Abstract
The present study has been undertaken to examine whether exposure to benzo(a)pyrene (BaP), a polycyclic aromatic hydrocarbon (PAH) compound influences the metabolism of fluoranthene (FLA), another PAH compound. Microsomes were isolated from the adipose tissue of mice that received 50 μg/kg BaP and incubated with FLA (3μM) alone; FLA in combination with BaP at equimolar concentrations and a control group that received nothing. Post-incubation, samples were extracted with ethyl acetate and analyzed for FLA metabolites by reverse-phase HPLC with fluorescence detection. The rate of FLA metabolism (pmol of metabolite/min/mg protein) was increased when microsomes from BaP-treated mice were exposed to FLA alone and FLA in combination with BaP, compared to controls. On the other hand, the difference in FLA metabolic rate between microsomes that were exposed to FLA + BaP was higher than the ones that received FLA. The microsomes from BaP-pretreated mice produced considerably higher proportion of FLA 2, 3-diol, and 2, 3 D FLA when microsomes were incubated with FLA. There were no differences in the FLA metabolite types formed when BaP-pretreated mice were co-incubated with BaP and FLA, than FLA alone. The enhanced biotransformation of FLA as a result of prior and concomitant exposure to BaP may have implications for assessment of risks arising from human exposure to PAH mixtures.
Keywords: Fluoranthene, Benzo(a)pyrene, Polycyclic aromatic hydrocarbons, Adipose microsomes, Metabolism
Introduction
Our environment is contaminated with a mixture of chemicals, a good majority of which are man-made while the others are of anthropogenic in origin. One family of these chemicals that are ubiquitous in the environment is polycyclic aromatic hydrocarbons (PAHs), which are released into the environment as a result of petrogenic and pyrolytic processes. The toxicity and carcinogenicity of PAHs are well established aspects (IPCS, 1998; Ramesh et al. 2004).
Humans and animals are seldom exposed to a single PAH compound but mixtures of PAHs through diet, personal- (smoking) and life style habits (cooking and home heating, using firewood) and occupational (firefighters, industrial workers, aircraft mechanics etc.) settings (IPCS, 1998; Ramesh et al. 2004). Some of these compounds are potent by nature, while others require addition of another PAH compound that manifest its effect (additivity) or to an enhanced effect than one would expect on the basis of additivity (synergism) or suppress the effect of other PAH compound (antagonism) or qualitatively different toxic effects when exposed together than when exposed alone (potentiation; Cassee et al., 1998; Kistos and Edler, 2005). The combination of PAHs can be binary, ternary, quaternary or more. Also, the patterns of exposure differ; they can be sequential or simultaneous exposures. To determine the effectiveness of these two exposure scenarios, we studied the in vitro metabolism of two PAH compounds benzo(a)pyrene (BaP) and fluoranthene (FLA) using microsomes. Microsomes are subcellular organelles that harbor drug metabolizing enzymes necessary for detoxification of chemicals (Ekins et al., 2000). These organelles along with cell cultures are being developed as in vitro screening tools to test the toxicity of PAHs in complex environmental mixtures (Krishnamurthi et al., 2007).
We did not dwell into the endpoints of toxicity or carcinogenesis; instead we focused on the metabolism of these chemicals, given the fact that metabolism drives the toxicity/carcinogenesis of any given environmental chemical. The metabolite profiles are expected to serve as predictors of toxic or carcinogenic responses that might ensue or the lack thereof upon exposure of cells to PAHs.
Being lipophilic, PAHs tend to accumulate in the adipose tissue (Moir et al., 1998; Walker et al., 2007). Continuous release of these compounds from adipose stores eventually leads to buildup of reactive metabolites in target tissues to induce toxic effects (Galván et al., 2005). Therefore, the rationale for this study was to assess the type of interactions between BaP and FLA in metabolism mediated by the adipose tissue microsomal system. Benzo(a)pyrene and FLA were chosen for this study because both were toxicants (Knuckles et al., 2001 and 2004). While the former PAH compound is an established carcinogen (IPCS, 1998), the latter one is a “non classifiable carcinogen [carcinogenicity class D as per EPA (2001) guidelines].
Materials and Methods
Exposure of mice to benzo(a)pyrene
Six-week-old male ApcMin mice, an animal model used for studying cancers of the gastrointestinal tract (Sattar et al., 1999; Harris et al., 2009a), purchased from Jackson Labs (Bar Harbor, ME) were used in this study. These mice weighing approximately 25 g were housed in-groups of 2–3 per cage, maintained on a 12/12 hour light/dark cycle (lights on at 0600 hour) and allowed free access to rodent chow (NIH-31 open formula diet, National Institutes of Health, MD) and water. All animals were allowed a seven-day acclimation period prior to being randomly assigned to a control (n = 5) or treatment group (n = 5). Treatment consisted of 50 μg/kg of BaP (97% pure, unlabeled; Sigma Chemical Co., St.Louis, MO) dissolved in peanut oil (Sigma). Mice that received no vehicle (peanut oil) and BaP served as controls. The test chemical was administered daily via oral gavage for 60 days. This dose has been chosen on the basis of human dietary relevance of orally administered BaP and the induction of colon tumors in ApcMin mice at this dose, reported earlier from our laboratory (Harris et al., 2009a). The care and husbandry of mice used in this study was in conformity with the guidelines (ILAR, 1996) that regulate the humane care and use of laboratory animals for research.
