Abstract
Rationale
Recent studies have demonstrated that nicotine can enhance operant responding for other nonpharmacological reinforcing stimuli. However, the nature of the reinforcement-enhancing effect of nicotine remains largely unknown.
Objective
The present study determined the dose dependency of the ability of nicotine to increase lever-pressing responses maintained by a compound visual stimulus (VS) in rats and examined its sensitivity to pharmacological antagonism of nicotinic acetylcholine receptors (nAChRs).
Materials and methods
Male Sprague–Dawley rats were trained in daily 1-h sessions to lever press for delivery of a VS (1 s lever light on and 60 s house light off) on a fixed ratio 5 schedule. During these sessions, eight scheduled response-independent intravenous infusions of nicotine (total amount: 0, 0.06, 0.12, 0.24, 0.48 mg kg−1 h−1) were delivered. In pharmacological tests, a nonselective nAChR antagonist mecamylamine, α4β2-selective antagonist dihydro-β-erythroidine (DHβE), and α7-selective antagonist methyllycaconitine (MLA) were administered in different groups of rats 30 min before the session.
Results
The VS maintained a moderate level of lever-pressing responses and nicotine dose-dependently increased responses for the VS presentations. Preteatment of mecamylamine and DHβE but not MLA significantly attenuated the nicotine-enhanced responding. However, mecamylamine had no effect on responding for the VS in rats that received scheduled saline infusions.
Conclusions
These results demonstrate dose dependency of the reinforcement-enhancing effect of nicotine and suggest that activation of the α4β2- but not α7-containing nAChRs may mediate this effect.
Keywords: Nicotine, Nonpharmacological stimuli, Reinforcement-enhancing effect, Nicotinic acetylcholine receptors, Mecamylamine, Dihydro-β-erythroidine, Methyllycaconitine
Introduction
In the standard self-administration procedure, nicotine delivery is typically accompanied by contingent presentation of an environmental stimulus such as a cue light or a light plus tone, and response rates tend to be robust (Corrigall and Coen 1989; Donny et al. 1995; Chiamulera et al. 1996; Shoaib et al. 1997; Watkins et al. 1999). However, when nicotine is self-administered without accompanying stimuli, response rates are much lower (Donny et al. 2003; Palmatier et al. 2006; Chaudhri et al. 2007). These findings suggest that the stimuli accompanying nicotine delivery play a critical role in maintaining reliable levels of nicotine self-administration. Goldberg et al. 1981 found a 50% decrease in intravenous (i.v.) nicotine self-administration in squirrel monkeys when a brief visual stimulus (VS) that had been associated with the drug was omitted. Recently, work from our laboratory has shown that nicotine synergizes with a compound VS to produce response rates substantially higher than those produced by either nicotine alone or the stimulus alone and that this synergism also results when nicotine is administered noncontingently (independent of the recipient rat's behavior) in a yoked design (Donny et al. 2003; Chaudhri et al. 2007). These findings are in agreement with those of Cohen et al. 2005, who reported that nicotine enhances responding for a tone/light stimulus and Olausson et al. 2004 who found that nicotine increased operant responding for the delivery of a stimulus that had become a conditioned reinforcer via prior Pavlovian conditioning. Taken together, these findings indicate that in addition to its primary reinforcing actions, nicotine can enhance the reinforcing effects of other non-nicotine stimuli (Caggiula et al. 2001; Chiamulera 2005; Chaudhri et al. 2006).
