Abstract
The rapid and complete digestion of proteins is important when protein characterization by hydrogen deuterium exchange (HDX) is coupled with mass spectrometry. We developed a single-pump, on-line, high pressure digestion system that relies on UPLC technology to aid in the digestion of proteins. Two model proteins, Aβ1–42 and NBSA, were used to demonstrate the efficacy of the high pressure system. Both model proteins readily aggregate and are difficult to digest under normal conditions. Our high pressure system successfully digests these proteins into small, overlapping peptides. The extra information afforded by overlapping peptides allows us to pinpoint HDX protection to protein segments smaller than the digested peptide. The calculated average segment length (ASL) for both model proteins decreased by 2-fold for high pressure digestion compared to digestion at ambient pressure.
Keywords: High pressure, pepsin, protein digestion, hydrogen deuterium exchange, mass spectrometry
Bottom-up proteomics has become the approach for protein identification. The coupling of enzyme proteolysis with tandem mass spectrometry enables the identification of proteins in digests from mixtures even as complex as those from cell lysates.1 A common limitation of the bottom-up approach, however, is insufficient sequence coverage. In a single proteolysis, identified peptides can represent less than 50% of the protein sequence, decreasing confidence in the identification and restricting complete protein characterization.2
In structural proteomics, mapping by hydrogen deuterium exchange mass spectrometry (HDX) has become a powerful complement to bottom-up proteomics because it probes protein-protein and protein-ligand interactions.3,4,5 An assessment of HDX at the peptide level can be achieved by using proteolysis followed by peptide analysis. A more detailed picture, even at the amino-acid level, can be achieved by proteolyzing to small, overlapping peptides.5,6 Another approach, tandem MS, can be hampered owing to scrambling of the deuterium label upon CID; this may be avoidable by using electron-capture based activation (i.e., ECD, ETD).7–8 All approaches, however, must be fast to avoid back exchange, must accommodate the quenching conditions, pH 2.5, 0 °C, and must maximize sequence coverage.
Pepsin is the most widely used protease for HDX because it digests quickly at low pH. Smaller, overlapping peptides can be achieved by increasing protease concentration and/or increasing digestion times. Increasing the protease concentration when digesting in solution, however, leaves behind a large protease signal in the mass spectrum; and this signal can obscure peptides of interest. Increasing digestion time leads to more back exchange of the reversible label, and, thus, dilutes the information obtainable from fast exchanging peptides.
Conducting the digestion at high pressure may be a useful approach to increasing enzymatic activity. Application of high pressure is effective in biotechnology and food technology where it is used for sterilization and processing.9 For example at pressures ranging from 800–4000 bar (~11,000–58,000 psi), viruses are inactivated,10 complex structures are dissociated,11 proteins are denatured,12 and microorganisms inactivated.13,14 There is also evidence that high pressure enhances enzymatic hydrolysis by trypsin.15,16 Although the mechanism is not fully understood, kinetic studies show that at elevated pressure, the enzyme-reaction equilibria are shifted towards substrate hydrolysis.17,18 Pressure-induced protein denaturation may also play role in increasing enzyme activity by allowing better access to the protein.15
To increase digestion coverage and reduce the average segment length without negatively impacting the HDX experiment, we developed an on-line, high pressure digestion system using UPLC technology. Unlike a previous on-line digestion system,16 we use a single gradient pump to pressurize the system and to deliver sample for MS analysis. This allows the digestion, chromatography, and MS analysis to be performed rapidly without increasing significantly the back exchange of the system. We describe the system in this communication and present a preliminary evaluation for the Aβ peptide and a mutant of the capsid protein from HIV virus.
EXPERIMENTAL SECTION
Materials and Reagents
Pepsin, formic acid, and HPLC-grade solvents were obtained from Sigma Aldrich (St. Louis, MO). Aβ 1–42 was purchased from American Peptides (Sunnyvale, CA). NBSA was expressed and purified in the Peter Prevelige laboratory (University of Alabama-Birmingham) by using the protocol for wild-type capsid protein.19 Urea refolding of the protein was used to separate the protein from inclusion bodies.
Digestion Procedure
Pepsin was reconstituted in HPLC-grade water with 0.1% formic acid. Aβ 1–42 was reconstituted in 10 mM NH4OH, pH 10. The peptide was diluted in 50 mM Tris pH 7.4 to give a final concentration 0.75 mg/mL. NBSA was dialyzed into 50 mM Na2HPO4 and diluted to a final concentration of 0.1 mg/mL. The proteins were acidified to mimic HDX quench conditions by the addition of 0.2% formic acid. All components from the UPLC injector through the C18 column were submerged in a 0 °C ice bath. Equal volumes of pepsin (final concentration 0.025 mg/mL) in 0.2% formic acid and protein were mixed and immediately loaded onto a 0° C, 20 μL sample loop for digestion. Digestion times, 2 min for Aβ 1–42 and 3 min for NBSA, were the same for high pressure and low pressure experiments.