Preparation of Microsomes
Microsomal fractions were prepared, and processed as described by Schenkman and Jansson (1999) with some modifications. The mesenteric adipose tissue (white adipose tissue that blankets the peritoneum connecting parts of intestine) were removed following which, they were washed in chilled (4°C) isotonic saline. Each tissue sample was individually cut into small pieces using sterile scalpel blades, minced separately with a fine pair of scissors and thoroughly mixed to obtain a homogenous mixture of minced tissue sample per animal. One gram of each minced sample was chilled in isotonic saline for 5 min prior to being homogenized in two volumes of sucrose-TKM buffer (sucrose 0.25 M, Tris 80 mM, KCl 25 mM, MgCl2 5 mM, pH 7.4). Each minced tissue sample homogenate was centrifuged at 10, 000 X g for 10 min, supernatant harvested and subjected to a second centrifugation at 15, 000 X g for 15 min to pellet down nuclei and mitochondria. Each resultant supernatant was centrifuged at 100, 000 X g for 60 min at 4°C following which, the cytosolic supernatant and microsomal pellet were separated. Each microsomal pellet was rinsed twice with 5 mL of sucrose-TKM buffer and resuspended in 5 mL of the same buffer. The microsomes were aliquoted into cryovials (Wheaton Science Products, Millville, NJ), and stored frozen at −20°C until used for studying FLA/BaP metabolism. Protein content of each microsomal preparation was determined according to the method of Bradford (1976).
Microsomal Incubations and Metabolism Studies
Prior to starting the present studies, pilot studies were conducted in our laboratory to establish the optimal conditions for microsomal protein concentrations, substrate concentrations and time of incubation of reaction mixtures in the assay. The results (data not shown here) showed that reaction rates were consistent with linearity of metabolism occurring in the first 15min, at microsomal protein and substrate concentrations of 0.5mg/ml and 5 μM, respectively. Hence these conditions were used for the present study.
Before conducting metabolism studies, microsomes were thawed at room temperature and 120 μL of the microsomal pellet resuspended in TKM buffer (final protein concentration 0.5 mg/mL) were added to 5 mL of cocktail containing NADPH (0.72 mM), EDTA (100 mM), KPO4 (100 mM), MgCl2 6H2O (3.75 mM). Samples per animal were preincubated for 2 min at 37°C in a tissue shaker (Precision Scientific Instruments, Chicago, IL). Treatment consisted of exposure in vitro to 3μM BaP (CAS No. 50-32-8; 98% pure, Sigma) or FLA (CAS No. 206-44-0; 98% pure, Sigma) dissolved in dimethyl sulfoxide. After a 15 min incubation at 37°C, the reaction was stopped with 8 mL of ethyl acetate containing butylated hydroxytoluene (0.2 mg/mL). Benzo(a)pyrene/fluoranthene metabolites were extracted twice with ethyl acetate and the extracts were analyzed by reverse phase HPLC for BaP/FLA metabolites as described previously (Ramesh et al., 2001, Walker et al. 2006). Metabolite standards for these two toxicants were obtained from the National Cancer Institute Chemical Carcinogen Repository (Midwest Research Institute, Kansas City, MO).
The two sets of BaP and FLA incubation experiments (FLA in the presence of BaP and vice versa) were not done simultaneously, but at different times. Controls were processed in parallel with experimental samples under identical assay conditions. Dimethyl sulfoxide [vehicle for FLA/BaP] was used as a negative control while reaction mixtures without FLA or BaP or microsomes served as positive controls. The rates of metabolism [formation of FLA/BaP metabolites] were expressed as pmol of product formed per min per mg of microsomal protein. Since FLA or BaP and their metabolites are suspected carcinogens, they were handled in accordance with NIH guidelines for preventing exposure of laboratory personnel to this chemical (NIH, 1981).
Statistical analysis
Data on microsomal mean FLA/ BaP metabolite concentrations were analyzed by ANOVA with repeated measures and the differences among means were tested for significance using the Openstat 3 software (an open source statistics package developed by William G. Miller, Iowa State University).