Little is known about the neurobiological mechanisms underlying the ability of nicotine to enhance the reinforcement maintained by a reinforcing VS. Therefore, the present study was designed (1) to characterize dose dependency of the reinforcement-enhancing effect of nicotine and (2) to examine whether this effect is sensitive to pharmacological blockade of nicotinic acetylcholine receptors (nAChRs). Specifically, rats were trained to press a lever for presentation of a moderately reinforcing VS while i.v. nicotine infusions of various doses were administered in a response-independent manner. The number and timing of nicotine infusions were scheduled throughout the sessions based on the pattern of nicotine infusions obtained in our previous studies in which rats self-administered nicotine without accompanying stimuli (Palmatier et al. 2006; Chaudhri et al. 2007). This i.v. nicotine regimen has been documented in our laboratory to produce reliable reinforcement-enhancing effect. Moreover, it would allow us to understand the reinforcement-enhancing effect of nicotine in a context of nicotine self-administration in rats and ultimately smoking behavior in humans. In pharmacological tests, rats were tested for the effect of pretreatment with a nonselective nAChR antagonist mecamylamine (Takayama et al. 1989) on the reinforcement-enhancing effect of nicotine in the rats that received scheduled nicotine infusions. Moreover, mecamylamine was also tested in the rats that received scheduled saline infusions to examine whether nonselective blockade of the nAChRs interferes with the intrinsic reinforcing properties of the VS. To further determine involvement of activation of subtypes of the nAChRs in mediating the reinforcement-enhancing effect of nicotine, dihydro-β-erythroidine (DHβE), an α4β2-selective antagonist (Williams and Robinson 1984; Egan and North 1986; Rapier et al. 1990; Wang et al. 1991), and methyllycaconitine (MLA), an α7-selective antagonist (Macallan et al. 1988; Alkondon et al. 1992), were tested in the rats that received scheduled nicotine infusions.
Materials and methods
Subjects
Male Sprague–Dawley rats (Harlan Farms) weighing 225–250 g upon arrival were used. Animals were individually housed in a humidity- and temperature-controlled (21–22°C) colony room on a reversed 12:12 h light/dark cycle (lights off at 7:00 a.m., on at 7:00 p.m.) with unlimited access to water. After 1 week of habituation, during which time food was made available ad libitum, the rats were placed on a food restriction regimen throughout all experiments as described below. All training and experimental sessions were conducted during the dark phase at the same time each day (9:00 A.M.–3:00 p.m.). All experimental procedures were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Apparatus
All experimental sessions were conducted in experimental chambers located inside sound-attenuating, ventilated cubicles (Med Associates, St. Albans, VT). The chambers were equipped with two response levers on one side panel and with a 28-V white light above each lever as well as a house light on the top of the chambers. Between the two levers was a food receptacle. I.v. nicotine injections were delivered by a drug delivery system with a syringe pump (Med Associates, model PHM100-10 rpm). Experimental events and data collection were controlled by an interfaced computer and software (Med Associates, MED-PC 2.0).
Food training
After 1 week of habituation to the colony room, animals were trained to respond for food reinforcement to facilitate learning of operant responses. Briefly, after overnight food deprivation, rats were allowed to consume 75 food pellets (45 mg) in a single magazine-training session. The following day, they were hand shaped to press the right (active) lever for 75 food pellets on a fixed ratio (FR) 1 schedule in a single session. Responses on the left (inactive) lever had no scheduled consequences. The criterion for completion of food training was to successfully earn 75 pellets. If rats failed to meet the criterion, they were again hand shaped on the following day. All rats completed food training in 2 days. There was no VS presented during food training.
Surgery
After food training, rats were anesthetized with halothane and implanted with jugular catheters as described previously (Caggiula et al. 2001). The rats were allowed at least 7 days to recover from surgery, and the cannulae were flushed twice a day with 0.1 ml of sterile saline containing heparin (30 U/ml), Timentin (66.67 mg/ml), and streptokinase (8,333 U/ml) to maintain catheter patency and prevent infection. Thereafter, the catheters were flushed with the heparinized saline before and after sessions throughout the experiments.
Operant responses for presentations of the VS
Rats were trained in daily 1-h sessions to press the right lever for presentations of the VS. The sessions were initiated with extension of the two levers and illumination of a white house light. Once the rats reached the FR requirement at the active lever, the VS was presented that consisted of the onset for 1 s of a light above the active lever followed by the offset of the house light for 1 min, indicating a timeout period during which responses were recorded but not reinforced by the VS. An FR1 schedule was used for days 1–5, an FR2 for days 6–8, and an FR5 for the remainder of the experiment. Responses at the inactive lever were recorded but had no programmed consequence. Experimental sessions were conducted 5 days a week.
Scheduled nicotine infusions
During each 1-h session, eight i.v. infusions of nicotine were automatically administered independently of the animal's behavior. Nicotine infusions were dispensed by the drug delivery system in a volume of 0.1 ml in approximately 1 s. The number and timing of nicotine infusions were based on the pattern of nicotine infusions obtained in previous studies in which rats self-administered nicotine without accompanying stimuli (Palmatier et al. 2006; Chaudhri et al. 2007) and thereby scheduled across sessions at the following time points (in seconds): 30–78, 120–180, 250–450, 520–770, 830–1,060, 1,240–1,480, 1,750–1,940, and 2,100–2,300. If the scheduled infusion time happened to coincide with a VS delivery, this infusion was delayed at least 30 s so that there was no fortuitous association of nicotine infusion with VS presentation.
