Abstract
Tissue engineering creates biological tissues that aim to improve the function of diseased or damaged tissues. To enhance the function of engineered tissues there is a need to generate structures that mimic the intricate architecture and complexity of native organs and tissues. With the desire to create more complex tissues with features such as developed and functional microvasculature, cell binding motifs and tissue specific morphology, tissue engineering techniques are beginning to focus on building modular microtissues with repeated functional units. The emerging field known as modular tissue engineering focuses on fabricating tissue building blocks with specific microarchitectural features and using these modular units to engineer biological tissues from the bottom up. In this review we will examine the promise and shortcomings of “bottom-up” approaches to creating engineered biological tissues. Specifically, we will survey the current techniques for controlling cell aggregation, proliferation and extracellular matrix deposition, as well as approaches to generating shape-controlled tissue modules. We will then highlight techniques utilized to create macroscale engineered biological tissues from modular microscale units.
1. Introduction
There is a substantial unmet demand for tissues to repair injured, degenerated or congenitally defected tissues. The field of tissue engineering has emerged to fill the void where neither native physiology nor purely artificial implantable materials can sufficiently replace or repair these damaged tissues [1]. While tissues such as bone [2] or skin [3, 4] can effectively repair a small injury given sufficient time, many tissues such as myocardium [5] and cartilage [6] do not regenerate properly without intervention.
Traditional tissue engineering strategies typically employ a “top-down” approach, in which cells are seeded on a biodegradable polymeric scaffold, such as poly (glycolic acid) (PGA) [7–10] (Figure 1). In top-down approaches, the cells are expected to populate the scaffold and create the appropriate extracellular matrix (ECM) and microarchitecture often with the aid of perfusion [7], growth factors [8] and/or mechanical stimulation [9, 11]. However, despite advances in surface patterning [12, 13] or use of more biomimetic scaffolding, such as decellularized ECM templates [14], top-down approaches often have difficulty recreating the intricate microstructural features of tissues.
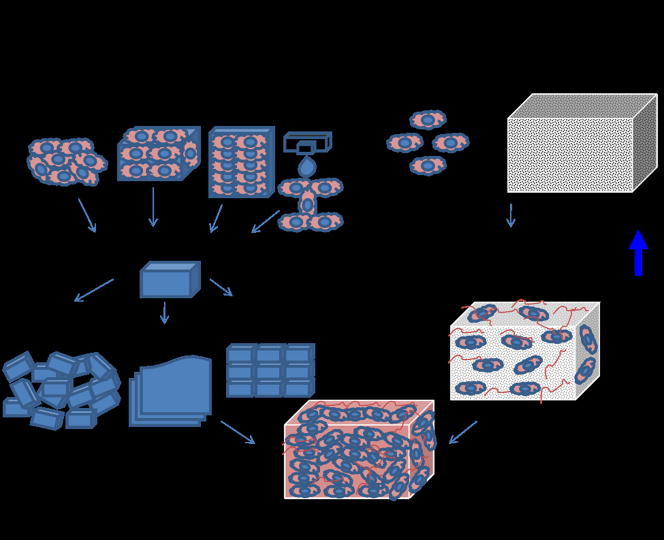
Figure 1.
Bottom-up & Top-down approaches to tissue engineering. In the bottom-up approach there are multiple methods for creating modular tissues, which are then assembled into engineered tissues with specific microarchitectural features. In the top-down approach, cells and biomaterial scaffolds are combined and cultured until the cells fill the support structure to create an engineered tissue.
Modular tissue engineering aims to address the challenge of recreating biomimetic structures by designing structural features on the microscale to build modular tissues that can be used as building blocks to create larger tissues (Figure 1). These modules can be created a number of ways, such as through self-assembled aggregation [15], microfabrication of cell-laden hydrogels [16], creation of cell sheets [17] or direct printing of tissues [18]. Once created, these modules can be assembled into larger tissues through a number of methods such as random packing [19–22], stacking of layers [17, 23] or directed assembly [24]. There is a strong biological basis for this bottom-up approach as many tissues are comprised of repeating functional units, such as the lobule in the liver [25]. By mimicking native microstructural functional units, bottom-up approaches aim to create more biomimetic engineered tissues.
The historical roots of the modular approach trace back to microencapsulation techniques, such as encapsulating pancreatic islet cells in alginate gels for transplantation in diabetic rats [26]. Although these studies were not conceived as modular tissue engineering, each cell-containing microcapsule behaves as a tissue module performing a particular function. Packing the microcapsules created an artificial biological structure, however traditional microencapsulation techniques did not create tissue-like structures and lacked the microvasculature and architecture of native tissues. Current techniques for creating modular tissues draw from research in microencapsulation and microfabrication as well as traditional cell and tissue culture procedures. By creating modular tissues with more physiological microarchitectural features, bottom-up tissue engineering aims to provide more guidance on the cellular level to direct tissue morphogenesis.
The following review will highlight the current techniques for creating modular engineered tissues using bottom-up tissue engineering principles. We will describe approaches to engineering modular tissues by classifying the techniques that utilize only cells and cell-produced materials as well as approaches using cell/biomaterial combinations. Subsequently, we will highlight the approaches used to direct the formation of larger engineered tissues from microscale building blocks.
2. Control of cell aggregation
Controlling cell aggregation is a key component of creating modular tissues with controlled microarchitecture. By restricting the geometry of cell aggregation, engineers can create more controlled environments to study cell behaviors (Figure 2, Panels A–C) [15, 27–29]. This control can be enacted in many ways, such as seeding cells in microwells [30] or channels [31], micromolding cells in hydrogels [16] or culturing cells in sheets [17, 23].