Results
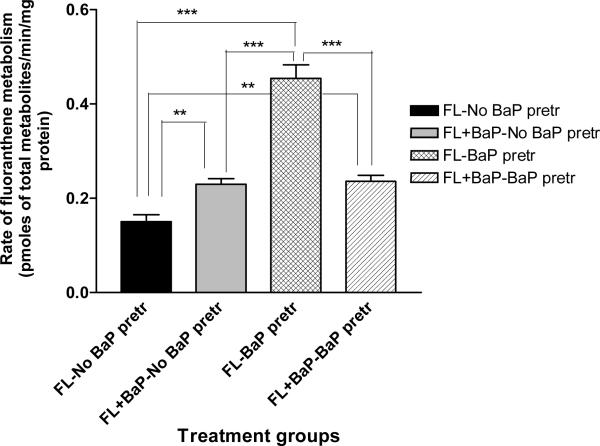
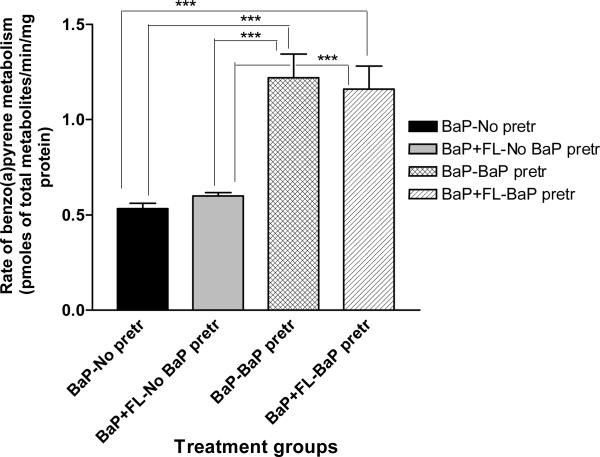
The total concentrations of FLA/ BaP metabolites produced by the mouse adipose tissue microsomes were shown in Figures 1 & 2. Microsomes from BaP-pretreated mice when incubated with FLA alone generated significantly greater (p < 0.05) concentrations of FLA metabolites when compared to microsomes from the other treatment categories (FLA with no BaP pre-treatment; FLA + BaP with no BaP pre-treatment; FLA + BaP with BaP pre-treatment; Figure 1). Similarly, microsomes from BaP-pretreated mice when incubated with BaP alone generated significantly greater (p < 0.05) concentrations of BaP metabolites when compared to other treatment categories (BaP with no BaP pre-treatment; BaP + FLA with no BaP pre-treatment; BaP + FLA with BaP pre-treatment; Figure 2).
Figure 1.
Rate of fluoranthene metabolism in adipose microsomes of ApcMin mice pre-exposed to benzo(a)pyrene. Explanations of the abbreviations are as follows; FL-no BaP pretr: FLA metabolite concentrations from microsomes of unexposed mice incubated with 3μM FLA alone; FL + BaP-no BaP pretr: FLA metabolite concentrations from microsomes of unexposed mice incubated with 3μM FLA + 3μM BaP; FL-BaP pretr: FLA metabolite concentrations from microsomes of BaP (50 μg/kg)-pretreated mice incubated with 3μM of FLA alone; FLA + BaP-BaP pretr: FLA metabolite concentrations from microsomes of BaP (50 μg/kg)-pretreated mice incubated with 3μM each of FLA and BaP. Values are expressed as mean concentration of total metabolites ± SE. Data from triplicate determination (variability was <10%) of five individual animals from each treatment group were compared. Asterisks denote statistical significance (*** p < 0.001, ** p < 0.01) in FLA metabolite concentrations among the treatment categories.
Figure 2.
Rate of benzo(a)pyrene metabolism in adipose microsomes of ApcMin mice pre-exposed to benzo(a)pyrene. Explanations of the abbreviations are as follows; BaP-no pretr: BaP metabolite concentrations from microsomes of unexposed mice incubated with 3μM BaP alone; BaP + FL-no BaP pretr: BaP metabolite concentrations from microsomes of unexposed mice incubated with 3μM FLA + 3μM BaP; BaP-BaP pretr: BaP metabolite concentrations from microsomes of BaP (50 μg/kg)-pretreated mice incubated with 3μM of BaP alone; BaP + FLA-BaP pretr: BaP metabolite concentrations from microsomes of BaP (50 μg/kg)-pretreated mice incubated with 3μM each of BaP and FLA. Values are expressed as mean concentration of total metabolites ± SE. Data from triplicate determination (variability was <10%) of five individual animals from each treatment group were compared. Asterisks denote statistical significance (*** p < 0.001, ** p < 0.01) in BaP metabolite concentrations among the treatment categories.
To see whether the quantitative differences in BaP and FLA metabolite concentrations in the above-mentioned incubation regimens were also extended to qualitative differences, we have identified the respective metabolites of the above-mentioned toxicants in all the treatment categories.
