Abstract
The study was designed to evaluate the combination treatment of methylselenocysteine (MSeC) and docetaxel and to delineate the underlying mechanism associated with observed in vitro synergy between MSeC and docetaxel in prostate cancer cells. Cells were treated with different concentrations and schedules (concurrent or sequential) of MSeC and docetaxel alone or in combination. Cell growth/death was assessed with sulforhodamine B assay, trypan blue assay, and time-lapse video. Loewe synergism/antagonism model was used to determine whether the combination effect was additive, synergistic, or antagonistic. Apoptosis and caspase-3 activity were evaluated with cell death ELISA assay and caspase activity assay, respectively. Synergy between MSeC and docetaxel was further assessed in the presence and absence of z-VAD-fmk, a pan-caspase inhibitor. Effect of MSeC and docetaxel alone or in combination on the cellular expression of the antiapoptotic protein survivin was measured with Western blot analyses. Pretreatment with MSeC was crucial to enhance docetaxel antitumor activity. The enhanced antitumor activity of the sequential combination treatment of MSeC and docetaxel (MSeC/docetaxel) was highly synergistic. Apoptosis increased after MSeC/docetaxel, compared with each drug alone or concurrent treatment. Pretreatment with z-VAD-fmk converted the synergy into antagonism, suggesting that the synergy is caspase-dependent apoptosis. The survivin level was down-regulated following MSeC/docetaxel treatment when compared with each drug alone. In conclusion, pretreatment with MSeC was essential to markedly sensitize cells to docetaxel. The synergy between MSeC and docetaxel in C2G prostate cancer cells is associated with increased level of caspase-dependent apoptosis and decreased level of survivin.
Introduction
Prostate cancer is the most commonly diagnosed cancer in men in the United States (1). One in six American men will be diagnosed with prostate cancer during their lifetime (2). The American Cancer Society estimates that there will be ~234,460 new cases of prostate cancer in the United States and ~27,350 men will die from this disease in the year 2006 (1). Current treatments for prostate cancer include surgery, radiation, and hormone therapy. However, treatment options for late-stage metastatic disease are at best palliative. Progression of prostate cancer occurs in almost all patients treated with androgen deprivation therapy. For patients initially diagnosed with metastatic prostate cancer, the median survival after onset of progression is 10 to 24 months. New therapeutic modalities are needed to improve the response rate of chemotherapeutic agents and lessen their toxicity, which will ultimately translate into improved efficacy against late-stage aggressive disease.
Docetaxel is an anticancer drug that binds to free tubulin and promotes assembly of stable microtubules while concurrently inhibiting their disassembly (3-7), resulting in inhibition of cell division. Docetaxel is being used to treat different types of cancer including breast, non–small-cell lung, ovarian, and prostate cancer. The major side effects of docetaxel include myelosuppression, alopecia, neurotoxicity, diarrhea, rash, sore mouth, and fluid retention, with myelosuppression being the most common dose-limiting toxicity.
Selenium is being evaluated as a chemopreventive agent in patients with prostate, colon, and lung cancer with promising results. Methylselenocysteine (MSeC) is a selenium-containing compound. Unlike L-selenomethionine and selenized yeast currently used in clinical prevention trials (8, 9), MSeC binds poorly to general body proteins and is activated in one step by β-lyase to the presumed active metabolite methylselenol (10). Many epidemiologic observations and clinical trials support the hypothesis that selenium protects against the risk of prostate cancer (11). The strongest evidence for a protective effect of selenium emerges from the Nutritional Prevention of Cancer Trial, a randomized study of oral selenized yeast in patients with nonmelanoma skin cancer. After 4.5 years of follow-up, the incidence of prostate cancer decreased by two thirds in men taking an equivalent of 200 μg selenium per day (selenized yeast) compared with placebo. Duffield-Lillico et al. (12) extended the follow-up to 7.45 years and showed an even greater decrease in prostate cancer incidence. Based on these findings, a large-scale Selenium and Vitamin E Cancer Prevention Trial is under way (13). Several mechanisms have been proposed for the chemopreventive activity of selenium, including induction of apoptosis, inhibition of angiogenesis, and arrest of cell cycle (14-17). The goal of this study is to investigate whether MSeC at a nontoxic dose improves the efficacy of docetaxel for treatment of prostate cancer.
In this study, we found that pretreatment of C2G prostate cancer cells with MSeC followed by docetaxel is critical for achieving synergy. The synergy was associated with induction of apoptotic cell death, which was associated with caspase-3 activation and down-regulation of antiapoptotic protein survivin.
Materials and Methods
Cell Culture
The colonal TRAMP cell line C2G was isolated and obtained from the TRAMP C2 cell lines by limited dilution cloning (18). TRAMP C2G is a metastatic cell line with 100% tumorigenicity. Cells were cultured in DMEM (Life Technologies, Bethesda, MD) supplemented with insulin and 10 nmol/L DHT, 10% fetal bovine serum, and 25 units/mL penicillin-streptomycin. The cell line was tested for Mycoplasma every 3 months with the Mycoplasma Plus PCR Primer Set (Stratagene, La Jolla, CA).
Drugs and Treatments
MSeC and Z-VAD-fmk were obtained from Sigma-Aldrich (St. Louis, MO). L-Methioninase was purchased from Wako Chemical USA, Inc. (Richmond, VA). Docetaxel was purchased from Novartis International AG (Basel, Switzerland) in an 80-mg bottle. All MSeC treatment schedules included L-methioninase at 0.05 units/mL.
Five groups of C2G cells were studied to assess trypan blue assay, time-lapse video, caspase-3 activity, detection of apoptotic cell death, and survivin expression by Western blotting. These treatment groups were (i) untreated control; (ii) MSeC for 24 hours at IC25 (4 μmol/L) after MSeC being activated with nontoxic dose of L-methioninase at 0.05 units/mL; (iii) docetaxel for 2 hours at IC10 (0.01 μmol/L) or IC90 concentrations (60 μmol/L); (iv) concurrent combination treatment of MSeC and docetaxel (MSeC + docetaxel) with the same individual drug concentration for 2 hours; and (v) sequential combination treatment of MSeC and docetaxel (MSeC/docetaxel) applied with the same individual drug concentration and treatment time, with docetaxel treatment following MSeC treatment.