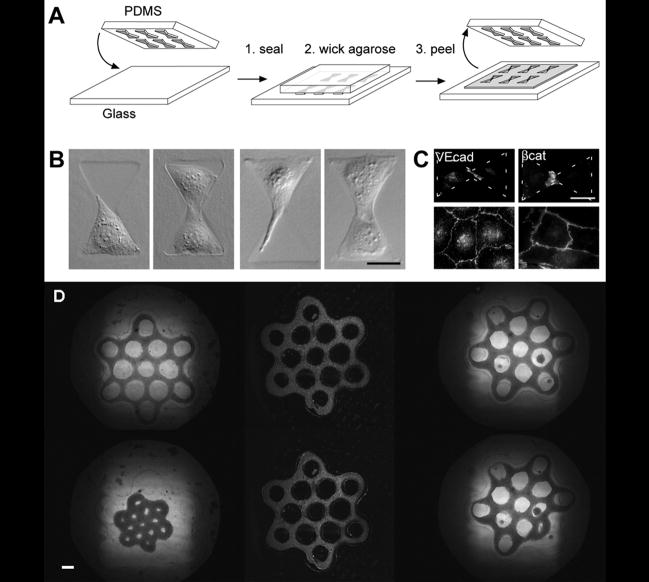
Figure 2.
Control of cell aggregation. Patterned stamps are used to create confined regions for cells to aggregate and interact (A, B, C), scale: 25 μm, reproduced with permission from Journal of Cell Science [29]. Use of larger geometric patterns such as connected tori (D) create modular tissues of specific cell and ECM architecture, scale = 400 μm [15]. Copyright 2007 by Fedn of Am Societies for Experimental Bio (FASEB). Reproduced with permission of Fedn of Am Societies for Experimental Bio (FASEB) in the format Journal via Copyright Clearance Center.
2.1 Micromolds as templates to control cell aggregation
Adaptation of similar techniques was used to create modular tissues by seeding cells in microwells to form spheroids made by primary [15] or stem cells [30] and their own secreted extracellular matrix (ECM). Researchers then created larger more intricate modular structures such as linear channels [31] to form more tissue-like structures. One major advantage of this technique is the ability to create modular tissues of specific shape and geometry where cells have created their own microarchitecture through ECM remodeling and cell migration/aggregation. However, one major limitation of this technique is that some cell types are unable to produce sufficient ECM, migrate or form cell-cell junctions.
Researchers in the Morgan group demonstrated that cell aggregation could be controlled to not only form spheroids [32], but also individual and connected tori [15] (Figure 2C). The resulting connected tori yielded honeycomb structures, demonstrating the potential for creating larger modular tissues of defined geometry using cells cultured in replica molds.
2.2 Sheet-based tissue modules
Another technique for creating modular tissues manipulates the cell culture environment to create modular cell sheets. Through optimizing the conditions leading to cell proliferation, and ECM production in cell culture, researchers have successfully created large cell sheet modules. By stacking these cell/ECM sheet modules and allowing the modules to remodel and interconnect over time, researchers have been able to create tissues with robust mechanical properties and cell-cell contacts. Using cell sheets as the building blocks to create engineered tissues layer-by-layer, researchers have successfully created functional blood vessels [17, 23], as well as in vivo patches to repair ocular [33], bladder [34] and other defects [35].
2.3. Cell-laden hydrogels
Since many cell types cannot produce sufficient ECM to create robust microtissues, researchers began mixing cells with natural and artificial hydrogels to form modular tissues of specific geometries and mechanical properties [36–42]. The most common techniques embed cells within photopolymerizable polymers such as poly (ethylene glycol) [43, 44] and other materials [45–47], temperature sensitive hydrogels [48] or self assembling peptide gels which can mimic ECM structure [38–42, 49]. To create microtissues, cells are mixed with hydrogel precursors, inserted into molds and polymerized either by using UV light [16] or incubation [50]. Alternatively cell-laden hydrogel modular tissues can be created directly by passing UV light through a photomask, initiating the crosslinking process within the polymer only within the areas where the UV light penetrates through the mask [51]. This technique bypasses the need for creating a mold and is also amenable with high throughput microfluidic techniques [24].
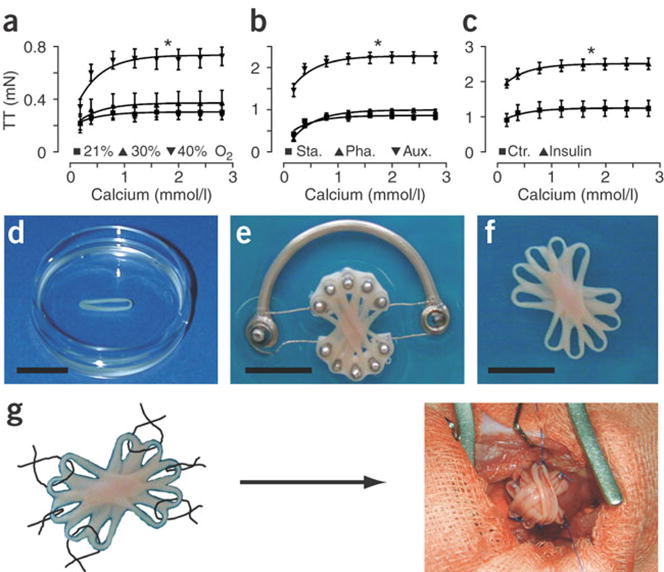
One example of this technique created modular rings of primary neonatal rat cardiac cells in collagen or matrigel. These rings were then further cultured in contact with multiple other engineered cardiac bands to form a composite graft, which could be conditioned to further improve cell alignment and contractile function (Figure 3). The resultant cardiac grafts significantly improved cardiac function in rat myocardial infarct models [5, 52, 53].