Dose–response study
Rats were randomly divided into five groups (n=8–11) for different nicotine doses of a total mount of 0 (saline control), 0.06, 0.12, 0.24, and 0.48 mg kg−1 3h−1 calculated as free base. That is, the different groups received eight scheduled injections of nicotine with the unit doses of 0, 0.0075, 0.015, 0.03, and 0.06 mg/kg per infusion. All groups received i.v. infusions at the same schedule described above. Animal grouping and the events of the VS presentation and nicotine infusion described above were implanted from the first daily session after recovery from surgery. After completion of 20 daily sessions, nicotine dose was switched to 0.24 mg kg−1 h−1 or 0.03 mg/kg per infusion for seven daily sessions to determine whether or not operant behavior would change accordingly, another indication of dose dependency.
Effect of mecamylamine
In rats that received scheduled nicotine infusions
After completion of the dose–response study, a subset of animals (n=8) that had patent catheters remained on the daily sessions with the scheduled infusions of nicotine at 0.03 mg/kg per infusion. These rats were habituated to subcutaneous saline injection 30 min before the session for 3 days. Then, 30 min before sessions, rats received subcutaneous injections of mecamylamine (0, 0.5, 1, 2 mg/kg) using a within-subject design. The eight rats were randomly designated into four sets (n=2 for each). Each set of rats received one of the four orders of drug dose combination: 0–0.5–1–-2, 0.5–1–2–0, 1–2–0–0.5, and 2–0–0.5–1. As such, both animals and the order of drug doses were counterbalanced in a pseudorandomized manner. Test sessions were conducted under conditions identical to that described above and arranged in such a manner that there were 2 days of nondrug sessions between testing to eliminate possible carryover effects of the drug; that is, rats received mecamylamine pretreatment on days 31, 34, 37, and 40.
In rats that received scheduled saline infusions
The saline rats (n=10) from the dose–response study that responded for the VS but without nicotine (saline infusions) were tested after pretreatment with mecamylamine at its highest dose (2 mg/kg) or saline vehicle. Pretreatments were given 30 min before each session in a counterbalanced order so that all rats received both saline and mecamylamine.
Effect of DHβE and MLA on nicotine-enhanced responding
A separate set of rats was trained to lever press for presentation of the VS with the eight scheduled response-independent infusions of nicotine at 0.03 mg/kg per infusion exactly the same as described above. After matched with the number of sessions received by the rats for mecamylamine tests, these rats were randomly divided into two groups for testing the effect of DHβE and MLA. In one group (n=12), DHβE (0, 1, 3, and 9 mg/kg) was subcutaneously administered 30 min before tests by using a within-subject design in a pseudorandomized order as described above for mecamylamine tests. The other group of rats (n=11) in a similar manner received intraperitoneal administration of MLA (0 and 10 mg/kg) 30 min before tests. The test sessions were arranged with 2 days of nondrug sessions in between.
Statistical analyses
The number of responses on the active and inactive levers in the dose–response study was separately analyzed by using two-way analysis of variance (ANOVA) with repeated measures (nicotine dose as the between-subject factor). The mean number of responses averaged across the last three sessions (sessions 18 to 20) of the FR5 phase was analyzed by one-way ANOVA. The data obtained from mecamylamine, DHβE, and MLA tests were separately analyzed by using one-way ANOVA with subsequent Newman–Keuls post-hoc tests to verify the significant difference among individual means.
Results
Dose dependence of the reinforcement-enhancing effect of nicotine
In saline (zero nicotine) rats, response-contingent presentation of the VS sustained a substantial level of lever-pressing responses (Fig. 1). Averaged across the last three sessions (sessions 18 to 20) of the FR5 schedule phase, rats emitted 74.5±9.3 responses at the active lever and 3.7±0.5 responses at the inactive lever (Fig. 2). Correspondingly, animals earned 13.4±1.9 presentations of the VS during the daily 1-h sessions, indicating the moderately reinforcing property of the VS.
Fig. 1.