Growth Inhibition Assay
Inhibition of cell growth for individual drug was determined with the total protein sulforhodamine B assay as described by Skehan et al. (19). Briefly, 1,000 cells per well were seeded onto 96-well plates. After 24 hours, exponentially growing cells were treated with MSeC and L-methioninase alone for 24 hours, docetaxel alone for 2 hours, which was diluted in culture medium, and combination of MSeC and docetaxel. Cells were incubated in a drug-free medium for 8 doubling times after drug exposure, fixed with 10% trichloroacetic acid, washed with water, and stained with sulforhodamine B. Unbound dye was removed with 1% acetic acid and protein-bound dye was extracted with Tris base. The absorbance was measured at 570 nm with an automated Bio-Kinetics reader (model EL 340, Bio-Tek Instruments, Winooski, VT). The results of cell growth inhibition after the combination treatments (sequential and concurrent) were normalized to MSeC alone–treated cells at IC25 for 24 hours. Antiproliferative activities were expressed as drug concentrations that induced inhibition of cell growth by 50% or 90% compared with cell growth of untreated controls (IC50 and IC90 values).
Trypan Blue Cell Death Assay
The cytotoxic effect of the combination treatment on cells was evaluated with trypan blue assay. After treatment with MSeC and docetaxel alone or in combination, cells were washed with PBS and admission/exclusion of dye was assayed daily for a total of 5 days after drug treatment. A collection of supernatants and adherent cells obtained by trypsinization was incubated in 0.2% trypan blue (Mediatech, Inc., Herndon, VA) and pipetted onto a hematocytometer and manually counted under a microscope at x100 magnification. The percentage of cells admitting trypan blue dye to the total number of cells was determined by counting three different fields for each experimental condition, which was done in triplicates.
Time-Lapse Video Analysis
The cytotoxic effect of the combination treatment was further confirmed with time-lapse video as described by Slocum et al. (20). Digital photographic records (.tiff format) of cells in T-25 flasks were made with a charge-coupled device camera (MTI 72) every 1.44 minutes (1,000 digital photos/d) via an image capture card in a PC (Dell Dimension XPS T800r) running ImagePro Plus version 5 (MediaCybernetics, Silver Spring, MD). The camera was attached to a Nikon inverted-optics (model TMS) microscope housed in a 37°C incubator in a 5% CO2/95% air atmosphere. The tiff files were assembled into sequence files for playback as digital movies or timed digital pictures, and thus the cellular response to treatment with MSeC and docetaxel alone or in combination was analyzed. In this way, the cells were under continuous observation, and events such as cell lysis, release from the substrate, cell swelling (consistent with necrotic type of cell death), or cell shrinkage, cessation of membrane movement, and cell breakage into smaller fragments forming apoptotic bodies (consistent with apoptotic cell death) could be assessed through time.
Synergism and Antagonism Studies
Drug interaction was evaluated in 96-well plates with growth inhibition assay as described by Faessel et al. (21). Briefly, each of the five plates included 11 wells for control, MSeC, docetaxel, and each of the five combinations of MSeC plus docetaxel in constant ratio (1:4, 1:2, 1:1, 2:1, and 4:1). The serial dilution was randomized as described by Faessel et al. (22, 23). Inhibition of cell growth by the combination treatment was determined with the same sulforhodamine B assay described above and data analysis was done by using Excel software applying Universal Response Surface Approaches as described by Levasseur et al. (24). Assuming the Hill model for concentration effect of each drug alone and the Loewe additivity model, a Loewe synergism/Loewe antagonism model is derived (25). Estimated interaction variable α with its SE is calculated. Synergy is indicated when α is positive and antagonism is indicated when α is negative. Additive effect is observed when the 95% confidence interval of α is zero. The software package SYNFIT (FORTRAN, Bellevue, WA) was used for fitting procedure. The absolute value of α will result in a degree of bowing either above (antagonism) or below (synergism) the diagonal additive line.
Isobologram Analysis
Hill model as described by Levasseur et al. (24) was used. Briefly, individual fitting of experimental data of the concentration-effect curve for each drug alone and for each constant ratio combination of MSeC and docetaxel was done. The effect values of 10%, 25%, 50%, 75%, and 90% with accompanying confidence interval were plotted on a normalized isobologram.
Caspase-3 Activity Assay
Cells were treated with MSeC and docetaxel alone or in combination, washed with ice-cold PBS, harvested, and suspended in lysis buffer. After incubation on ice for 1 minute, the homogenate was centrifuged at 4°C for 30 minutes. The clear lysate was stored at −20°C for later use in the assay. Briefly, cell lysates and positive control are added to a 96-well plate coated with monoclonal antibody, which was prepared according to the instructions of the manufacturer (Roche Applied Science, Indianapolis, IN). Plate with samples was incubated at 37°C for 1 hour, rinsed thrice with incubation buffer, and substrate was added to each sample. After incubation at 37°C for 2 hours, the fluorescence intensity of activated caspase-3 is measured with a fluorescence plate reader at excitation wavelength of 370 to 425 nm and emission wavelength of 490 to 530 nm.
Apoptotic Cell Death Detection by ELISA
Cells were treated with MSeC, docetaxel, and combination of MSeC and docetaxel with and without pretreatment with z-VAD-fmk. In vitro apoptotic cell death was assessed in untreated and drug-treated cells with ELISA Plus kit (Roche Applied Science) following the recommended procedures of the manufacturer. The concept of the ELISA Plus kit is binding of peroxidase-conjugated monoclonal antibody to nuleosome single-stranded and double-stranded DNA, and binding of biotin-labeled monoclonal antibody to nucleosome histones (H1, H2A, H2B, H3, and H4). The reaction is a “quantitative sandwich-enzyme-immunoassay” reaction (Roche Applied Science). Samples were immediately analyzed to preserve the ELISA signal using a plate reader to determine the absorbance intensity.