Figure 3.
Modular cardiac tissue engineering. Native cardiomyocytes are mixed with ECM (collagen or Matrigel) in ring shaped molds. These rings are then assembled together on a cyclic stretching devices to condition the rings and allow them to integrate with the other rings creating an aligned tissue for implantation on the cardiac surface, scale: 10mm [5]. Adapted by permission from Macmillan Publishers Ltd: Nature Medicine, copyright 2006.
3. Techniques for assembling engineered tissue building blocks
One of the major challenges of bottom-up tissue engineering is to assemble modular tissues with specific microarchitectures into macroscale biomimetic engineered tissues. The challenge is to retain the microarchitecture and cellular behavior of modular tissues, while creating engineered tissues with robust mechanical properties to withstand clinical implantation and interact appropriately with the native tissues. Some of the specific issues facing the field are: creating materials with biomimetic mechanical properties, integration of modular units to form robust integrated tissues, creation of functional microvasculature, improving manufacturing techniques to scale up production to tissue-level dimensions and successfully testing these tissues in the appropriate in vivo environment.
Here we highlight five examples of promising techniques for creating and assembling modular tissues into engineered biological tissues, examining the advantages and disadvantages of each technique. These techniques highlight the most current research in creating modular tissues with improved higher order structures and assembly, functional vasculature, and the ability to reliably manufacture and evaluate these tissues in vivo.
3.1 Additive photocrosslinking of cell-laden hydrogels
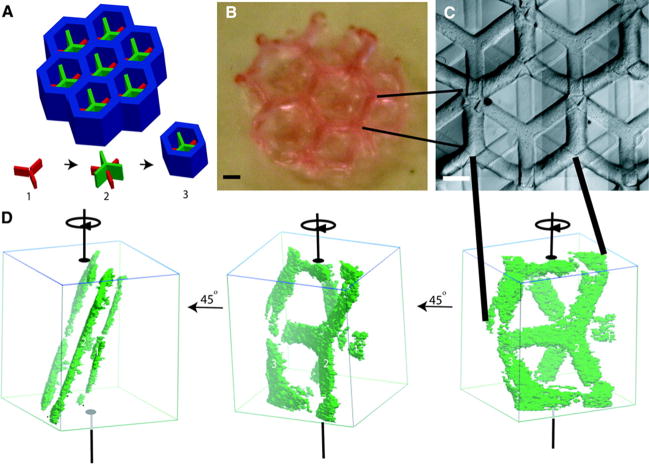
Additive photocrosslinking uses 2D arrays of cells and ECM/polymers as the building blocks to create tissues layer-by-layer [54, 55]. Using additive photopolymerization, dual layer modular tissues were created to fabricate living tissues of cellular hydrogels for hepatic tissue engineering. Hepatocytes were isolated from adult female Lewis rats then mixed with liquid PEG functionalized with RGD motifs. This mixture was inserted into a sealed chamber and polymerized by UV exposure through a photomask in the shape of an array of 3 pointed stars (Figure 4A1). The spacer height was then doubled and a 2nd layer was created on top of the first extending the full spacer height (Figure 4A2). Finally the spacer was increased again, and the mask was changed to create a honeycomb enclosure around each individual 2-layer unit to create an engineered hepatic microtissue. This technique successfully increased hepatocyte adhesion and urea production with the created microarchitecture combined with the RGD motif.
Figure 4.
Hepatic tissue engineering using additive photopolymerization. Multiple layers are deposited sequentially (red, then green, then blue, A) to create multilayer hepatic tissues (B), scale: 1mm, (C), scale: 500 μm, (D) [25]. Copyright 2007 by Fedn of Am Societies for Experimental Bio (FASEB). Reproduced with permission of Fedn of Am Societies for Experimental Bio (FASEB) in the format Journal via Copyright Clearance Center.
While this technique successfully creates modular tissues with geometries similar to the native hepatocyte environment, one potential drawback of this technique is the inability to restrict the thickness of subsequent layers. As the cell-hydrogel mixture must fill the entire volume of the chamber, and it is not possible to restrict the depth of UV polymerization, each newly created layer must be the full height of the chamber, potentially limiting the number of layers achievable. However, if the goal is to create layered structures of the same geometrical pattern, this restriction would be of minimal impact.
3.2 Random packing of encapsulated modules
A major limitation in many engineered tissues is the lack of functional microvasculature to ensure proper blood perfusion and connection with surrounding tissues. Despite progress in using microfabrication techniques to improve the vascularization of engineered tissues [56, 57] this limitation is still present in many engineered tissues.
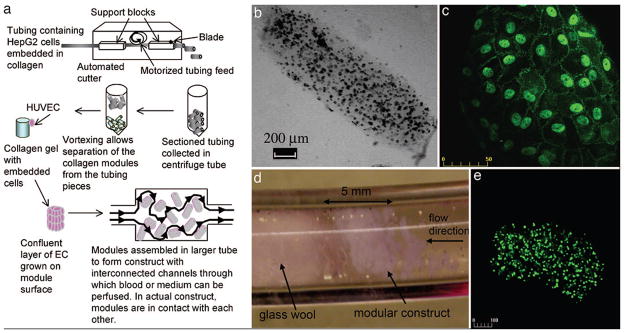
In an alternative approach to creating capillary networks, a random packing system was developed to create tortuous channels of perfusable modular tissues. Cylindrical, perfused tissues were created by assembling cell-laden collagen units together within perfused tubing [19–22]. First, modular constructs were created by mixing collagen with or without HepG2 cells and allowing the mixture to reach gelation to create cylindrical tissue modules. The gels were then collected in media and seeded with HUVEC cells until a confluent layer formed around the modules. The HUVEC-HepG2 modules were then collected into tubing with a porous plug then perfusion was initiated to allow the cell-laden modules to compact, remodel and form a porous, perfusable tissue. Tissue constructs were perfused with whole blood to demonstrate the ability to withstand clotting, while maintaining high viability of the HUVEC and encapsulated HepG2 cells (figure 5).