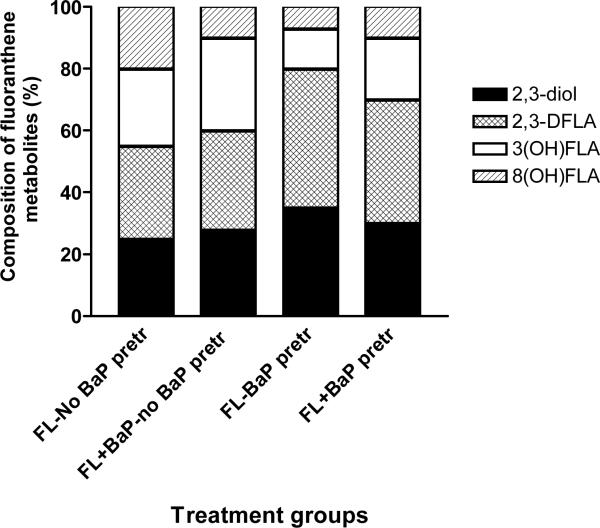
The FLA metabolites generated from adipose tissue microsomes were FLA 2,3-diol, trans-2, 3-dihydroxy-1, 10b-epoxy-1, 2, 3, l0b tetrahydro FLA (2, 3 D FLA), 3-hydroxy FLA, and 8-hydroxy FLA. The relative distribution (concentration) of individual FLA metabolites among total metabolites was represented as percentages and shown in Figure 3. Microsomes from BaP-pretreated mice produced considerably higher proportion of FLA 2, 3-diol, and 2, 3 D FLA when microsomes were incubated with FLA alone and FLA in combination with BaP when compared to microsomes from mice that received no prior BaP treatment.
Figure 3.
Metabolite composition of fluoranthene in adipose microsomes of ApcMin mice pre-exposed to benzo(a)pyrene. Values are expressed as the percentage of individual metabolite types among the total metabolites (sum of individual concentrations of FLA2, 3-diol, trans-2,3-dihydroxy-1,10b-epoxy-1,2,3,10b tetrahydro FLA [2, 3D FLA], 3-hydroxy FLA, and 8-hydroxy FLA) formed (n = 5 for each treatment category). Explanations of the abbreviations are as follows; FL-no BaP pretr: FLA metabolite types from microsomes of unexposed mice incubated with 3μM FLA alone; FL + BaP-no BaP pretr: FLA metabolite types from microsomes of unexposed mice incubated with 3μM FLA + 3μM BaP; FL-BaP pretr: FLA metabolite types from microsomes of BaP (50 μg/kg)-pretreated mice incubated with 3μM of FLA alone; FLA + BaP-BaP pretr: FLA metabolite types from microsomes of BaP (50 μ/kg)-pretreated mice incubated with 3μM each of FLA and BaP.
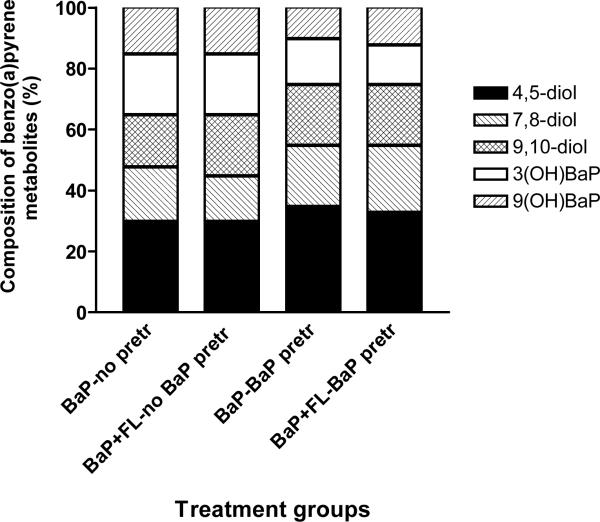
The BaP metabolites produced from adipose tissue microsomes were BaP 9, 10-diol, BaP 4,5-diol, BaP 7, 8-diol, 3-hydroxy and 9-hydroxy BaP. The relative distribution (concentration) of individual BaP metabolites among total metabolites represented as percentages is shown in Figure 4. Microsomes from BaP-pretreated mice produced considerably higher proportion of BaP 9, 10-diol, BaP 4,5-diol, and BaP 7, 8-diol when microsomes were incubated with BaP alone and BaP in combination with FLA when compared to microsomes from mice that received no prior BaP treatment.
Figure 4.
Metabolite composition of benzo(a)pyrene in adipose microsomes of ApcMin mice pre-exposed to benzo(a)pyrene. Values are expressed as the percentage of individual metabolite types among the total metabolites (sum of individual concentrations of BaP 9, 10-diol; BaP 4,5-diol; BaP 7,8-diol; 3-hydroxy and 9-hydroxy BaP) formed (n = 5 for each treatment category). Explanations of the abbreviations are as follows; BaP-no pretr: BaP metabolite types from microsomes of unexposed mice incubated with 3μM BaP alone; BaP + FL-no BaP pretr: BaP metabolite types from microsomes of unexposed mice incubated with 3μM FLA + 3μM BaP; BaP-BaP pretr: BaP metabolite types from microsomes of BaP (50 μg/kg)-pretreated mice incubated with 3μM of BaP alone; BaP + FLA-BaP pretr: BaP metabolite types from microsomes of BaP (50 μg/kg)-pretreated mice incubated with 3μM each of BaP and FLA.