Digestion apparatus
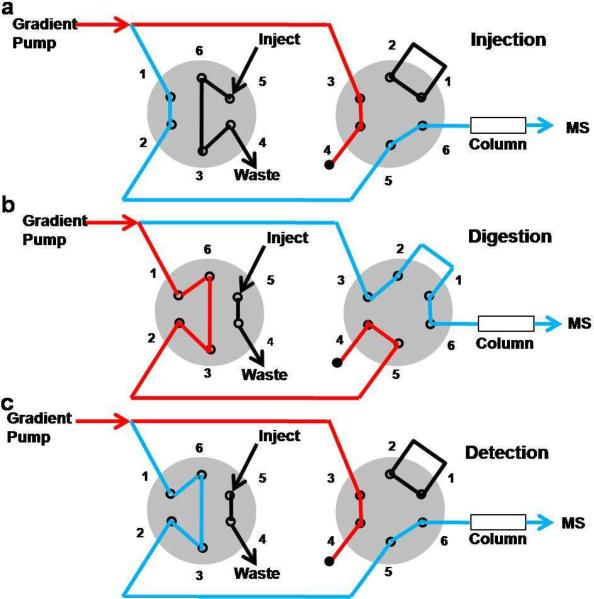
The on-line high pressure digestion system (Figure 1) utilized a 10,000 psi rated NanoAcquity UPLC (Waters, Milford, MA). The high-pressure configuration consists of two 20,000 psi rated, six-port injections valves (VICI Valco, Houston, TX). A constant flow was kept through the system. The protein and protease solution were injected into the sample loop in the injection mode (Figure 1a). The valve was switched to the digestion mode where the sample flow was blocked at position B4 (Figure 1b). A separate flow through a 1.7 μm, 1 × 50 mm, Acquity UPLC BEH C18 column (Waters, Milford, MA) created a back pressure on the system. The digestion was performed in the sample loop at a pressure of 9000–9600 psi. After digestion, the valves were switched, and the flow path directed the sample onto the column (Figure 1c). After column separation, production spectra were collected on a LTQ-Orbitrap XL (Thermo-Fisher, Waltham, MA). The MASCOT search engine was used to identify peptides from the MS/MS data.20 The average segment length (ASL) for the digestion was calculated as described below.
Figure 1.
Schematic of the on-line, high-pressure digestion system. The on-line digestion system has three modes, (a) injection, (b) digestion, and (c) detection. The blue line indicates the continuous flow through the system. The red lines indicate the flow that is blocked at position B4.
Average segment length
A segment corresponds to the shortest stretch of amino acids that is deduced from peptide overlap (see explanation in Figure 2a). All residues of the protein not observed are considered one non-deducible segment.
The average segment length (ASL) is calculated using equation 1. As the segments become shorter, the ASL becomes smaller, and the experiment becomes closer to revealing single residue HDX information.
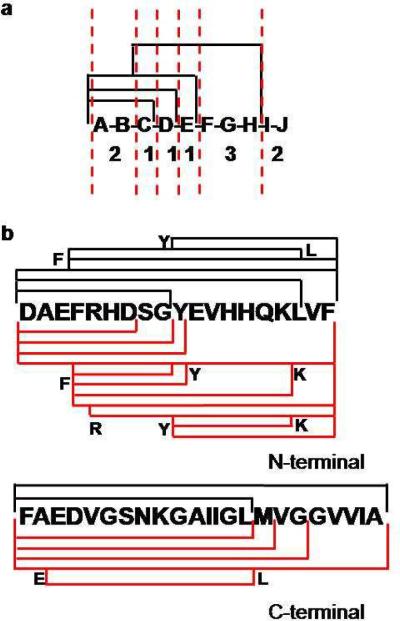
Figure 2.
Segment length determination. (a) Means to determine the segment length: the observed peptides are shown with black lines; the dashed red lines show the delineation of the segments based on the amount of overlap of the peptides. The numbers show the lengths of each segment. Peptide IJ is considered a non-deducible segment. (b) Digestion coverage of Aβ1–42 with (red lines) and without (black lines) high pressure. The amino acid at the beginning and end of some peptides are highlighted to reduce ambiguity.