Figure 5.
Assembly of modular tissues in perfusable engineered tissues. HepG2 cells are encapsulated in collagen, cast into modular units then coated with a confluent layer of HUVEC cells and packed inside a perfused tube (A). The resultant tissue consists of interconnected cell modules (B–E) which are perfusable and non-coagulative due to the endothelial (HUVEC) cells [20].
This simple solution to creating a perfusable tissue would be very effective in tissues whose primary function is filtration, such as the liver or kidney, especially when envisioned as an external device or a self-contained implantable device. However, as a technique for creating microvasculature there are potential shortcomings such as the lack of mechanical integrity in the absence of an enclosure. Also, as the secondary cell types must persist and remain effective when fully encapsulated by endothelial cells this could potentially limit the secondary cell types for this application.
3.3 Tissue printing
Similar in concept to additive photopolymerization, organ printing creates 2-D arrays of cells and ECM/polymer [42] which can be built layer by layer into tissues [18, 58–60]. While photopolymerization creates layers all at once, organ printing deposits cells in small groups using similar technology to traditional printing systems. The advantage of this technique is the high potential level of control in cell and ECM placement and alignment to create engineered tissues with a wide array of properties and geometries.
Replacing ink with cells suspended in liquid ECM or self assembled ECM mimics, a specific pattern is designed and printed onto a substrate, then the resultant cell aggregate is cultured to allow the cells and ECM to integrate into tissue structures. This technique can be used to create individual patterned cell groups, or more intricate 2D arrays of cells (Figure 6) [18, 42]. In addition, it is possible to print subsequent layers once the printed layers have sufficient culture time, creating 3D arrays of cells and tissues.
Figure 6.
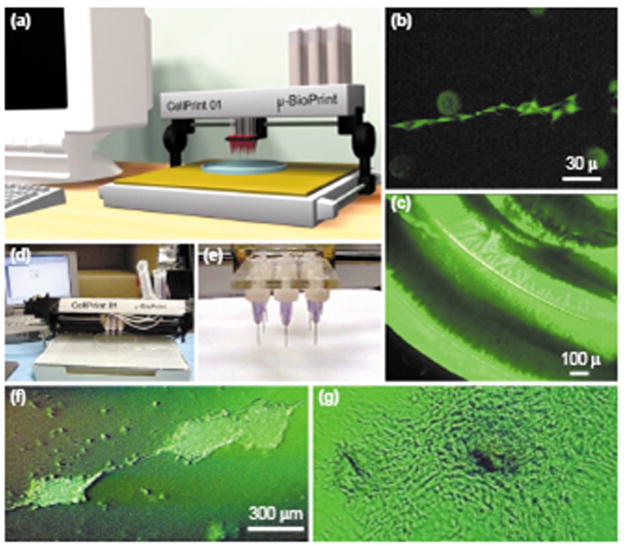
Organ printing. The desired pattern is input into the computer attached to the cell printing device (A, D, E), individual cells and ECM are deposited (B) in larger geometrical patterns (C, F, G) [18]. Reprinted from TRENDS in Biotechnology, 21/4, Mironov, V., T. Boland, T. Trusk, G. Forgacs and R.R. Markwald, Organ printing: computer-aided jet-based 3D tissue engineering, Pages 157–61, Copyright (2003), with permission from Elsevier.
While this technique could be effective in creating modular 2D tissues, one disadvantage of this technique is the impact on cell viability in the time elapsed between creation of subsequent tissue layers. Also, as in sheet based tissue engineering, layer to layer binding and integrity could be difficult to achieve. Additionally, not all cell types will respond positively to the conditions potentially required for this technique.
3.4 Directed assembly of tissue modules
Recent research from our group has demonstrated the ability to use directed assembly techniques to create higher order tissue structures using UV crosslinked hydrogel modular microtissues [24]. This technique addresses the challenges of creating higher order structures when the tissue modules used are fragile or otherwise difficult to manipulate, while also suggesting potential methods for microfluidic or automated techniques for creating engineered tissues from modular microtissues.
By harnessing the surface tension characteristics of hydrophilic hydrogels we assembled modular tissues into higher order structures (Figure 7). First, cell-laden rectangular hydrogels were created in different aspect ratios through photopolymerization with a photomask. These microtissue modules were then collected in hydrophobic mineral oil causing the hydrophilic hydrogels to aggregate together to form tissues of varying dimensions, which could then be solidified through a secondary UV polymerization step. Varying the aspect ratio of the modules demonstrated the ability to control the ultimate size and shape of the resulting aggregate tissues as the number of hydrogels per tissue increased proportionally with the module aspect ratio. Future applications of more intricate structures were demonstrated with lock and key shapes, indicating the potential versatility of this technique.
Figure 7.