Discussion
The concentrations of BaP or FLA (3μM) used in the present study are higher than the actual concentrations (0.03–0.09 ng/g wet tissue) reported for healthy humans (Beach et al., 2000; Goldman et al., 2001). Nonetheless, these concentrations were chosen because of their relevance to environmental levels of these PAHs in hazardous waste sites, former industrial sites, and exposure concentrations for “at risk” populations such as smokers and occupationally exposed individuals (IPCS, 1998; Ramesh et al., 2004).
Unlike BaP, the absence of a bay region in FLA (Phillips & Grover, 1994) affects the binding of this compound to the Ah receptor (Sjögren et al., 1996), which mediates the induction of cytochrome P450 enzymes. Therefore, FLA requires metabolic activation to become biologically reactive from a toxicity/carcinogenesis standpoint; hence BaP was used as an inducer prior to FLA exposure.
The lack of a significant difference in metabolite concentrations between the FL + BaP –no BaP pre-treatment and BaP pre-treatment group indicates that the PAH substrates may compete for the same enzymes that metabolizes these chemicals. Similarly, there was no significant difference in metabolite concentrations between BaP + FLA –no BaP pre-treatment and BaP alone without any pre-treatment groups. Also, the difference in metabolite concentrations between BaP alone with BaP-pretreatment group and BaP + FLA with BaP pretreatment groups was not significant. These results further suggest that BaP pretreatment may be beneficial when FLA alone is incubated without BaP because of competitive inhibition of FLA by BaP.
The differential metabolic behavior of BaP and FLA could be explained by the relative ability of these toxicants to induce drug metabolizing enzyme (cytochrome P450) activity, with BaP apparently showing more potency. Fluoranthene has been reported to be a weak inducer of monooxygenase enzymes; at least 100 times less effective compared to BaP in rodent liver microsomes (vanDuuren and Goldschmidt, 1976). Cytosolic receptor binding affinity (Piskorska-Pliszczynska et al., 1986) and rat hepatoma cell bioassays (Willett et al., 1997) also revealed that FLA was not a potent inducer of aryl hydrocarbon hydroxylase and 7-ethoxyresorufin O-deethylase activities, respectively.
The induction of drug metabolizing enzymes by subchronic exposure to BaP may have enhanced microsomal metabolism of FLA, as seen when microsomes from mice that have not received any BaP were exposed to FLA alone versus microsomes from mice that received prior BaP exposure. The BaP-induced effect was also evident when it was co-exposed with FLA. This characteristic explains the synergistic responses observed when various PAH compounds were co-exposed with BaP. Benzo(a)pyrene was found to act as a comutagen in a binary mixture of BaP and benzo(e)pyrene in the Salmonella typhimurum strain TA98/microsome assay (Haas et al., 1981). In mouse skin bioassays, mixtures of anthracene, chrysene, pyrene, fluoranthene and phenanthrene were reported to have a greater tumor latency period compared to a decreased latency period when BaP was added to the mixture (Warshawsky et al., 1993). Binary mixtures of dibenzo(a,e)pyrene, dibenzo[a,l]pyrene, dibenzo[a,h]anthracene, benzo[b]fluoranthene, fluoranthene or 1-methylphenanthrene with BaP showed synergistic interaction in DNA binding potencies compared to individual compounds (Hughes and Phillips, 1990). On the other hand, binary or ternary mixtures of BaP, anthracene and chrysene were reported to reduce the renal cytotoxic potential of individual compounds (Falahatpisheh et al., 2004). These findings lend credence to our supposition that the presence of another PAH compound in a mixture will have a bearing on the metabolism of the chemical in question.
Our observations that prior exposure to BaP induces the microsomal metabolism of BaP to precursors of reactive metabolites are in agreement with that of Vaca et al. (1992) who reported similar findings. Fluoranthene, however presents a contrasting picture in that though this PAH compound could be induced by BaP in our studies, but not in Vaca et al. (1992). The incongruity between our findings and Vaca et al. (1992) could be attributed to differences in the BaP dose used for induction of P450 monooxygenase activity (20 mg BaP/kg bw/day for 4 days by I.P. injection in Vaca et al., 1992 versus 50 μg BaP/kg bw/day for 60 days by oral gavage in our study), type of microsomes (hepatic microsomes of Vaca et al., 1992 versus adipose microsomes in our study), and exposure concentrations of BaP and FLA (100 μM for Vaca et al., 1992 versus 3 μM in our study). Also, the possibility of differences in modes of action at different doses for these toxicants cannot be ruled out.
The relative importance of various BaP and FLA metabolites detected and quantified in the binary exposure situation can be discussed in the context of their ability to induce toxicity or carcinogenesis.