Western Blot Analysis
Untreated control and treated C2G cells were lysed in radioimmunoprecipitation assay buffer [50 mmol/L Tris-HCl (pH 8), 100 mmol/L NaCl, 0.5% SDS, 0.5% sodium deoxycholate, 0.5% NP40, 10 mmol/L DTT, 1 mmol/L phenylmethylsulfonyl fluoride, and 0.4 trypsin inhibitor unit of aprotinin], and the protein content was determined with the Bio-Rad detergent-compatible protein assay (Hercules, CA). A total of 50 μg of protein lysate was subjected to 12.5% SDS-PAGE and transferred onto a nitrocellulose membrane (Bio-Rad). Western blotting was done as previously described (26) with antisurvivin monoclonal antibody from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA) using the Renaissance Chemiluminescence Reagent Kit (Perkin Elmer Life Science Products, Boston, MA). Anti-β-actin monoclonal antibody (Sigma-Aldrich) was used to show equal protein loading.
Statistical Analysis
The calculated mean values ± SD were compared using unpaired t test. P < 0.05 is considered statistically significant.
Results
Enhancement of Docetaxel Antitumor Activity Is MSeC Schedule Dependent
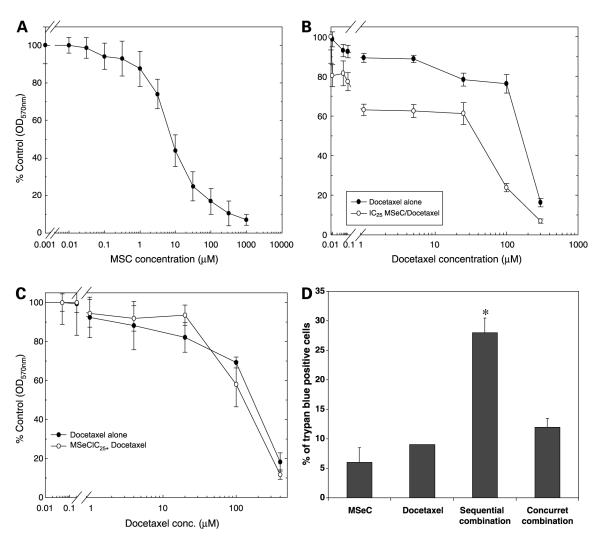
Inhibition of cell growth induced by MSeC or docetaxel was assessed by sulforhodamine B assay at 5 days after treatment with each drug alone (Fig. 1A and B). Cell growth after the combination treatments of MSeC and docetaxel was evaluated. Cells were treated with docetaxel for 2 hours at multiple concentrations (serial dilution) with and without MSeC pretreatment for 24 hours at different doses (IC10, IC25, and IC50). Pretreatment with MSeC at IC25 enhanced docetaxel inhibition of cell growth (Fig. 1B), as well as pretreatment with MSeC at the other two concentrations (data not shown). To determine whether pretreatment with MSeC was essential for achieving inhibition of cell growth, cells were treated with docetaxel alone or concurrently with MSeC. The concurrent combination treatment did not induce docetaxel inhibition of cell growth (Fig. 1C), indicating the importance of the pretreatment with MSeC shown in Fig. 1B.
Figure 1.
The effects of MSeC and docetaxel, alone or in combination, on cell growth and cell death. Cells were treated with MSeC alone for 24 h (A)or docetaxel alone for 2 h (B) and incubated in drug-free medium for 5 d. Cell growth was assessed with the total protein sulforhodamine B assay. Pretreatment of cells with MSeC for 24 h at concentration that inhibits 25% of cell growth (IC25) followed by multiple concentrations of docetaxel resulted in enhancement of docetaxel inhibition of cell growth (B). C, cells were subjected to a concurrent treatment of MSeC and docetaxel with the same MSeC concentration (IC25). The concurrent treatment did not enhance docetaxel inhibition of cell growth, which suggests that enhancement of docetaxel inhibition of cell growth is MSeC schedule dependent. D, cells were treated with IC25 of MSeC alone for 24 h, IC90 of docetaxel alone for 2 h, sequential combination of MSeC for 24 h followed by docetaxel for 2 h, and concurrent combination of MSeC and docetaxel for 2 h. After staining and normalizing to untreated control, pretreatment with MSeC (sequential combination) significantly increased percentage of cells admitting trypan blue when compared with each drug alone or with the concurrent combination treatment. *, P < 0.05, significantly different when compared with all other treatment groups.
To determine whether the effect of the combination treatment on cells was cytostatic or cytotoxic, cells treated with MSeC (IC25), docetaxel (IC90), sequential (MSeC/docetaxel), or concurrent combination treatment (MSeC + docetaxel) were assessed for cell death with trypan blue assay. After normalization to untreated control, percentage of cells admitting trypan blue was significantly (P < 0.05) higher after MSeC/docetaxel treatment when compared with all other treatment groups (Fig. 1D). A daily assessment of cells viability showed that the sequential combination treatment significantly induced (P < 0.05) cell death, in excess of 90% after 5 days of treatment, when compared with docetaxel alone treatment (Fig. 2A). However, large amounts of dead cells debris were present in some samples, suggesting that cells were degenerating after death and being lost to analysis. Thus, to avoid this artifact of undetected cell loss, which can occur when intermittent assays are employed at limited time points, cells were subjected to continuous observation using time-lapse video. Continuous time-lapse video records indicated that after the sequential combination of MSeC/docetaxel, in excess of 95% of cells showed marked shrinkage, loss of refractivity, breakage into small fragments (apoptotic bodies), and cessation of all membrane movement, consistent with cell death by an apoptotic mechanism (Fig. 2B). Docetaxel alone resulted in ~20% of cells being viable at day 5 after treatment (data not shown). Thus, the increased effect of docetaxel when used in combination with MSeC cannot be due to cytostatic or growth-slowing effects on the population, but rather due to an increase in apoptotic cell death among most of the cells treated.
