaled better cognitive processing speed, an ability to keep track of many individuals at once, including a large number of personal relationships, and advanced memory for social events over long periods of time. Dunbar [20] has specifically implicated language functions in human societies as crucial for social cohesion, yet nonverbal, emotional communication has also been implicated in social bonding [5••,22]. In fact, the part of the brain that shows the strongest relationship with group complexity is the newer region of the neocortex, not phylogenetically older regions such as the amygdala or areas of visual cortex [23]. Moreover, it has recently been suggested that maintaining group cohesion in species such as the chimpanzee, that live in a fission-fusion society (see glossary) characterized by temporal and geographical distance, is far more demanding in terms of computational and cognitive load than a group that preserves a constant grouping structure, as is found in Cercopithecines [24].
There are two neuroanatomical routes to facial movement: first, a route through the facial nucleus in the pons of the brainstem, which regulates spontaneous, or emotional, facial movement; second, voluntary movements can be produced through activity in the facial representation area of the motor cortex. There is recent evidence linking the evolution of facial expressions and elaboration of the facial musculature to each of these particular brain regions [25••,26••]. The volume of the facial nucleus, for example, was larger than would be predicted based on standard phylogenetic regression in great apes and humans, again suggesting greater spontaneous or emotional control of the facial musculature in Homonoids compared to that in other primate species [26••]. Moreover, Sherwood et al. [25••,26••] have observed phylogenetic differences in the organization of neurons enriched with neurofilament protein in the primary motor cortex among nonhuman primates. Species with more elaboration in the mimetic facial muscles and greater repertoire of facial expressions, such as great apes and humans, showed a greater density of these neurons that might contribute to greater dexterity and more voluntary control of the facial region [25••]. Thus, there appears to be a phylogenetic relationship between facial expression repertoire and specializations in the neural control of facial movement. This is important for the social brain hypothesis in that the evolution of neural specializations controlling complex facial movements might have received increased selective pressure in our closest ancestors.
The evolution of greater flexibility in emotional communications with an underlying neural basis, such as those described above, might have played an important role in the evolution of socially derived emotions, or self-conscious emotions, such as guilt, shame, pride and embarrassment. These emotions are considered to be unique human specializations because they require some degree of social awareness, such as theory of mind (see glossary), that enables an understanding of how one's actions are perceived by others. At its most basic level, this type of awareness is mostly unconscious and occurs as a form of emotional contagion, or basic form of empathy, that results simply from watching the behavior of others (for an evolutionary account see Preston and de Waal [27]). This type of emotional awareness functions to coordinate activity among group members, facilitate social cohesion and motivate conciliatory tendencies, and is likely to play a key role in coordinating social behaviors in large-brained social primates, such as Homonoids. There is evidence in the animal world for contagious yawning, scratching and emotional behavior, including play and aggression [28–30].
A neural substrate for this process might occur in the form of mirror neurons, which have been anointed by some as a neural basis underlying empathy [27]. Mirror neurons are located in the prefrontal cortex and respond not only to actions performed by an individual but also when that individual watches the same action performed by others. This extends to passive viewing of facial expressions, such as lip smacking, which selectively activates premotor areas on the right side of the brain, leading researchers to suppose that empathy is based on a motoric representation of action and expression [31,32]. Therefore, as opposed to responding exclusively to motor movements involving the hand, mirror neurons have selective properties for facial movement. Finally, self-conscious emotions are often linked to specific activation in the anterior cingulate cortex. This region contains a special class of neurons referred to as ‘spindle cells’, or ‘Von Economo neurons’ [33••]. The density of these cells is greatest in humans, but they are also prevalent in the great apes — bonobos, chimpanzees, gorillas and orangutans, but not in monkeys [34]. Although the exact function of these neurons is not known, their recent phylogenetic emergence and their location in the prefrontal cortex strongly supports their involvement in social bonding, decision formation, and social emotions [33••]. There is also evidence that the volume of the anterior cingulate cortex that contains Von Economo neurons is reduced, and their precise neuroanatomical location is abnormal in autistic individuals. This finding, although tentative, supports the described function of these neurons, as autism is characterized by impaired social communication and lack of empathy [33••].