Hydroxyfluoranthenes (3-,8-hydroxyfluoranthenes, and 2,3-dihydro-2,3-dihydroxy fluoranthene) are not mutagenic per se, but may be oxidized to potentially mutagenic metabolites such as dihydrodiols, mediated by the microsomal epoxide hydrolase. One of the metabolites, the FLA 2,3-diol undergoes metabolism to form FLA 2,3-diol-1,10b-epoxides. These epoxides bind to DNA (Babson et al., 1986) and hemoglobin (Hutchins et al., 1988) to a considerable extent. The enhanced production of FLA diol metabolites in microsomal incubations of FLA in the presence of BaP indicate a greater potency of BaP as an inducer of drug metabolizing enzymatic activities.
Microsomal metabolism of BaP generates the trans-4,5-, trans-7,8-, and trans-9,10-dihydrodiols along with 3 and 9 (OH) BaP metabolites. The trans-dihydrodiols are oxidized to bay-region diol epoxides, which bind with DNA. For e.g., the 7,8-diol relative to 4,5-diol and 9,10-diol bind to DNA strongly and form stable DNA adducts, which perturb nuclear functions such as transcription and replication ultimately leading to neoplasia (Luch, 2006). Though the 3-and 9-hydroxy BaP are considered to be a part of the detoxification pathway, these metabolites of BaP have been implicated in DNA damage, contributing to unscheduled DNA synthesis (Chen et al., 2000).
The FLA and BaP metabolite types identified in our study are in accord with published literature, which used microsomes from intestinal, hepatic and extra-hepatic tissues (Walker et al., 2006; Smith et al., 2007; Harris et al., 2009b). However, the variation in proportion of specific BaP/FLA metabolic types generated from the adipose microsomes could be attributed to the competition for microsomal metabolizing enzymes and the relative stability of the metabolites of one compound versus the other. In real world-complex PAH mixtures, BaP and FLA may not present in the same relative proportion as were used in laboratory studies. This limitation notwithstanding, as a result of enhanced biotransformation of one compound versus the other, the reactive metabolites that were generated in greater proportion may have the potential to react with cellular macromolecules leading to toxicity or carcinogenesis (Staal et al., 2007). From a human health standpoint, the individual and interactive toxicity of PAHs that stem from the plethora of metabolites they generate may have implications as these compounds are emanated from the Superfund sites and their metabolites possess immunosuppressive characteristics (Silkworth et al., 1995). Future studies will use animal models to target the mechanisms that underlie the differential metabolic behavior of BaP and FLA mixtures.
Acknowledgement
This research was supported by the National Institutes of Health (NIH) grants 1S11ES014156-01A1 (AR), 1RO3CA130112-01 (AR), 5T32HL007735-12 (Meharry) and 1F31ES017391-01 (DLH).
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Babson JR, Russo-Rodriguez S, Rastetter WH, Wogan GN. Invitro DNA-binding of microsomally-activated fluoranthene: evidence that the major product is a fluoranthene N2 – deoxyguanosine adduct. Carcinogenesis. 1986;7:859–865. doi: 10.1093/carcin/7.6.859. [DOI] [PubMed] [Google Scholar]
- Beach JB, Pellizzari E, Keever JT, Ellis L. Determination of benzo(a)pyrene and other polycyclic aromatic hydrocarbons (PAHs) at trace levels in human tissues. J. Anal. Toxicol. 2000;24:670–677. doi: 10.1093/jat/24.8.670. [DOI] [PubMed] [Google Scholar]
- Bradford M. A rapid and sensitive method for the quantification of microgram quantities of protein, utilizing the principle of dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cassee FR, Groten JP, van Bladeren PJ, Feron VJ. Toxicological evaluation and risk assessment of chemical mixtures. Crit. Rev. Toxicol. 1998;28:73–101. doi: 10.1080/10408449891344164. [DOI] [PubMed] [Google Scholar]
- Chen JK, Wu ZL, Liu YG, Lei YX. Effects of metabolites of benzo(a)pyrene on unscheduled DNA synthesis in BALB/3T3 cell line. Chemosphere. 2000;41:139–142. doi: 10.1016/s0045-6535(99)00401-4. [DOI] [PubMed] [Google Scholar]
- Ekins S, Ring BJ, Grace J, McRobie-Belle DJ, Wrighton SA. Present and future invitro approaches for drug metabolism. J. Pharmacol. Toxicol. Meth. 2000;44:313–324. doi: 10.1016/s1056-8719(00)00110-6. [DOI] [PubMed] [Google Scholar]