Profiles of responses on the active (top) and inactive (below) levers. Note that the doses of nicotine in the figure legend are the total amount of nicotine rats received during the 1-h sessions. FR5:0.24 means that nicotine dose was switched to 0.24 mg kg−1 h−1 (0.03 mg/kg per infusion) in all nicotine-infused rats. See text for statistical details
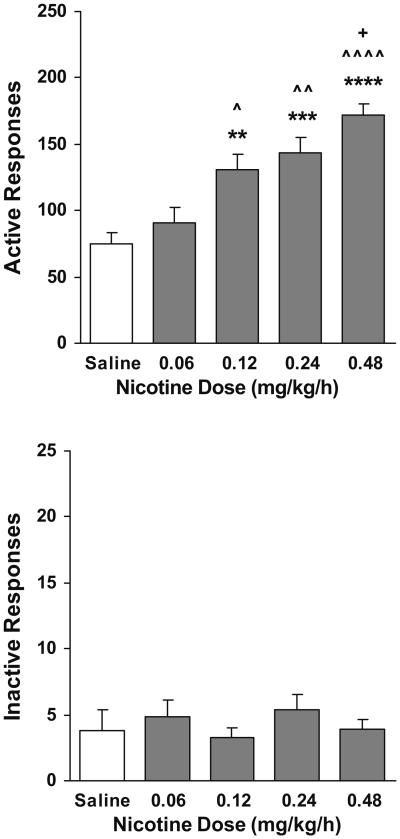
Fig. 2.
Dose-dependent effect of nicotine administered in a response-independent manner on responses on the active (top) and inactive (below) levers (n=8–11). The numbers of responses were averaged across the last three sessions (sessions 18 to 20) of the FR5 phase. **p< 0.01, ***p<0.001, and ****p<0.0001 different from saline group. ^p<0.05, ^^p<0.01, and ^^^^p<0.0001 different from the 0.06 mg kg−1 h−1 group. +p<0.05 different from the 0.12 mg kg−1 h−1 group
As shown in Fig. 1, top panel, response-independent i.v. infusions of nicotine dose-dependently increased lever pressing for the VS as compared to saline infusions. The mean of both the active and the inactive lever responses for each rat across the last three sessions of each FR schedule phases (sessions 3 to 5 on FR1, sessions 6 to 8 on FR2, and sessions 18 to 20 on FR5) was calculated, and a repeated-measures ANOVA on these means yielded significant main effect of dose (F[4, 86]=17.63, p<0.0001), lever (F[1, 86]=524.41, p<0.0001), and FR schedule phase (F[2, 172]=289.74, p<0.0001) as well as significant interactions of dose×phase (F[8, 172]=6.13, p<0.0001), lever×phase (F[2, 172]=301.37, p<0.0001), dose×lever (F[4, 86]=14.57, p<0.0001), and dose×lever×phase (F[8, 172]=6.31, p<0.0001). Further repeated-measure ANOVA analyses of the active response data across each FR schedule phase revealed significant main effect of dose (F[4, 43]=6.71, p<0.001 in FR1, F[4, 43]=7.50, p<0.001 in FR2, and F[4, 43]=21.42, p<0.0001 in FR5) and session (F[4, 172]=19.68, p<0.0001 in FR1, F[2, 86]=10.45, p<0.0001 in FR2, and F(11, 473)=16.59, p<0.0001 in FR5). There was a significant dose×session interaction in FR1 (F[16, 172]=1.77, p<0.05) but not FR2 or FR5. Similar analyses on the inactive lever responses produced neither main effect and nor interactions (details not shown).
When a single nicotine dose (0.24 mg kg−1 h−1 or 0.03 mg/kg per infusion) replaced other doses, the number of active lever responses approached to similar levels within three sessions as shown in the FR5:0.24 phase of Fig. 1, indicating that the active lever-pressing responses closely followed the change of nicotine dose. A repeated-measures ANOVA on the active response data across this phase revealed neither significant difference among the four nicotine-injected groups nor group×session interaction. Similar analysis on the inactive lever responses showed no difference across nicotine-injected groups. This data from a different angle support the dose dependency of the reinforcement-enhancing effect of nicotine.