Conclusions
Accumulating evidence now suggests that the increased cognitive demands from complex group living, particularly the number of individual relationships, might have been a driving force behind the evolution of neural specializations for socio-emotional communication in primates. Specifically, Hominoids possess more elaborate control of their facial movements, greater number and complexity of facial expressions and perhaps even a neural basis for emotional representation and comprehension in the form of mirror neurons and spindle cells. The next few years have the potential to reveal many more behavioral and neural specializations as our understanding of social cognition in Hominoid species becomes clearer. One important direction for future research is to investigate the relationship between facial expressions and emotion. Although it is clear that the production of facial expressions is associated with predictable behavioral outcomes, little is known about whether nonhuman primates attribute an emotional disposition to individuals who produce these expressions. Such a question has important implications for the evolution of theory-of-mind, the ability to attribute mental states to others. Theory of mind is thought to be a unique human specialization but it might have an evolutionary precursor in basic empathy, the ability to understand the emotional states of others, which unlike mental states, can be understood through observable behavior, that is, facial expressions.
Acknowledgments
This paper was made possible by support from RR-00165 from the NIH/NCRR National Institutes of Health/National Center for Research Resourses to the Yerkes National Primate Research Center, NIMH-R01-MH068791 to LA Parr and the Center for Behavioral Neuroscience, Emory University (NSF-IBN-9876754). The Yerkes National Primate Research Center is fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Glossary
- Social Brain Hypothesis
This hypothesis states that relative brain size has increased in the primate order as a result of the selection pressures to solve the problems associated with living in social groups.
- Fission-fusion society
Social groups of individuals composed of smaller groups that fission (split off from the main group) and fuse (rejoin) in line with social and ecological conditions.
- Homologous trait
Similarity in a structure or behavior that can be attributed to a common ancestor, compared with an analogous trait that is similar in form but not due to common descent.
- Theory of mind
The ability to attribute mental states, that is, thoughts, intuitions, drives, to others and to reflect on one's own mental state.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Parr LA, Waller B. The evolution of human emotion. In: Kaas J, editor. Evolution of the Nervous System. Vol. 5. Elsevier; in press. [Google Scholar]
- 2.Huber E. The Evolution of Facial Musculature and Facial Expression. The Johns Hopkins Press; 1931. [Google Scholar]
- 3.Burrows AM, Waller BM, Parr LA, Bonar CJ. Muscles of facial expression in the chimpanzee (Pan troglodytes): Descriptive, ecological and phylogenetic contexts. J Anat. doi: 10.1111/j.1469-7580.2006.00523.x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4••.Parr LA, Cohen M, de Waal FBM. Influence of social context on the use of blended and graded facial displays in chimpanzees. Int J Primatol. 2005;26:73–103. [Google Scholar]; The authors present a structural analysis of chimpanzee facial displays documenting the occurrence and context of expressions ‘blended’ between two parent types. Rather than being associated with a new context (which would imply a new, distinct function), these blended expressions tend to occur in contexts similar to only one of the parent expressions. Thus, blended expressions reflect motivational and emotional conflicts, as such increasing our understanding of the relationship between emotional state and expressive behavior.
- 5••.Waller B, Dunbar RIM. Differential behavioural effects of silent bared teeth display and relaxed open mouth display in chimpanzees (Pan troglodytes) Ethology. 2005;111:129–142.1. [Google Scholar]; Through behavioral observations, this study demonstrates how chimpanzee facial signals function to increase affinitive behavior in dyadic interactions. The authors propose that the silent bared-teeth display and relaxed open-mouth display function to facilitate social bonding and the maintenance of social groups.