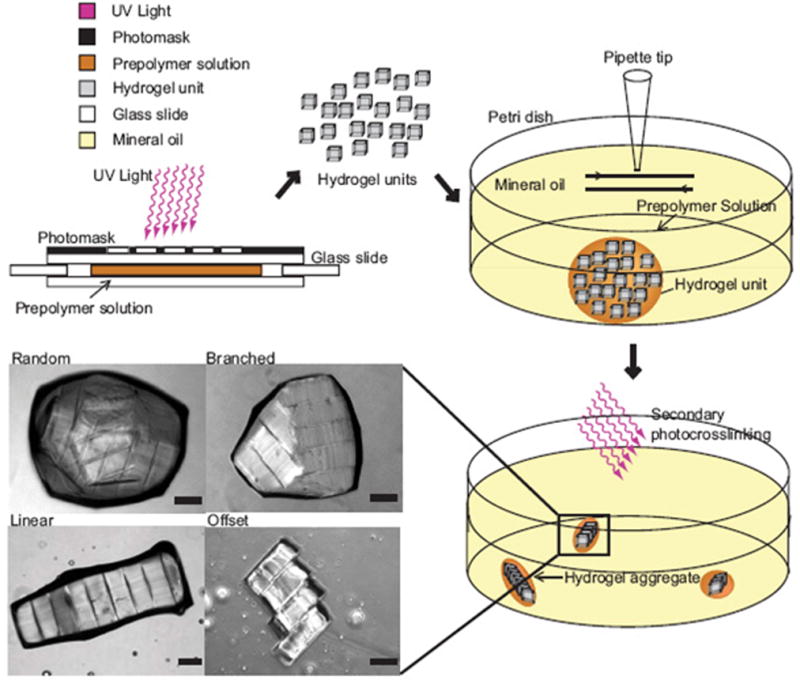
Directed assembly of modular tissues. Rectangular cell/hydrogel modules were created directly by photopolymerization using UV through a photomask, then allowed to aggregate and self-assemble in a hydrophobic media (mineral oil), scale: 200μm. After assembly a 2nd UV polymerization solidified the structures, which varied in size and organization based on the module geometry [24].
Some potential advantages of this system are the ability to reproducibly direct the structure of tissues based on the module geometry without the need for complicated assembly and manipulation procedures. However, there are practical limits to the size of structures achievable, and as seen in figure 7, the potential for random or uncontrolled structures still exists. The development of controlled bioreactors, as well as optimal module sizes and shapes, are promising approaches to solve some of these challenges.
3.5 Cell sheet technologies
One technique that has successfully addressed the insufficient mechanical properties and cell alignment in engineered modular tissues is cell sheet engineering. By creating sheets of cells under conditions that encourage ECM production, engineered modular tissues made using these techniques have shown mechanical properties on the order of native tissues [17].
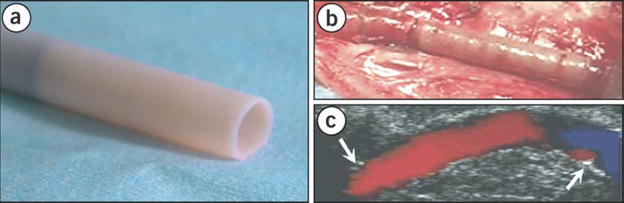
One of the pioneering works in cell sheet tissue engineering created blood vessels over a decade ago [17]. In this work, smooth muscle cells and fibroblasts were cultured separately under overconfluent conditions. Over time the cells had deposited sufficient ECM to be able to create sheets of smooth muscle and fibroblasts, which were then rolled around a cylindrical mandrel and cultured for at least 8 weeks. The resultant construct was then seeded with endothelial cells to yield vascular-like tissues. In early studies, vessels were validated in a canine model, demonstrated burst strength similar to saphenous veins and were responsive to vasoactive agents ex vivo [61]. Newer techniques using only fibroblast sheets and endothelial cells from human patients (now under the company Cytograft®) have shown great promise as dialysis access grafts in human trials [23, 62] (Figure 8).
Figure 8.
Creation of human blood vessel from cell sheet technology. Fibroblast cells are harvested from the patient, expanded into cell sheets, wrapped and cultured around a cylindrical mandrel to create robust, blood vessels (A). After seeding w/harvested endothelial cells, the grafts were tested as AV shunts for dialysis patients (B, C), where they performed well through multiple puncture wounds [23, 62]. Adapted by permission from Macmillan Publishers Ltd: Nature Medicine, copyright 2006.
The major advantages of this technique are the native-like mechanical properties, combined with the entirely biological makeup of the sheets and tissues achievable from small biopsies. In addition the created engineered tissues have shown significant success in clinical applications in animal, primate and human trials. Some potential limitations, however, are the limited possible geometries that can be fabricated using sheets as well as reported leaking between layers indicating insufficient joining between layers [17]. In addition, cell sheets can only be created by cells that are both proliferative and produce sufficient ECM.
4. Conclusions and future directions
While some of the techniques outlined in this manuscript to control the microarchitecture of modular tissues are well documented, techniques for creating larger engineered tissues from these modules are still relatively new. Some of the major challenges to overcome in the future are increasing the spatial resolution to create even smaller, more precise modules to better recreate biomimetic micro- and nanostructures, while also improving assembly techniques to create larger, more robust tissues without sacrificing the desired microstructural features. Another major challenge will be the potential integration of bottom-up techniques with more traditional top-down approaches to create more complex tissues than are currently achievable using either technique alone by optimizing the advantages of each technique. Modular tissue engineering possesses immense potential for advancing the fields of tissue engineering while bringing us closer to the ultimate goal of creating clinically relevant engineered tissues for human implantation and the treatment of disease.