RESULTS AND DISCUSSION
We chose the amyloid β-peptide 1–42 (Aβ 1–42) as a model system for the single-pump on-line high pressure digestion system. This 42-residue peptide has a high propensity for fibril formation21 and is difficult to digest.22 Aggregation of the protein was induced by decreasing the pH from 10 to 7.4 where it readily aggregates. The peptide was digested by pepsin with and without high pressure. In the absence of high pressure, eight peptides were observed with an ASL of 6.0 residues (Figure 2). Conversely, significantly more (16) peptides were observed with digestion at high pressure, and the ASL is 3.5. In the N-terminal region, residues 1–19, the number of observed peptides doubled with high pressure. Peptides with 6 and 7 residues that overlapped with longer peptides were observed for the high-pressure digest. This combination of short, overlapping peptides greatly increases HDX information, sometimes even to the amino-acid level.
NMR studies show that the C-terminus of the Aβ1–42 peptide has reduced flexibility compared Aβ 1–40, an amyloid β-peptide with a lower competence for fibril formation.23 This reduced flexibility may contribute to decreased protease digestion in the C- terminal region of Aβ1–42 at low pressure where only two peptides were observed with an ASL of 11.5 amino acid residues. Conversely, in the high pressure digest, five peptides were produced in the C- terminal region of Aβ1–42, and a significant decrease in the ASL to 4.6 residues was observed.
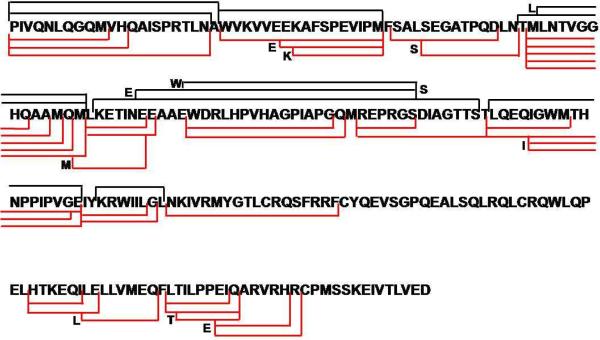
The NBSA protein, an HIV-1 capsid mutant protein, assembles into a higher order oligomer that has implications for the design of novel anti-HIV drugs. We are using HDX to characterize the protein-protein interactions in the assembled form of the protein. Pepsin digestion yielded 10 peptides with an ASL of 15.8 residues (Figure 3). No peptides from the C- terminal domain of the protein were found. This low coverage, which hampered the identification of protein-protein interaction sites, can be attributed to acid-induced protein aggregation. Increasing protease concentration or digestion times did not enhance the digestion coverage. When submitted to our on-line high-pressure digestion system, however, the number of peptides observed was 35, almost four times as many as at ambient pressure. Moreover, the digest ASL decreased 2.5 times to 6.0 residues. Further studies showed the increased pressure did not adversely affect back exchange of the deuterium label (see supplemental data).
Figure 3.
Coverage map of NBSA. Digestion coverage of NBSA with (red lines) and without (black lines) high pressure. The amino acids at the beginning and end of some peptides are highlighted.
CONCLUSIONS
We developed an on-line, high-pressure digestion system that facilitates the rapid and more complete digestion of proteins. The system utilizes UPLC technology to improve protease digestion via the application of dynamic high pressure. The high-pressure digestion system affords higher coverage and increased amino acid information for proteins that are difficult to proteolyze under normal conditions. Given that more smaller peptides are produced at high pressure, this approach increases the residue-level information of HDX studies without turning to tandem MS, where H/D scrambling is a concern, or to electron-capture based activation such as ECD and ETD, where special instrumentation is needed.
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr. Peter E. Prevelige, Jr. from the University of Alabama-Birmingham for a gift of the NBSA fusion protein (Grant number AI44624). This work was funded by a NIH National Center for Research Resources grant (Grant number 2P41RR000954) and a Pfizer Global Research and Development postdoctoral fellowship for Lisa Jones.