- 6.van Hooff JARAM. A comparative approach to the phylogeny of laughter and smiling. In: Hinde RA, editor. NonVerbal Communication. Cambridge University Press; 1972. [Google Scholar]
- 7.Preuschoft S, van Hooff JARAM. Homologizing primate facial displays: A critical review of methods. Folia Primatol (Basel) 1995;65:121–137. doi: 10.1159/000156878. [DOI] [PubMed] [Google Scholar]
- 8•.Panksepp J. Beyond a joke: from animal laughter to human joy? Science. 2005;308:62–63. doi: 10.1126/science.1112066. [DOI] [PubMed] [Google Scholar]; The emerging field of affective neuroscience triangulates the study of behavior, brain substrates and affective experience to support the hypothesis that internal emotional states are a universal feature of animal minds. The author discusses the body of empirical work supporting the experience of positive affect (joy) in animals, including rats, dogs and primates.
- 9.Ekman P. An argument for basic emotions. Cogn Emotion. 1992;6:169–200. [Google Scholar]
- 10.Seyfarth RM, Cheney DL. Meaning and emotion in animal vocalizations. Ann N Y Acad Sci. 2003;1000:32–55. doi: 10.1196/annals.1280.004. [DOI] [PubMed] [Google Scholar]
- 11.Gouzoules S, Gouzoules H, Marler P. Rhesus monkey (Macaca mulatta) screams: representational signalling in the recruitment of agonistic aid. Anim Behav. 1984;32:182–193. [Google Scholar]
- 12.Seyfarth RM, Cheney DL, Marler P. Monkey responses to three different alarm calls: Evidence of predator classification and semantic communication. Science. 1980;210:801–803. doi: 10.1126/science.7433999. [DOI] [PubMed] [Google Scholar]
- 13.Zuberbuhler K, Noe R, Seyfarth RM. Diana monkey long-distance calls: messages for conspecifics and predators. Anim Behav. 1997;53:589–604. [Google Scholar]
- 14.Crockford C, Boesch C. Context-specific calls in wild chimpanzees, Pan troglodytes verus: analysis of barks. Anim Behav. 2003;66:115–125. [Google Scholar]
- 15.Marler P. Social organization, communication, and graded signals: the chimpanzee and the gorilla. In: Hinde RA, Bateson PPG, editors. Growing Points in Ethology. Cambridge University Press; 1976. pp. 239–279. [Google Scholar]
- 16.Zuberbuhler K. A syntactic rule in forest monkey communication. Anim Behav. 2002;63:293–299. [Google Scholar]
- 17••.Slocombe K, Zuberbuhler K. Agonistic screams in wild chimpanzees (Pan troglodytes schweinfurthii) vary as a function of social role. J Comp Psychol. 2005;119:67–77. doi: 10.1037/0735-7036.119.1.67. [DOI] [PubMed] [Google Scholar]; The authors found that chimpanzees give acoustically distinct screams depending on whether the caller is the aggressor or victim in an agonistic encounter. These differences were primarily associated with the shape of the second half of the call. Whether others take advantage of these acoustical differences in responding to aggressive encounters, providing evidence for referential communication, remains to be determined.
- 18•.Parr LA. Perceptual basis for multimodal cues in chimpanzee affect recognition. Anim Cogn. 2004;7:363–371. doi: 10.1007/s10071-004-0207-1. [DOI] [PubMed] [Google Scholar]; In this study, chimpanzees were required to match facial expression videos by selecting a still photo that matched either the visual, auditory or both (multimodal) cues presented in a video. Subjects showed a strong preference for visual cues associated with screams, and auditory cues associated with pant-hoots and laughter. The results suggest that the presence of multimodal signals does not necessarily increase recognition and that each channel provides nonredundant information.