Figure 2.
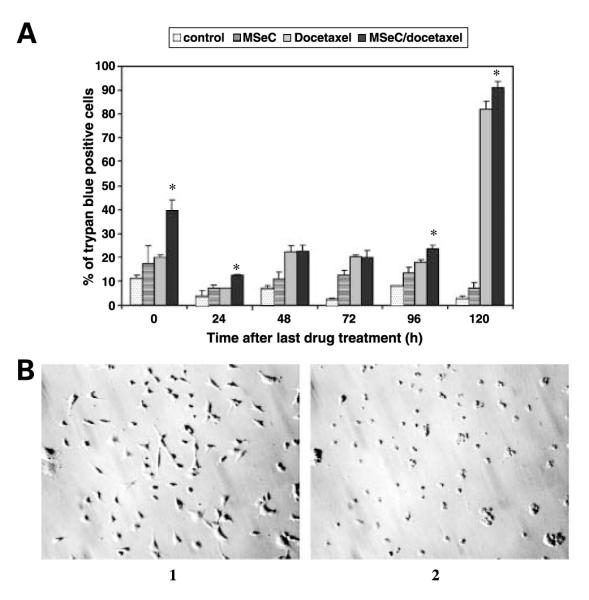
Cell death assessments after the combination treatment of MSeC/docetaxel. Cells were treated with MSeC and docetaxel alone or in combination, stained with trypan blue, and evaluated daily for dye admission/exclusion. A, the sequential combination significantly increased (P < 0.05) percentage of cells admitting trypan blue when compared with each drug alone, indicating more cell death. B, cells were continuously observed with time-lapse video. 1, still digital picture of cells after 24 h of MSeC (IC25) treatment, before adding docetaxel, showing normal profile of cells. 2, picture of the same field, 5 d after treatment with 2 h docetaxel (IC90), showing that >95% of cells exhibited marked shrinkage, loss of cells, and breakage into smaller parts (apoptotic bodies), which is consistent with cell death by an apoptotic mechanism. *, P < 0.05, significantly different when compared with all other treatment groups.
These results indicate that in vitro pretreatment with MSeC is crucial for the enhancement of docetaxel antitumor activity and is in accordance with the in vivo reported data by Cao et al. (27), which showed the requirement of MSeC pretreatment to enhance the antitumor efficacy of multiple chemotherapeutic agents.
Enhanced Docetaxel Antitumor Activity by the Sequential Combination Treatment Is a Synergistic Effect
The enhanced docetaxel antitumor activity by MSeC was further investigated to determine whether this effect was an additive or a synergistic effect. Cells treated with MSeC/docetaxel were analyzed using custom SYNFIT generated graph of Hill and Loewe additivity models. The analyses show that the enhanced docetaxel antitumor activity by MSeC was a synergistic effect (increased degree of bowing below the diagonal additive line; Fig. 3). Administration of MSeC and docetaxel at different percentages of their individual concentrations that achieve IC90 or IC50 effect resulted in synergy with more bowing degree at IC90 doses than at IC50 doses. However, when docetaxel and MSeC are given at different percentages of their individual IC10 concentrations, the combination treatment yielded an additive effect (Fig. 3), suggesting that the docetaxel concentration is also important for the synergistic effect.
Figure 3.
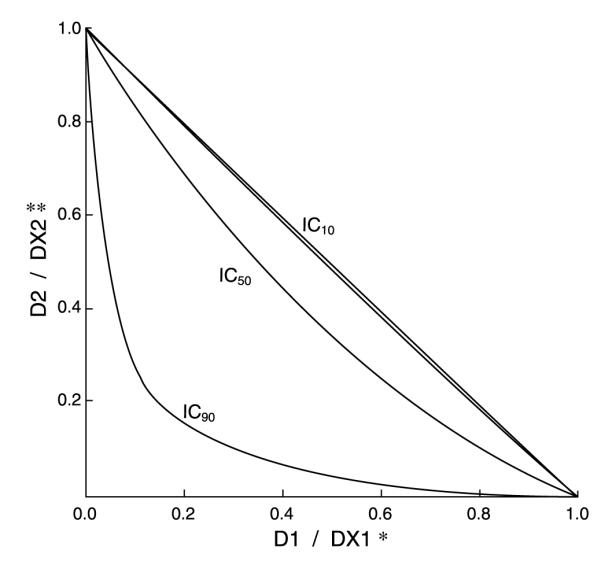
Synergistic effect between MSeC and docetaxel. A SYNFIT custom graph shows the degree of bowing of the additive line corresponding with the absolute value of antagonism/synergism variable α. *, D1 / DX1 is the dose of MSeC in combination over the dose of MSeC when applied as a drug alone that achieves an X effect. **, D2 / DX2 is the dose of docetaxel in combination over the dose of docetaxel when applied as a drug alone that achieves an X effect. The inward bowing of the straight additive line is an indication of the combination synergistic effect that achieves 90% or 50% inhibition of cell growth by the combination treatment. The sequential combination treatment was highly synergistic, indicated by the degree of inward bowing.