REFERENCES
- (1).Link AJ, Eng J, Schieltz DM, Carmack E, Mize GJ, Morris DR, Garvik BM, Yates JR., 3rd Nat Biotechnol. 1999;17:676–682. doi: 10.1038/10890. [DOI] [PubMed] [Google Scholar]
- (2).Kelleher NL, Lin HY, Valaskovic GA, Aaserud DJ, Fridriksson EK, McLafferty FW. Journal of the American Chemical Society. 1999;121:806–812. [Google Scholar]
- (3).Englander SW, Kallenbach NR. Quarterly Reviews of Biophysics. 1983;16:521–655. doi: 10.1017/s0033583500005217. [DOI] [PubMed] [Google Scholar]
- (4).Nemirovskiy O, Giblin DE, Gross ML. J Am Soc Mass Spectrom. 1999;10:711–718. doi: 10.1016/S1044-0305(99)00036-7. [DOI] [PubMed] [Google Scholar]
- (5).Engen JR, Smith DL. Methods Mol Biol. 2000;146:95–112. doi: 10.1385/1-59259-045-4:95. [DOI] [PubMed] [Google Scholar]
- (6).Englander JJ, Del Mar C, Li W, Englander SW, Kim JS, Stranz DD, Hamuro Y, Woods VL., Jr. Proc Natl Acad Sci U S A. 2003;100:7057–7062. doi: 10.1073/pnas.1232301100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Rand KD, Zehl M, Jensen ON, Jorgensen TJD. Analytical Chemistry. 2009;81:5577–5584. doi: 10.1021/ac9008447. [DOI] [PubMed] [Google Scholar]
- (8).Zehl M, Rand KD, Jensen ON, Jorgensen TJD. Journal of the American Chemical Society. 2008;130:17453–17459. doi: 10.1021/ja805573h. [DOI] [PubMed] [Google Scholar]
- (9).Gross M, Jaenicke R. Eur J Biochem. 1994;221:617–630. doi: 10.1111/j.1432-1033.1994.tb18774.x. [DOI] [PubMed] [Google Scholar]
- (10).Bradley DW, Hess RA, Tao F, Sciaba-Lentz L, Remaley AT, Laugharn JA, Jr., Manak M. Transfusion. 2000;40:193–200. doi: 10.1046/j.1537-2995.2000.40020193.x. [DOI] [PubMed] [Google Scholar]
- (11).Jaenicke R, Ludemann HD, Schade BC. Biophysics of Structure and Mechanism. 1981;7:195–203. [Google Scholar]
- (12).Samarasinghe SD, Campbell DM, Jonas A, Jonas J. Biochemistry. 1992;31:7773–7778. doi: 10.1021/bi00149a005. [DOI] [PubMed] [Google Scholar]
- (13).Hoover DG, Metrick C, Papineau AM, Farkas DF, Knorr D. Food Technology. 1989;43:99–107. [Google Scholar]
- (14).Metrick C, Hoover DG, Farkas DF. Journal of Food Science. 1989;54:1547–&. [Google Scholar]
- (15).Lopez-Ferrer D, Petritis K, Hixson KK, Heibeck TH, Moore RJ, Belov ME, Camp DG, Smith RD. Journal of Proteome Research. 2008;7:3276–3281. doi: 10.1021/pr7008077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Lopez-Ferrer D, Petritis K, Lourette NM, Clowers B, Hixson KK, Heibeck T, Prior DC, Pasa-Tolic L, Camp DG, 2nd, Belov ME, Smith RD. Anal Chem. 2008;80:8930–8936. doi: 10.1021/ac800927v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bruins ME, Janssen AEM, Boom RM. Journal of Molecular Catalysis B-Enzymatic. 2006;39:124–127. [Google Scholar]
- (18).Northrop DB. Biochimica Et Biophysica Acta-Protein Structure and Molecular Enzymology. 2002;1595:71–79. doi: 10.1016/s0167-4838(01)00335-1. [DOI] [PubMed] [Google Scholar]
- (19).Lanman J, Sexton J, Sakalian M, Prevelige PE., Jr. J Virol. 2002;76:6900–6908. doi: 10.1128/JVI.76.14.6900-6908.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Perkins DN, Pappin DJ, Creasy DM, Cottrell JS. lectrophoresis. 1999;20:3551–3567. doi: 10.1002/(SICI)1522-2683(19991201)20:18<3551::AID-ELPS3551>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- (21).Masters CL, Simms G, Weinman NA, Multhaup G, McDonald BL, Beyreuther K. Proc Natl Acad Sci U S A. 1985;82:4245–4249. doi: 10.1073/pnas.82.12.4245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Burdick D, Soreghan B, Kwon M, Kosmoski J, Knauer M, Henschen A, Yates J, Cotman C, Glabe C. J Biol Chem. 1992;267:546–554. [PubMed] [Google Scholar]
- (23).Riek R, Guntert P, Dobeli H, Wipf B, Wuthrich K. Eur J Biochem. 2001;268:5930–5936. doi: 10.1046/j.0014-2956.2001.02537.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.