- 19.Partan S, Marler P. Communication goes multimodal. Science. 1999;283:1272–1273. doi: 10.1126/science.283.5406.1272. [DOI] [PubMed] [Google Scholar]
- 20.Dunbar RIM. Coevolution of neocortex size, group size and language in humans. Behav Brain Sci. 1993;16:681–735. [Google Scholar]
- 21.Dunbar RIM. The social brain hypothesis. Evol Anthropol. 1998;6:178–190. [Google Scholar]
- 22.Spoor JR, Kelly JR. The evolutionary significance of affect in groups: communication and group bonding. Group Process Intergroup Relat. 2004;7:398–412. [Google Scholar]
- 23.Joffe TH, Dunbar RIM. Visual and socio-cognitive information processing in primate brain evolution. Proc Biol Sci. 1997;264:1303–1307. doi: 10.1098/rspb.1997.0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barrett L, Henzi P, Dunbar RIM. Primate cognition: from ‘what now?’ to ‘what if?’. Trends Cogn Sci. 2003;7:494–497. doi: 10.1016/j.tics.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 25••.Sherwood CC, Holloway RL, Erwin JM, Hof PR. Cortical orofacial motor representation in Old World monkeys, great apes and humans. Brain Behav Evol. 2004;63:82–106. doi: 10.1159/000075673. [DOI] [PubMed] [Google Scholar]; The authors analyzed the microstructural organization of the primary motor cortex (BA 4) to explore its role in the communication systems of 12 Anthropoid primates. Great apes and humans showed increased thickness of layer III and an overall lower cell volume density compared to Old World monkeys. These differences might provide an anatomical substrate for the increased fine motor control of facial expressions exhibited by Homonoids.
- 26••.Sherwood CC, Hof PR, Holloway RL, Semendeferi K, Gannon PJ, Frahm HD, Zilles K. Evolution of the brainstem orofacial motor system in primates: A comparative study of trigeminal, facial and hypoglossal nuclei. J Hum Evol. 2005;48:45–84. doi: 10.1016/j.jhevol.2004.10.003. [DOI] [PubMed] [Google Scholar]; The authors present a comparative neuroanatomical investigation of the craniofacial motor nuclei in primates. The investigation revealed no differences between suborders in the trigeminal (V: mastication) or hypoglossal (XIII: tongue) nuclei, but great ape and human facial (VII) nuclei were significantly larger than predicted. This specialization in the great apes might indicate increased utilization of the facial muscles for social communication, and reflect greater ability for expression in these species.
- 27.Preston S, de Waal FBM. Empathy: Its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–72. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- 28.Anderson JR, Myowa-Yamakoshi M, Matsuzawa T. Contagious yawning in chimpanzees. Proc Biol Sci. 2004;271:S468–470. doi: 10.1098/rsbl.2004.0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nakayama K. Observing conspecifics scratching induces a contagion of scratching in Japanese monkeys (Macaca fuscata) J Comp Psychol. 2004;118:20–24. doi: 10.1037/0735-7036.118.1.20. [DOI] [PubMed] [Google Scholar]
- 30.Parr LA, Hopkins WD. Brain temperature asymmetries and emotional perception in chimpanzees, Pan troglodytes. Physiol Behav. 2000;71:363–371. doi: 10.1016/s0031-9384(00)00349-8. [DOI] [PubMed] [Google Scholar]
- 31.Leslie KR, Johnson-Frey SH, Grafton ST. Functional imaging of the face and hand imitation: towards a motor theory of empathy. Neuroimage. 2004;21:601–607. doi: 10.1016/j.neuroimage.2003.09.038. [DOI] [PubMed] [Google Scholar]
- 32.Ferrari PF, Gallese V, Rizzolatti G, Fogassi L. Mirror neurons responding to the observation of ingestive and communicative mouth actions in the monkey ventral premotor cortex. Eur J Neurosci. 2003;17:1703–1714. doi: 10.1046/j.1460-9568.2003.02601.x. [DOI] [PubMed] [Google Scholar]
- 33••.Allman JM, Watson KK, Tetreault NA, Hakeem AY. Intuition and autism: a possible role for Von Economo neurons. Trends Cogn Sci. 2005;9:367–373. doi: 10.1016/j.tics.2005.06.008. [DOI] [PubMed] [Google Scholar]; This paper reviews the neuroanatomy of the Von Economo neurons, specific to layer 5 of the anterior cingulate cortex and frontoinsular cortex. The authors suggest that the recent phylogenetic appearance and ontogenetic pattern of these neurons implicates them as a lead candidate for neurodevelopmental disorders, such as autism. Their location suggests an involvement in social bonding, social emotions and reward.
- 34.Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]