Sequential Combination Treatment Induces Caspase-Dependent Apoptosis
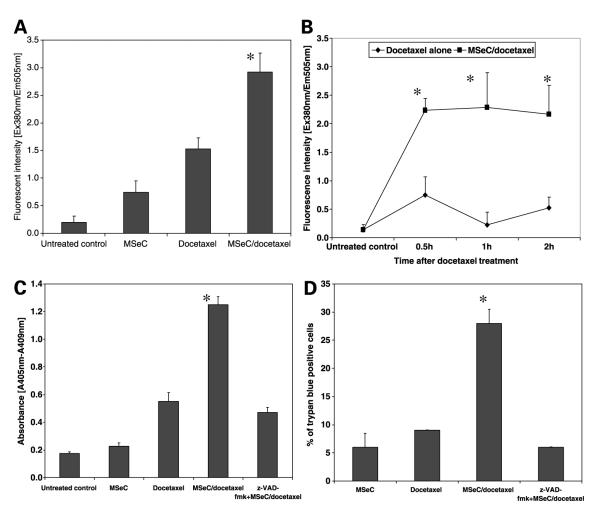
To determine whether the observed combination synergy is related to the induction of caspase-dependent apoptosis, four treatment groups of untreated control, MSeC alone, docetaxel alone, and MSeC/docetaxel were evaluated for caspase-3 activity by caspase-3 activity assay. Cells were treated with MSeC for 24 hours and then with docetaxel for 2 hours. Immediately after drug treatment, MSeC/docetaxel treatment significantly increased caspase-3 activity (P < 0.0001) when compared with untreated control, MSeC alone, or docetaxel alone (Fig. 4A). Significant induction of caspase-3 activity was observed at 30 minutes (P = 0.003), 1 hour (P = 0.03), and 2 hours (P = 0.005) after MSeC/docetaxel treatment, when compared with docetaxel alone (Fig. 4B). Induction of caspase-3 activity by MSeC/docetaxel treatment suggests an induction of a favorable pre-apoptotic condition.
Figure 4.
The sequential combination treatment of MSeC and docetaxel induces caspase-3 activity and apoptotic cell death. Cells were treated with MSeC alone, docetaxel alone, and sequential combination of MSeC/docetaxel. The sequential combination treatment induces caspase-3 activity immediately (A) and at different time points (B) after drug treatments when compared with each drug alone treatment. C, the sequential combination treatment significantly induced apoptotic cell death when compared with each drug alone or pretreatment with z-VAD-fmk. D, treated cells were stained with trypan blue to assess cell death. Pretreatment with z-VAD-fmk significantly reduced percentage of cells admitting the dye, linking apoptosis to cell death. *, P < 0.05, significantly different when compared with all other treatment groups.
The induction of caspase-3 activity was further investigated to determine the effect of the combination treatment on apoptotic cell death with a cell death detection ELISA kit. The same four treatment groups were assessed for apoptosis in the presence and absence of z-VAD-fmk, a pan-caspase inhibitor. MSeC/docetaxel treatment significantly induced apoptotic cell death (P = 0.002) when compared with treatment with each drug alone (Fig. 4C). Treatment with z-VAD-fmk significantly (P < 0.05) reduced the induction of apoptosis in MSeC/docetaxel treatment group (Fig. 4C), indicating the significance of induction of caspase-dependent apoptosis by MSeC/docetaxel treatment.
To confirm that the induction of caspase-3 activity and apoptosis were related to cell death, cells were assessed with trypan blue assay after MSeC/docetaxel treatment in the presence and absence of z-VAD-fmk. MSeC/docetaxel treatment significantly (P < 0.05) increased percentage of cells admitting trypan blue when compared with treatment with each drug alone (Fig. 4D). However, in the presence of z-VAD-fmk, the percentage of cells admitting trypan blue was significantly (P < 0.05) reduced when compared with MSeC/docetaxel treatment group without z-VAD-fmk (Fig. 4D). These data indicate that treatment with z-VAD-fmk protects cells from death caused by MSeC/docetaxel treatment.
Synergy between MSeC and Docetaxel Is Caspase-Dependent Apoptosis
To test whether the observed MSeC/docetaxel synergy is dependent on induction of caspase-3 activity and apoptotic cell death, cells were treated with MSeC/docetaxel in the presence of pretreatment with z-VAD-fmk (Fig. 5). Pretreatment with z-VAD-fmk reversed the observed synergy between MSeC and docetaxel (inward bowing blots in Fig. 3), resulting in antagonism as shown by the change in the degree of bowing to outward bowing plots (Fig. 5). The reversal of the synergy by z-VAD-fmk further suggests that the MSeC/docetaxel synergy is apoptosis dependent and regulated by caspases.
Figure 5.
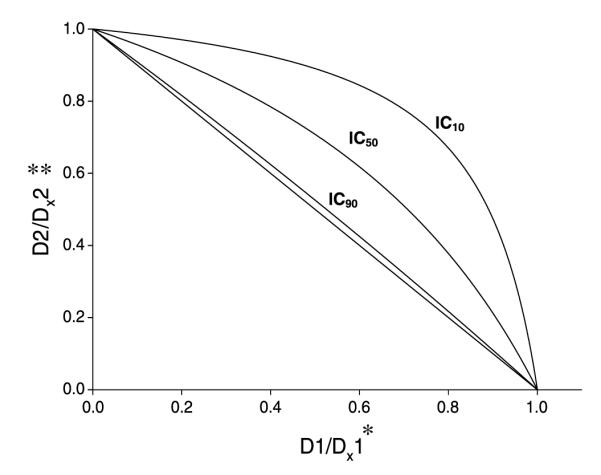
MSeC and docetaxel synergy is caspase-dependent apoptosis synergy. Cells were assessed for the combination synergy with pretreatment of z-VAD-fmk using SYNFIT generated graph of Hill and Loewe additivity models. *, D1 / DX1 is the dose of MSeC in combination over the dose of MSeC when applied as a drug alone that achieves an X effect. **, D2 / DX2 is the dose of docetaxel in combination over the dose of docetaxel when applied as a drug alone that achieves an X effect. After pretreatment with z-VAD-fmk, the synergistic inward bowing of the combination treatment lines (seen without z-VAD-fmk pretreatment in Fig. 3) changed to antagonistic outward bowing lines in all concentrations. This conversion from synergy to antagonism with blocking of all caspases through pretreatment with z-VAD-fmk suggests that the combination synergy is caspase-dependent apoptosis.
Survivin Down-Regulation after the Sequential Combination Treatment
To determine whether MSeC/docetaxel treatment affects the level of survivin expression, cells from the four treatment groups of untreated control, MSeC, docetaxel, and MSeC/docetaxel were analyzed for survivin expression at 24 hours after docetaxel treatment. MSeC doses were fixed at IC25 but two doses of docetaxel, at IC90 (Fig. 6A) and at IC10 (Fig. 6B), were used alone or in combination. MSeC/docetaxel treatment significantly down-regulated survivin expression, compared with each drug alone, when docetaxel was administered at IC90 concentration that produced synergy (Fig. 6A). After treatment with docetaxel at IC10 concentration that produced additivity, MSeC/docetaxel treatment did not change the level of survivin when compared with each drug alone (Fig. 6B). These data indicate that the synergy between MSeC and docetaxel is associated with decreased level of survivin.