Figure 2 shows the number of lever responses that were averaged across the final three sessions (sessions 18 to 20) on the FR5 schedule when rats received nicotine injections at different doses. There was a systemic increase in the number of active lever responses with increasing dose of nicotine. One-way ANOVA analysis confirmed the significant group (dose) effect (F[4, 43]=14.71, p<0.001), and subsequent Newman–Keuls post-hoc tests verified the significant increase in the number of responses in nicotine-injected groups as compared to saline rats (p<0.0001 for 0.48 mg kg−1 h−1, p<0.001 for 0.24 mg kg−1 h−1 and p<0.01 for 0.12 mg kg−1 h−1) and systemic increase as nicotine dose increased (statistical details are shown in Fig. 2, top). However, there was no significant change in the number of inactive lever responses (Fig. 2, below).
Effect of mecamylamine on nicotine-enhanced responses
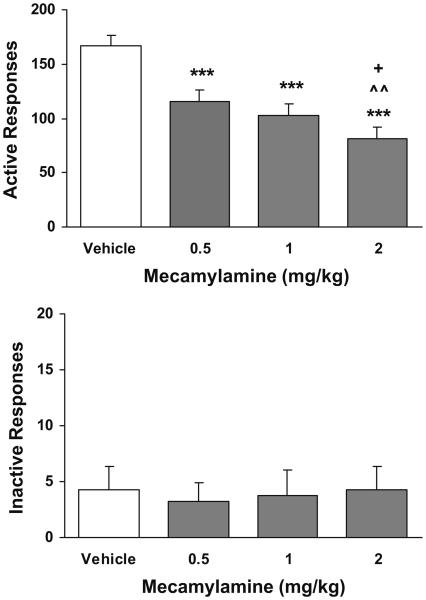
Pretreatment with mecamylamine significantly attenuated responses at the active lever as shown in Fig. 3, top. One-way ANOVA analysis yielded significant dose effect (F(3, 21)=23.82, p<0.001). Subsequent Newman–Keuls post-hoc tests verified the significant decreases in the number of responses in the mecamylamine-treated conditions as compared to the saline control condition (p<0.001 for all three doses). The number of responses under the highest dose (2 mg/kg) condition was significantly lower than that under 0.5 (p<0.01) and 1 mg/kg (p<0.05). There was no significant change in the number of inactive lever responses (Fig. 3, below).
Fig. 3.
Effect of mecamylamine on lever responses in rats (n=8) that received scheduled nicotine infusions. ***p<0.001 different from vehicle. ^^p<0.01 different from 0.5 mg/kg. +p<0.05 different from 1 mg/kg
Effect of mecamylamine in saline rats
In saline (zero nicotine) rats (n=10), pretreatment with mecamylamine (at its highest dose, 2 mg/kg) did not change lever-pressing responses for presentation of the VS (Fig. 4). There was no difference in the number of responses between mecamylamine vs vehicle condition (p>0.05).
Fig. 4.
Effect of mecamylamine on lever responses in rats (n=10) receiving no nicotine infusions
Effect of DHβE and MLA on nicotine-enhanced responses
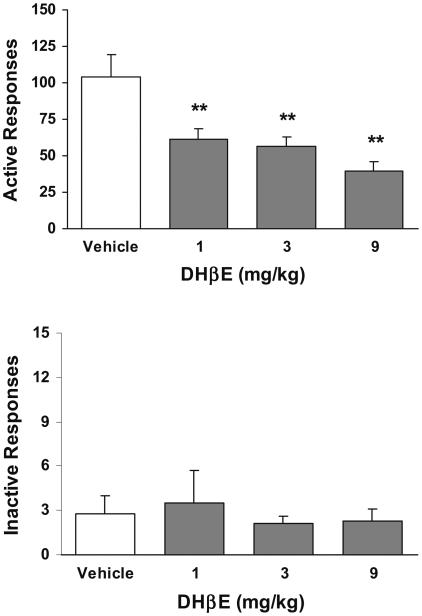
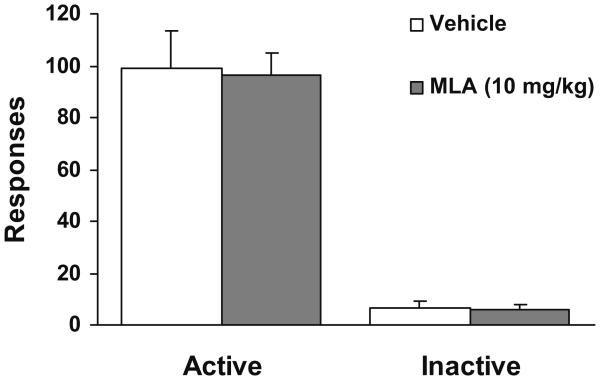
Pretreatment with DHβE substantially decreased the number of active lever responses (Fig. 5, top). One-way ANOVA analysis yielded a significant dose effect (F(3, 44)=8.24, p<0.05). Further Newman–Keuls post-hoc tests verified that the number of responses under all three doses of DHβE was significantly (p<0.01) lower than saline control condition. However, as shown in Fig. 6, pretreatment with MLA did not change the nicotine-enhanced responding because there was no difference in the number of active lever responses between MLA vs the saline control condition (p>0.05). Inactive lever responses in both drug groups remained at low levels indistinguishable across different dose conditions (Fig. 5, below, and Fig. 6).