References
- 1.Langer R, Vacanti JP. Tissue engineering. Science. 1993;260(5110):920–6. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 2.Hillsley M, Frangos J. Review: Bone Tissue Engineering: The Role of Interstitial Fluid Flow. Biotech Bioeng. 1994;43:575–81. doi: 10.1002/bit.260430706. [DOI] [PubMed] [Google Scholar]
- 3.Martin P. Wound healing--aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 4.Bello YM, Falabella AF, Eaglstein WH. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol. 2001;2(5):305–13. doi: 10.2165/00128071-200102050-00005. [DOI] [PubMed] [Google Scholar]
- 5.Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky A, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nat Med. 2006 Apr;12(4):452–8. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- 6.Caplan AI, Elyaderani M, Mochizuki Y, Wakitani S, Goldberg VM. Principles of cartilage repair and regeneration. Clin Orthop. 1997;342:254–69. [PubMed] [Google Scholar]
- 7.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, Langer R. Functional arteries grown in vitro. Science. 1999;284(5413):489–93. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
- 8.Gooch KJ, Blunk T, Courter DL, Sieminski AL, Vunjak-Novakovic G, Freed LE. Bone morphogenetic proteins-2, -12, and -13 modulate in vitro development of engineered cartilage. Tissue Eng. 2002;8(4):591–601. doi: 10.1089/107632702760240517. [DOI] [PubMed] [Google Scholar]
- 9.Boublik J, Park H, Radisic M, Tognana E, Chen F, Pei M, Vunjak-Novakovic G, Freed LE. Mechanical properties and remodeling of hybrid cardiac constructs made from heart cells, fibrin, and biodegradable, elastomeric knitted fabric. Tissue Eng. 2005 Jul–Aug;11(7–8):1122–32. doi: 10.1089/ten.2005.11.1122. [DOI] [PubMed] [Google Scholar]
- 10.Tranquillo RT. The tissue-engineered small-diameter artery. Ann N Y Acad Sci. 2002;961:251–4. doi: 10.1111/j.1749-6632.2002.tb03094.x. [DOI] [PubMed] [Google Scholar]
- 11.Gopalan SM, Flaim C, Bhatia SN, Hoshijima M, Knoell R, Chien KR, Omens JH, McCulloch AD. Anisotropic Stretch-Induced Hypertrophyin Neonatal Ventricular Myocytes Micropatterned on Deformable Elastomers. Biotech and Bioeng. 2003;81(5 March 5):578–87. doi: 10.1002/bit.10506. [DOI] [PubMed] [Google Scholar]
- 12.Daxini SC, Nichol JW, Sieminski AL, Smith G, Gooch KJ, Shastri VP. Micropatterned Polymer Surfaces Improve Retention of Endothelial Cells Exposed to Flow-Induced Shear Stress. Biorheology. 2005;43(1):45–55. [PubMed] [Google Scholar]
- 13.Hahn MS, Miller JS, West JL. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv Mater. 2006;18(20):2679–84. [Google Scholar]
- 14.Schaner PJ, Martin ND, Tulenko TN, Shapiro IM, Tarola N, Leichter RF, Carabasi RA, Dimuzio PJ. Decellularized vein as a potential scaffold for vascular tissue engineering. J Vasc Surg. 2004;40(1):146–53. doi: 10.1016/j.jvs.2004.03.033. [DOI] [PubMed] [Google Scholar]
- 15.Dean DM, Napolitano AP, Youssef J, Morgan JR. Rods, tori, and honeycombs: the directed self-assembly of microtissues with prescribed microscale geometries. FASEB. 2007;21:4005–12. doi: 10.1096/fj.07-8710com. [DOI] [PubMed] [Google Scholar]
- 16.Yeh J, Ling Y, Karp JM, Gantze J, Chandawarkard A, Eng G, Blumling J, Langer R, Khademhosseini A. Micromolding of shape-controlled, harvestable cell-laden hydrogels. Biomaterials. 2006;27:5391–8. doi: 10.1016/j.biomaterials.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 17.L’Heureux N, Paquet S, Labbe R, Germain L, Auger FA. A completely biological tissue-engineered human blood vessel. FASEB J. 1998;12(1):47–56. doi: 10.1096/fasebj.12.1.47. [DOI] [PubMed] [Google Scholar]
- 18.Mironov V, Boland T, Trusk T, Forgacs G, Markwald RR. Organ printing: computer-aided jet-based 3D tissue engineering. TRENDS in Biotechnology. 2003;21(4):157–161. doi: 10.1016/S0167-7799(03)00033-7. [DOI] [PubMed] [Google Scholar]
- 19.McGuigan AP, Leung B, Sefton MV. Fabrication of cells containing gel modules to assemble modular tissue-engineered constructs. Nature Protocols. 2006;1(6):2963–70. doi: 10.1038/nprot.2006.443. [DOI] [PubMed] [Google Scholar]
- 20.McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proceedings of the National Academy of Sciences. 2006;103(31):11461–6. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGuigan AP, Sefton MV. Design and Fabrication of Sub-mm-Sized Modules Containing Encapsulated Cells for Modular Tissue Engineering. Tissue Engineering. 2007;13(5):1069–78. doi: 10.1089/ten.2006.0253. [DOI] [PubMed] [Google Scholar]