Figure 6.
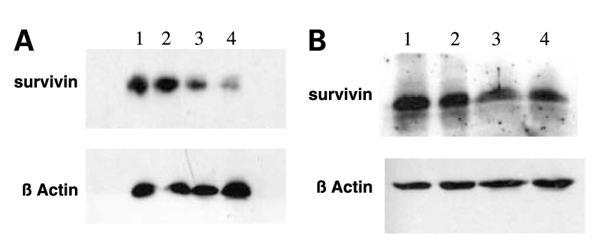
The sequential combination treatment down-regulates survivin expression. Cells treated with two different concentrations of docetaxel alone or in combination [IC90 (A) or IC10 (B) for 2 h] with a fixed concentration of MSeC (IC25 for 24 h). Survivin expression was assessed by Western blot analysis and loaded samples were numbered in both treatments from 1 to 4. A1 and B1, samples of untreated control; A2 and B2, samples of MSeC alone at IC25; A3 and B3, samples of docetaxel alone at IC90 and IC10, respectively; A4 and B4, samples of MSeC at IC25 in combination with docetaxel at IC90 and IC10, respectively. Down-regulation of survivin was associated with the combination treatment of MSeC and docetaxel at a concentration of docetaxel that achieved synergy (IC90) but not with a docetaxel concentration that achieved additivity (IC10).
Discussion
MSeC is being used as a chemotherapeutic modulator of different anticancer agents in different cancers (27). The reported increase in the therapeutic index of irinotecan in nude mice against multiple human tumor xenografts by Cao et al. led to the initiation of the concept of testing MSeC in combination with docetaxel against prostate cancer. It has been reported that organic selenium compounds (e.g., MSeC) and inorganic selenium compounds (e.g., sodium selenite) affect cell growth in multiple human cancer cell lines through different pathways (28, 29). Whereas most of the focus is on inorganic selenium compounds, this is the first report, to our knowledge, to investigate the combination treatment of organic selenium compound (MSeC) with docetaxel against prostate cancer. The goal of this study is to evaluate MSeC as a modulator of docetaxel efficacy and to understand the mechanism of the synergy between MSeC and docetaxel against prostate cancer. Understanding the mechanism of action of this combination regimen is important and could translate into improved clinical outcomes, thereby improving quality of life of men with prostate cancer.
In this study, we showed that pretreatment with MSeC is essential for the in vitro enhancement of docetaxel antitumor activity. At equitoxic concentration, pretreatment with MSeC decreased the concurrent combination dose that achieved 50% inhibition of cell growth (IC50 of concurrent combination) by 80%. In addition, at equimolar concentration, pretreatment with MSeC increased the inhibitory effect of the concurrent combination treatment by 30% (Fig. 1). Thus, MSeC enhancement of docetaxel antitumor activity is dependent on MSeC schedule.
This docetaxel enhanced inhibition of cell growth by the sequential combination treatment (MSeC/docetaxel) observed at the level of the population (sulforhodamine B assays) was determined to be due to an increase in apoptotic cell death and not due to cytostatic or growth-slowing effects. Trypan blue assay and time-lapse video showed that the vast majority of cells were trypan blue positive and were subject to an apoptotic type of death (shrinking, displaying total cessation of all membrane movement, and eventually breaking into smaller parts and forming apoptotic bodies; Figs. 1 and 2).
The enhanced docetaxel antitumor activity was further investigated to determine whether the combination effect is additive or synergistic. The result showed that the enhanced combination effect is synergistic when docetaxel and MSeC are given at different percentages of their individual concentrations that achieve IC90 or IC50 effects (Fig. 3). Specifically, administration of 20% of MSeC alone concentration that achieved 90% inhibition of cell growth (20% of IC90) and 15% of docetaxel alone concentration that achieved 90% inhibition of cell growth (15% of IC90) resulted in 90% combination inhibition of cell growth (IC90 of the combination; Fig. 3). In other words, 90% inhibition of cell growth with the combination treatment required 0.2 of MSeC and 0.15 of docetaxel of their individual IC90 doses. If the effect of the combination treatment of MSeC and docetaxel was additive, only 35% inhibition of cell growth would have been achieved. On the other hand, when docetaxel and MSeC were administered at different percentages of their individual IC10 concentrations, the combination effect was additive (Fig. 3).
Several reports documented the finding that MSeC induced apoptosis through different mechanisms in various cancer lines including leukemia, ovarian, and mammary cells (17, 30-34). In this study, to investigate the effect of the combination treatment (MSeC/docetaxel) on apoptosis and determine the mechanism of action behind the synergy, caspase-3 activity assay and detection of apoptotic cell death were done. MSeC/docetaxel treatment significantly increased the caspase-3 activity and apoptotic cell death when compared with each drug alone and untreated control (Fig. 4). The induced apoptotic cell death by MSeC/docetaxel treatment was significantly reduced when cells were pretreated with a pan-caspase inhibitor (Fig. 4). Conversion of synergy between MSeC and docetaxel to antagonism after pretreatment with a pancaspase inhibitor further confirms that caspase activation plays a critical role in the observed synergy (Fig. 5). In other words, blocking caspase-dependent apoptosis eliminates the synergy between MSeC and docetaxel.