- EPA Integrated Risk Information System (IRIS) National Center for Environmental Assesment. 2001 Available at: http://www.epa.gov/iris/index.html; last accessed May 2003. [Google Scholar]
- Falahatpisheh MH, Kerzee JK, Metz RP, Donnelly KC, Ramos KS. Inducible cytochrome P450 activities in renal glomerular mesangial cells: biochemical basis for antagonistic interactions among nephrocarcinogenic polycyclic aromatic hydrocarbons. Journal of Carcinogenesis. 2004;3:12. doi: 10.1186/1477-3163-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galván N, Teske DE, Zhou G, Moorthy B, MacWilliams PS, Czuprynski CJ, Jefcoate CR. Induction of CYP1A1 and CYP1B1 in liver and lung by benzo(a)pyrene and 7,12-dimethylbenz(a)anthracene do not affect distribution of polycyclic hydrocarbons to target tissue: role of AhR and CYP1B1 in bone marrow cytotoxicity. Toxicol. Appl. Pharmacol. 2005;202:244–257. doi: 10.1016/j.taap.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Goldman R, Enewold L, Pellizzari E, Beach JB, Bowman ED, Krishnan SS, Shields PG. Smoking increases carcinogenic polycyclic aromatic hydrocarbons in human lung tissue. Cancer Res. 2001;61:6367–6371. [PubMed] [Google Scholar]
- Haas BS, Brooks EE, Schumann KE, Dornfeld SS. Synergistic, additive, and antagonistic mutagenic responses to binary mixtures of benzo(a)pyrene and benzo(e)pyrene as detected by strains TA98 and TA100 in the Salmonella/microsome assay. Environ. Mutagen. 1981;3:159–166. doi: 10.1002/em.2860030207. [DOI] [PubMed] [Google Scholar]
- Harris DL, Washington MK, Hood DB, Roberts LJ, II, Ramesh A. Dietary fat-influenced development of colon neoplasia in ApcMin mice exposed to benzo(a)pyrene. Toxicologic Pathol. 2009a;37:938–946. doi: 10.1177/0192623309351722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DL, Huderson AC, Niaz MS, Ford JJ, Archibong AE, Ramesh A. Comparative metabolism of benzo(a)pyrene by ovarian microsomes of various species. Environ Toxicol. 2009b;24:603–609. doi: 10.1002/tox.20461. [DOI] [PubMed] [Google Scholar]
- Hughes NC, Phillips DH. Covalent binding of dibenzpyrenes and benzo[a]pyrene to DNA: evidence for synergistic and inhibitory interactions when applied in combination to mouse skin. Carcinogenesis. 1990;11:1611–1619. doi: 10.1093/carcin/11.9.1611. [DOI] [PubMed] [Google Scholar]
- Hutchins DA, Skipper PL, Naylor S, Tannenbaum SR. Isolation and characterization of the major fluoranthene-hemoglobin adducts formed in vivo in the rat. Cancer Res. 1988;48:4756–4761. [PubMed] [Google Scholar]
- ILAR . Guide for the care and use of laboratory animals. The National Academies Press; Washington DC: 1996. p. 140. [PubMed] [Google Scholar]
- IPCS . Environmental Health Criteria 202: Selected non-heterocyclic polycyclic aromatic hydrocarbons. International Programme on Chemical Safety, World Health Organization; Lyon, France: 1998. [Google Scholar]
- Knuckles ME, Inyang F, Ramesh A. Acute and subchronic oral toxicity of benzo(a)pyrene in F-344 rats. Tox. Sci. 2001;61:382–388. doi: 10.1093/toxsci/61.2.382. [DOI] [PubMed] [Google Scholar]
- Knuckles ME, Inyang F, Ramesh A. Acute and subchronic oral toxicity of fluoranthene in F-344 rats. Ecotoxicol. Environ. Saf. 2004;59:102–108. doi: 10.1016/S0147-6513(03)00110-6. [DOI] [PubMed] [Google Scholar]
- Kistos CP, Edler L. Cancer risk assessment for mixtures. In: Edler L, Kistos CP, editors. Recent Advances in Quantitative Methods in Cancer and Human Health Risk Assessment. John Wiley & Sons Ltd.; England: 2005. pp. 283–298. [Google Scholar]
- Krishnamurthi K, Devi SS, Chakrabarti T. The genotoxicity of priority polycyclic aromatic hydrocarbons (PAHs) containing sludge samples. Toxicol. Mech. Method. 2007;17:1–12. doi: 10.1080/15376510600943676. [DOI] [PubMed] [Google Scholar]
- Luch A, editor. The carcinogenic effects of polycyclic aromatic hydrocarbons. Imperial College Press; London: 2006. p. 489. [Google Scholar]
- Moir D, Viau A, Chu I, Withey J, McMullen E. Pharmacokinetics of benzoz(a)pyrene in the rat. J. Toxicol. Environ HealthA. 1998;53:507–530. doi: 10.1080/009841098159114. [DOI] [PubMed] [Google Scholar]
- NIH National Institutes of Health, US Government Printing Office; Washington, DC: Guidelines for the laboratory use of chemical carcinogens. 1981 NIH Publication No. 81-2385.