- 22.McGuigan AP, Sefton MV. The thrombogenicity of human umbilical vein endothelial cell seeded collagen modules. Biomaterials. 2008;29:2453–63. doi: 10.1016/j.biomaterials.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.L’Heureux N, McAllister TN, de la Fuente LM. Tissue-engineered blood vessel for adult arterial revascularization. New England Journal of Medicine. 2007;357(14):1451–3. doi: 10.1056/NEJMc071536. [DOI] [PubMed] [Google Scholar]
- 24.Du Y, Lo E, Ali S, Khademhosseini A. Directed assembly of cell-laden microgels for fabrication of 3D tissue constructs. Proceedings of the National Academy of Sciences. 2008;105(28):9522–7. doi: 10.1073/pnas.0801866105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsang VL, Chen AA, Cho LM, Jadin KD, Sah RL, DeLong S, West JL, Bhatia SN. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB. 2007;21:790–801. doi: 10.1096/fj.06-7117com. [DOI] [PubMed] [Google Scholar]
- 26.Lim F, Sun AM. Microencapsulation Islets as Bioartificial Endocrine Pancreas. Science. 1980;210(21):908–10. doi: 10.1126/science.6776628. [DOI] [PubMed] [Google Scholar]
- 27.Tien J, Nelson CM, Chen CS. Fabrication of aligned microstructures with a single elastomeric stamp. Proc Natl Acad Sci U S A. 2002;99(4):1758–62. doi: 10.1073/pnas.042493399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tien J, Chen CS. Patterning the cellular microenvironment. IEEE Eng Med Biol Mag. 2002;21(1):95–8. doi: 10.1109/memb.2002.993201. [DOI] [PubMed] [Google Scholar]
- 29.Nelson CM, Chen CS. VE-cadherin simultaneously stimulates and inhibits cell proliferation by altering cytoskeletal structure and tension. J Cell Sci. 2003 Sep 1;(116):3571–81. doi: 10.1242/jcs.00680. [DOI] [PubMed] [Google Scholar]
- 30.Karp JM, Yeh J, Eng G, Fukuda J, Blumling J, Suh KY, Cheng J, Mahdavi A, Borenstein J, Langer R, Khademhosseini A. Controlling size, shape and homogeneity of embryoid bodies using poly(ethylene glycol) microwells. Lab on a Chip. 2007;7:786–94. doi: 10.1039/b705085m. [DOI] [PubMed] [Google Scholar]
- 31.Khademhosseini A, Eng G, Yeh J, Kucharczyk PA, Langer R, Vunjak-Novakovic G, Radisic M. Microfluidic patterning for fabrication of contractile cardiac organoids. Biomed Microdevices. 2007;9:149–57. doi: 10.1007/s10544-006-9013-7. [DOI] [PubMed] [Google Scholar]
- 32.Napolitano AP, Chai P, Dean DM, Morgan JR. Dynamics of the Self-Assembly of Complex Cellular Aggregates on Micromolded Nonadhesive Hydrogels. Tissue Engineering. 2007;13(8):2087–94. doi: 10.1089/ten.2006.0190. [DOI] [PubMed] [Google Scholar]
- 33.Nishida K, Yamato M, Hayashida Y, Watanabe K, Maeda N, Watanabe H, Yamamoto K, Nagai S, Kikuchi A, Tano Y, Okano T. Functional bioengineered corneal epithelial sheet grafts from corneal stem cells expanded ex vivo on a temperature-responsive cell culture surface. Transplantation. 2004;77(3):379–85. doi: 10.1097/01.TP.0000110320.45678.30. [DOI] [PubMed] [Google Scholar]
- 34.Shiroyanagi Y, Yamato M, Yamazaki Y, Toma H, Okano T. Transplantable urothelial cell sheets harvested noninvasively from temperature-responsive culture surfaces by reducing temperature. Tissue Engineering. 2003;9(5):1005–12. doi: 10.1089/107632703322495646. [DOI] [PubMed] [Google Scholar]
- 35.Yamato M, Okano T. Cell sheet engineering. Materials Today. 2004 May;:42–7. [Google Scholar]
- 36.Khademhosseini A, Langer R. Microengineered hydrogels for tissue engineering. Biomaterials. 2007;28:5087–92. doi: 10.1016/j.biomaterials.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 37.Peppas N, Hilt JZ, Khademhosseini A, Langer R. Hydrogels in biology and medicine. Adv Mater. 2006;18:1–17. [Google Scholar]
- 38.Palmer LC, Stupp SI. Molecular Self-Assembly into One-Dimensional Nanostructures. Acc Chem Res. 2008 Aug 28; doi: 10.1021/ar8000926. p. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thornton PD, McConnell G, Ulijn RV. Enzyme responsive polymer hydrogel beads. Chem Commun (Camb) 2005 Dec 21;(47):5913–5. doi: 10.1039/b511005j. [DOI] [PubMed] [Google Scholar]
- 40.Zhang S. Fabrication of novel biomaterials through molecular self-assembly. Nature Biotech. 2003 Oct21;(10):1171–8. doi: 10.1038/nbt874. [DOI] [PubMed] [Google Scholar]
- 41.Zhang S, Holmes T, Lockshin C, Rich A. Spontaneous assembly of a self-complementary oligopeptide to form a stable macroscopic membrane. Proc Natl Acad Sci USA. 1993;90:3334–8. doi: 10.1073/pnas.90.8.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang S, Yan L, Altman M, Lassle M, Nugent H, Frankle F, Lauffenburger DAWGM, Rich A. Biological surface engineering: a simple system for cell pattern formation. Biomaterials. 1999;20:1213–30. doi: 10.1016/s0142-9612(99)00014-9. [DOI] [PubMed] [Google Scholar]
- 43.Koh WG, Revzin A, Pishko MV. Poly(ethylene glycol) hydrogel microstructures encapsulating living cells. Langmuir. 2002;18(7):2459–62. doi: 10.1021/la0115740. [DOI] [PubMed] [Google Scholar]