The role of survivin in control and drug resistance in various in vitro and in vivo models, including prostate cancer, was documented by many reports. In summary, the higher the survivin level, the less apoptotic response achieved after drug treatment (35-37). Furthermore, the relationship between survivin and the response to docetaxel was also documented in several published reports; briefly, the higher the survivin level, the more resistance to docetaxel treatment (38, 39). In this study, survivin level was down-regulated by MSeC/docetaxel treatment when MSeC was given at IC25 and docetaxel was given at IC90, which corresponded with the maximum synergy. However, MSeC/docetaxel treatment with MSeC at IC25 and docetaxel at IC10, which corresponded with additive effect, did not change the survivin level (Fig. 6). These results associate survivin expression level after MSeC/docetaxel treatment with the observed MSeC/docetaxel synergy. The lower the survivin level in C2G cells after the MSeC/docetaxel treatment, the more synergy is observed.
In summary, pretreatment with MSeC was essential to markedly sensitize cells to docetaxel. The synergy between MSeC and docetaxel in C2G prostate cancer cells is associated with increased level of caspase-dependent apoptosis and decreased level of survivin. Future studies will determine whether the mechanism of action of MSeC/docetaxel treatment down-regulating survivin is transcriptional or translational.
Acknowledgments
Grant support: Grants CA65761 and CA109481 and Comprehensive Cancer Center Support grant CA16056 from the National Cancer Institute (Bethesda, MD).
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–30. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- 2.Landis SH, Taylor M, Bolden S, Wingo PA. Cancer statistics. CA Cancer J Clin. 1999;49:8–31. doi: 10.3322/canjclin.49.1.8. [DOI] [PubMed] [Google Scholar]
- 3.Ringel I, Horwitz SB. Studies with RP 56976 (Taxotere): a semisynthetic analogue of Taxol. J Natl Cancer Inst. 1991;83:288–91. doi: 10.1093/jnci/83.4.288. [DOI] [PubMed] [Google Scholar]
- 4.Diaz JF, Andreu JM. Assembly of purified GDP-tubulin into micro-tubules induced by Taxol and Taxotere: reversibility, ligand stoichiometry, and competition. Biochemistry. 1993;32:2747–55. doi: 10.1021/bi00062a003. [DOI] [PubMed] [Google Scholar]
- 5.Dumontet C, Sikic BI. Mechanisms of action of and resistance to antitubulin agents: microtubule dynamics, drug transport, and cell death. J Clin Oncol. 1999;17:1061–70. doi: 10.1200/JCO.1999.17.3.1061. [DOI] [PubMed] [Google Scholar]
- 6.Pienta KJ. Preclinical mechanisms of action of docetaxel and docetaxel combinations in prostate cancer. Semin Oncol. 2001;28:3–7. doi: 10.1016/s0093-7754(01)90148-4. [DOI] [PubMed] [Google Scholar]
- 7.Beer TM, El-Geneidi M, Eilers KM. Docetaxel (Taxotere) in the treatment of prostate cancer. Expert Rev Anticancer Ther. 2003;3:261–8. doi: 10.1586/14737140.3.3.261. [DOI] [PubMed] [Google Scholar]
- 8.Clark L, Combs GJ, Turnbull B, et al. Nutritional Prevention of Cancer Study Group Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. JAMA. 1997;276:1957–63. [PubMed] [Google Scholar]; JAMA. 1997;277:1520. Erratum in: [Google Scholar]
- 9.Medina D, Thompson H, Ganther H, Ip C. Se-methylselenocysteine: a new compound for chemoprevention of breast cancer. Nutr Cancer. 2001;40:12–7. doi: 10.1207/S15327914NC401_5. [DOI] [PubMed] [Google Scholar]
- 10.Ip C, Dong Y, Ganther HE. New concepts in selenium chemo-prevention. Cancer Metastasis Rev. 2002;21:281–9. doi: 10.1023/a:1021263027659. [DOI] [PubMed] [Google Scholar]
- 11.Klein EA. Selenium: epidemiology and basic science. J Urol. 2004;171:S50–3. doi: 10.1097/01.ju.0000107837.66277.e9. discussion S53. [DOI] [PubMed] [Google Scholar]
- 12.Duffield-Lillico AJ, Dalkin BL, Reid ME, et al. Selenium supplementation, baseline plasma selenium status and incidence of prostate cancer: an analysis of the complete treatment period of the Nutritional Prevention of Cancer Trial. BJU Int. 2003;91:608–12. doi: 10.1046/j.1464-410x.2003.04167.x. [DOI] [PubMed] [Google Scholar]
- 13.Klein EA, Thompson IM, Lippman SM, et al. SELECT: the next prostate cancer prevention trial. Selenum and Vitamin E Cancer Prevention Trial. J Urol. 2001;166:1311–5. doi: 10.1016/s0022-5347(05)65759-x. [DOI] [PubMed] [Google Scholar]
- 14.Jiang C, Wang Z, Ganther H, Lu J. Caspases as key executors of methyl selenium-induced apoptosis (anoikis) of DU-145 prostate cancer cells. Cancer Res. 2001;61:3062–70. [PubMed] [Google Scholar]
- 15.Wang Z, Jiang C, Lu J. Induction of caspase-mediated apoptosis and cell-cycle G1 arrest by selenium metabolite methylselenol. Mol Carcinog. 2002;34:113–20. doi: 10.1002/mc.10056. [DOI] [PubMed] [Google Scholar]
- 16.Lu J. Apoptosis and angiogenesis in cancer prevention by selenium. Adv Exp Med Biol. 2001;492:131–45. doi: 10.1007/978-1-4615-1283-7_11. [DOI] [PubMed] [Google Scholar]
- 17.Ip C, Dong Y. Methylselenocysteine modulates proliferation and apoptosis biomarkers in premalignant lesions of the rat mammary gland. Anticancer Res. 2001;21:863–7. [PubMed] [Google Scholar]
- 18.Foster BA, Gingrich JR, Kwon ED, Madias C, Greenberg NM. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 1997;57:3325–30. [PubMed] [Google Scholar]