- Phillips DH, Grover PL. Polycyclic hydrocarbon activation: bay regions and beyond. Drug Metab Rev. 1994;26:443–467. doi: 10.3109/03602539409029808. [DOI] [PubMed] [Google Scholar]
- Piskorska-Pliszczynska J, Keys B, Safe S, Newman MS. The cytosolic receptor binding affinities and AHH induction potencies of 29 polynuclear aromatic hydrocarbons. Toxicol. Lett. 1986;34:67–74. doi: 10.1016/0378-4274(86)90146-3. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Inyang F, Hood DB, Knuckles ME. Aryl hydrocarbon hydroxylase activity in F-344 rats subchronically exposed to benzo(a)pyrene and fluoranthene through diet. J. Biochem. Mol. Toxicol. 2000;14:155–161. doi: 10.1002/(sici)1099-0461(2000)14:3<155::aid-jbt5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Inyang F, Hood DB, Archibong AE, Knuckles ME, Nyanda AM. Metabolism, bioavailability, and toxicokinetics of benzo(a)pyrene in F344 rats following oral administration. Exp. Toxic. Pathol. 2001;53:275–290. doi: 10.1078/0940-2993-00192. [DOI] [PubMed] [Google Scholar]
- Ramesh A, Walker SA, Hood DB, Guillen MD, Schneider H, Weyand EH. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons (review) Int. J. Toxicol. 2004;23:301–333. doi: 10.1080/10915810490517063. [DOI] [PubMed] [Google Scholar]
- Sattar A, Hewer A, Phillips DH, Campbell FC. Metabolic proficiency and benzo(a)pyrene DNA adduct formation in ApcMin mouse adenomas and uninvolved mucosa. Carcinogenesis. 1999;20:1097–1101. doi: 10.1093/carcin/20.6.1097. [DOI] [PubMed] [Google Scholar]
- Schenkman JB, Jansson I. Measurement of cytochrome P450. In: Maines MD, Costa LG, Hodgson E, Reed DJ, Sipes IG, editors. Current Protocols in Toxicology. John Wiley; Hoboken, New Jersey: 1999. pp. 4.1.1–4.1.14. [Google Scholar]
- Silkworth JB, Lipinskas T, Stoner CR. Immunosuppressive potential of several polycyclic aromatic hydrocarbons (PAHs) found at a Superfund site: new model used to evaluate additive interactions between benzo[a]pyrene and TCDD. Toxicology. 1995;105:375–386. doi: 10.1016/0300-483x(95)03235-8. [DOI] [PubMed] [Google Scholar]
- Sjogren M, Ehrenberg I, Rannug U. Relevance of different biological assays in assessing initiating and promoting properties of polycyclic aromatic hydrocarbons with respect to carcinogenic potency. Mut. Res. 1996;358:97–112. doi: 10.1016/0027-5107(96)00175-3. [DOI] [PubMed] [Google Scholar]
- Smith TL, Merry ST, Harris DL, Joe Ford J, Ike J, Archibong AE, Ramesh A. Species-specific testicular and hepatic microsomal metabolism of benzo(a)pyrene, an ubiquitous toxicant and endocrine disruptor. Toxicol In Vitro. 2007;4:753–758. doi: 10.1016/j.tiv.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Staal YCM, Hebels DGAJ, van Herwijnen MHM, Gottschalk RWH, van Schooten FJ, van Delft JHM. Binary PAH mixtures cause additive or antagonistic effects on gene expression but synergistic effects on DNA adduct formation. Carcinogenesis. 2007;28:2632–2640. doi: 10.1093/carcin/bgm182. [DOI] [PubMed] [Google Scholar]
- Vaca C, Tornquist M, Rannug U, Lindahl-Kiessling K, Ahnstrom G, Ehrenberg L. On the bioactivation and genotoxic action of fluoranthene. Arch. Toxicol. 1992;66:538–545. doi: 10.1007/BF01973383. [DOI] [PubMed] [Google Scholar]
- vanDuuren BL, Goldschmidt BM. Cocarcinogenic and tumor-promoting agents in tobacco carcinogenesis. J. Natl. Cancer Inst. 1976;56:1237–1242. doi: 10.1093/jnci/56.6.1237. [DOI] [PubMed] [Google Scholar]
- Walker SA, Whitten LB, Seals GB, Lee WE, Archibong AE, Ramesh A. Inter-species comparison of liver and small intestinal microsomal metabolism of fluoranthene. Food. Chem. Toxicol. 2006;44:380–387. doi: 10.1016/j.fct.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Walker SA, Addai AB, Mathis M, Ramesh A. Effect of dietary fat on metabolism and DNA adduct formation after acute oral exposure of F-344 rats to fluoranthene. J Nutr. Biochem. 2007;18:236–249. doi: 10.1016/j.jnutbio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Warshawsky D, Barkley W, Bingham E. Factors affecting carcinogenic potential of mixtures. Fundam. Appl. Toxicol. 1993;20:376–382. doi: 10.1006/faat.1993.1048. [DOI] [PubMed] [Google Scholar]
- Willett KL, Gardinali PR, Sericano JL, Wade TL, Safe SH. Characterization of the rat hepatoma cell bioassay for evaluation of environmental samples containing polynuclear aromatic hydrocarbons (PAHs) Arch. Environ. Contam. Toxicol. 1997;32:442–448. doi: 10.1007/s002449900211. [DOI] [PubMed] [Google Scholar]