- 44.Khademhosseini A, Yeh J, Jon S, Eng G, Suh KY, Burdick JA, Langer R. Molded polyethylene glycol microstructures for capturing cells within microfluidic channels. Lab Chip. 2004;4(5):425–30. doi: 10.1039/b404842c. [DOI] [PubMed] [Google Scholar]
- 45.Khademhosseini A, Eng G, Yeh J, Fukuda J, Blumling J, Langer R, Burdick JA. Micromolding of photocrosslinkable hyaluronic acid for cell encapsulation and entrapment. J Biomed Mater Res A. 2006;79(3):522–32. doi: 10.1002/jbm.a.30821. [DOI] [PubMed] [Google Scholar]
- 46.Fukuda J, Khademhosseini A, Yeo Y, Yang X, Yeh J, Eng G, et al. Micromolding of photocrosslinkable chitosan hydrogel for spheroid microarray and co-cultures. Biomaterials. 2006 doi: 10.1016/j.biomaterials.2006.05.044. [DOI] [PubMed] [Google Scholar]
- 47.Luo Y, Stoichet MS. A photolabile hydrogel for guided threedimensional cell growth and migration. Nat Mater. 2004;3(4):249–53. doi: 10.1038/nmat1092. [DOI] [PubMed] [Google Scholar]
- 48.Golden AP, Tien J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip. 2007;7(6):720–5. doi: 10.1039/b618409j. [DOI] [PubMed] [Google Scholar]
- 49.Nichol JW, Engelmayr GC, Jr, Cheng M, Freed LE. Co-culture induces alignment in engineered cardiac constructs via MMP-2 expression. Biochem Biophys Res Commun. 2008;373(3):360–5. doi: 10.1016/j.bbrc.2008.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGuigan AP, Bruzewicz DA, Glavan A, Butte M, Whitesides GM. Cell Encapsulation in Sub-mm Sized Gel Modules Using Replica Molding. PLoS ONE. 2008;3(5):1–11. doi: 10.1371/journal.pone.0002258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Revzin A, Russell RJ, Yadavalli VK, Koh WG, Deister C, Hile DD, Mellott MB, Pishko MV. Fabrication of Poly(ethylene glycol) Hydrogel Microstructures Using Photolithography. Langmuir. 2001;17:5440–7. doi: 10.1021/la010075w. [DOI] [PubMed] [Google Scholar]
- 52.Naito H, Melnychenko I, Didie M, Schneiderbanger K, Schubert P, Rosenkranz S, Eschenhagen T, Zimmermann WH. Optimizing engineered heart tissue for therapeutic applications as surrogate heart muscle. Circ. 2006 Jul 4;(1141 Suppl):I72–8. doi: 10.1161/CIRCULATIONAHA.105.001560. [DOI] [PubMed] [Google Scholar]
- 53.Eschenhagen T, Fink C, Remmers U, Scholz H, Wattchow J, Weil J, Zimmermann W, Dohmen HH, Schafer H, Bishopric N, Wakatsuki T, Elson EL. Three-dimensional reconstitution of embryonic cardiomyocytes in a collagen matrix: a new heart muscle model system. FASEB. 1997 Jul 11;(8):683–94. doi: 10.1096/fasebj.11.8.9240969. [DOI] [PubMed] [Google Scholar]
- 54.Tan W, Desai TA. Layer-by-layer microfluidics for biomimetic three-dimensional structures. Biomaterials. 2004;25(7–8):1355–64. doi: 10.1016/j.biomaterials.2003.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Liu VA, Bhatia SN. Three-dimensional photopatterning of hydrogels containing living cells. Biomed Microdev. 2002;4(4):257–66. [Google Scholar]
- 56.Borenstein JT, Terai H, King KR, Weinberg EJ, Kaazempur-Mofrad MR, Vacanti JP. Microfabrication technology for vascularized tissue engineering. Biomed Microdev. 2002;4(3):167–75. [Google Scholar]
- 57.Chrobak KM, Potter DR, Tien J. Formation of perfused, functional microvascular tubes in vitro. Microvasc Res. 2006;71(3):185–96. doi: 10.1016/j.mvr.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Federovich NE, Alblas J, DeWijn JR, Hennink WE, Verbout AJ, Dhert WJA. Hydrogels as Extracellular Matrices for Skeletal Tissue Engineering: State-of-the-Art and Novel Application in Organ Printing. Tissue Engineering. 2007;13(8):1905 – 25. doi: 10.1089/ten.2006.0175. [DOI] [PubMed] [Google Scholar]
- 59.Federovich NE, DeWijn JR, Verbout AJ, Alblas J, Dhert WJA. Three-Dimensional Fiber Deposition of Cell-Laden, Viable Patterned Constructs for Bone Tissue Printing. Tissue Engineering Part A. 2008;14(1):127–133. doi: 10.1089/ten.a.2007.0158. [DOI] [PubMed] [Google Scholar]
- 60.Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25:3707–15. doi: 10.1016/j.biomaterials.2003.10.052. [DOI] [PubMed] [Google Scholar]
- 61.L’Heureux N, Stoclet JC, Auger FA, Lagaud GJ, Germain L, Andriantsitohaina R. A human tissue-engineered vascular media: a new model for pharmacological studies of contractile responses. Faseb J. 2001;15(2):515–24. doi: 10.1096/fj.00-0283com. [DOI] [PubMed] [Google Scholar]
- 62.L’Heureux N, Dusserre N, Konig G, Victor B, Keire P, Wight TN, Chronos NA, Kyles AE, Gregory CR, Hoyt G, Robbins RC, McAllister TN. Human tissue-engineered blood vessels for adult arterial revascularization. Nat Med. 2006;12(3):361–5. doi: 10.1038/nm1364. [DOI] [PMC free article] [PubMed] [Google Scholar]