- 19.Skehan P, Storeng R, Scudiero D, et al. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–12. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 20.Slocum HK, Parsons JC, Winslow EO, et al. Time-lapse video reveals immediate heterogeneity and heritable damage among human ileocecal carcinoma HCT-8 cells treated with raltitrexed (ZD1694) Cytometry. 2000;41:252–60. doi: 10.1002/1097-0320(20001201)41:4<252::aid-cyto3>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 21.Faessel HM, Levasseur LM, Slocum HK, Greco WR. Parabolic growth patterns in 96-well plate cell growth experiments. In Vitro Cell Dev Biol Anim. 1999;35:270–8. doi: 10.1007/s11626-999-0071-z. [DOI] [PubMed] [Google Scholar]
- 22.Faessel HM, Slocum HK, Rustum YM, Greco WR. Folic acid-enhanced synergy for the combination of trimetrexate plus the glycinamide ribonucleotide formyl transferase inhibitor, AG2034-comparison across sensitive and resistant human tumor cell lines. Biochem Pharmacol. 1999;57:567–77. doi: 10.1016/s0006-2952(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 23.Faessel HM, Slocum HK, Jackson RC, et al. Super in vitro synergy between inhibitors of dihydrofolate reductase and inhibitors of other folate-requiring enzymes: the critical role of polyglutamylation. Cancer Res. 1998;58:3036–50. [PubMed] [Google Scholar]
- 24.Levasseur LM, Greco WR, Rustum YM, Slocum HK. Combined action of paclitaxel and cisplatin against wildtype and resistant human ovarian carcinoma cells. Cancer Chemother Pharmacol. 1997;40:495–505. doi: 10.1007/s002800050693. [DOI] [PubMed] [Google Scholar]
- 25.Greco WR, Park HS, Rustum YM. Application of a new approach for the quantitation of drug synergism to the combination of cis-diamminedichloroplatinum and 1-β-D-arabinofuranosylcytosine [see comment] Cancer Res. 1990;50:5318–27. [PubMed] [Google Scholar]
- 26.Guo B, Yin MB, Toth K, Cao S, Azrak RG, Rustum YM. Dimerization of mitochondrial Bax is associated with increased drug response in Bax-transfected A253 cells. Oncol Res. 1999;11:91–9. [PubMed] [Google Scholar]
- 27.Cao S, Durrani F, Rustum YM. Selective modulation of the therapeutic efficacy of anticancer drugs by selenium containing compounds against human tumor xenografts. Clin Cancer Res. 2004;10:2561–9. doi: 10.1158/1078-0432.ccr-03-0268. [DOI] [PubMed] [Google Scholar]
- 28.Jiang C, Wang Z, Ganther H, Lu J. Distinct effects of methylseleninic acid versus selenite on apoptosis, cell cycle, and protein kinase pathways in DU145 human prostate cancer cells. Mol Cancer Ther. 2002;1:1059–66. [PubMed] [Google Scholar]
- 29.Sinha R, Said TK, Medina D. Organic and inorganic selenium compounds inhibit mouse mammary cell growth in vitro by different cellular pathways. Cancer Lett. 1996;107:277–84. doi: 10.1016/0304-3835(96)04373-x. [DOI] [PubMed] [Google Scholar]
- 30.Jang BC, Choi ES, Im KJ, et al. Induction of apoptosis by Se-MSC in U937 human leukemia cells through release of cytochrome c and activation of caspases and PKC-δ: mutual regulation between caspases and PKC-δ via a positive feedback mechanism. Int J Mol Med. 2003;12:733–9. [PubMed] [Google Scholar]
- 31.Yeo JK, Cha SD, Cho CH, et al. Se-methylselenocysteine induces apoptosis through caspase activation and Bax cleavage mediated by calpain in SKOV-3 ovarian cancer cells. Cancer Lett. 2002;182:83–92. doi: 10.1016/s0304-3835(02)00075-7. [DOI] [PubMed] [Google Scholar]
- 32.Unni E, Singh U, Ganther HE, Sinha R. Se-methylselenocysteine activates caspase-3 in mouse mammary epithelial tumor cells in vitro. Biofactors. 2001;14:169–77. doi: 10.1002/biof.5520140122. [DOI] [PubMed] [Google Scholar]
- 33.Jung U, Zheng X, Yoon SO, Chung AS. Se-methylselenocysteine induces apoptosis mediated by reactive oxygen species in HL-60 cells. Free Radic Biol Med. 2001;31:479–89. doi: 10.1016/s0891-5849(01)00604-9. [DOI] [PubMed] [Google Scholar]
- 34.Kim T, Jung U, Cho DY, Chung AS. Se-methylselenocysteine induces apoptosis through caspase activation in HL-60 cells. Carcinogenesis. 2001;22:559–65. doi: 10.1093/carcin/22.4.559. [DOI] [PubMed] [Google Scholar]
- 35.Zhang M, Mukherjee N, Bermudez RS, et al. Adenovirus-mediated inhibition of survivin expression sensitizes human prostate cancer cells to paclitaxel in vitro and in vivo. Prostate. 2005;64:293–302. doi: 10.1002/pros.20263. [DOI] [PubMed] [Google Scholar]
- 36.Zhang M, Latham DE, Delaney MA, Chakravarti A. Survivin mediates resistance to antiandrogen therapy in prostate cancer. Oncogene. 2005;24:2474–82. doi: 10.1038/sj.onc.1208490. [DOI] [PubMed] [Google Scholar]
- 37.Kishi H, Igawa M, Kikuno N, Yoshino T, Urakami S, Shiina H. Expression of the survivin gene in prostate cancer: correlation with clinicopathological characteristics, proliferative activity and apoptosis. J Urol. 2004;171:1855–60. doi: 10.1097/01.ju.0000120317.88372.03. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi N, Asano K, Suzuki H, et al. Adenoviral infection of survivin antisense sensitizes prostate cancer cells to etoposide in vivo. Prostate. 2005;15:10–9. doi: 10.1002/pros.20232. [DOI] [PubMed] [Google Scholar]
- 39.Meng H, Lu CD, Sun YL, Dai DJ, Lee SW, Tanigawa N. Expression level of wild-type survivin in gastric cancer is an independent predictor of survival. World J Gastroenterol. 2004;10:3245–50. doi: 10.3748/wjg.v10.i22.3245. [DOI] [PMC free article] [PubMed] [Google Scholar]