Fig. 5.
Effect of DHβE on lever responses in rats (n=12) that received scheduled nicotine infusions. **p<0.01 different from vehicle
Fig. 6.
Effect of MLA on lever responses in rats (n=11) that received scheduled nicotine infusions
Discussion
This study examined the dose–response function describing the ability of nicotine to enhance lever-pressing responses for the moderately reinforcing VS, the reinforcement-enhancing effect of nicotine. In pharmacological tests, nonselective blockade of the nAChRs by mecamylamine attenuated nicotine-enhanced responses but did not change the reinforcing value of the VS, and importantly, pretreatment with DHβE but not MLA decreased nicotine-enhanced responses. These data indicate that activation of the nAChRs containing α4β2- but not α7-subunits is involved in mediation of the reinforcement-enhancing effect of nicotine.
In this study, rats responded at an active lever for presentations of a compound VS indicating the latter bears intrinsically reinforcing value. The VS may belong to the category of sensory stimuli that have long been evidenced to have reinforcing properties (Stewart 1960; Fowler 1971). Across the daily 1-h sessions, eight injections of i.v. nicotine were delivered in a response-independent manner, which were scheduled based on the pattern of nicotine infusions that happened in nicotine self-administration paradigms in our previous studies (Palmatier et al. 2006; Chaudhri et al. 2007). As previously shown, the VS (1 s turn on of lever light and 60 s turn off of house light) sustained substantial level of lever-pressing behavior, indicating its moderately reinforcing property (Caggiula et al. 2002; Donny et al. 2003; Palmatier et al. 2006; Chaudhri et al. 2007). Scheduled i.v. infusions of nicotine substantially enhanced the VS-maintained responding as compared to saline infusions. In light of the fact that nicotine has psychomotor stimulating effects, one may speculate that the response-enhancing effect might be due to its nonspecific stimulation of locomotor activity. However, the finding that responding at the inactive lever where no VS presented remained unchanged negates this possibility. Therefore, the noncontingently administered nicotine produced its enhancing effect selectively on operant responses that were reinforced by the moderately reinforcing VS. This finding confirms previous observations from our laboratory (Caggiula et al. 2002; Donny et al. 2003; Palmatier et al. 2006; Chaudhri et al. 2007). In another line of studies using brain self-stimulation reward procedures, nicotine has been found to decrease the threshold of electrical self-stimulation of the medial forebrain bundle, lateral hypothalamus, and ventral tegmental area and therefore potentiate the rewarding effects of electrical stimulation in these brain regions (Olds and Domino 1969; Pradhan and Bowling 1971; Newman 1972; Huston-Lyons and Kornetsky 1992; Ivanova and Greenshaw 1997; Wise et al. 1998; Harrison et al. 2002; Kenny and Markou 2006). Taken together, these data demonstrate that nicotine produces reinforcement-enhancing effect. More interestingly, this effect has been found to depend on the reinforcing value of the non-nicotine stimuli. Recent studies have shown that although nicotine does not produce enhancement effect on the low level of operant responses maintained by relatively neutral stimuli, after these stimuli were endowed with reinforcing value by being conditioned to sucrose pellets or water, nicotine significantly increased responses reinforced by these conditioned stimuli (Olausson et al. 2004; Liu et al. 2005). However, it should be noted that the reinforcement-enhancing effect may not be a unique feature of nicotine only. There has been evidence showing that other drugs also produce similar response enhancement. For instance, other psychostimulants (e.g., amphetamine, cocaine) and drugs of different pharmacological categories (e.g., opiates) as well have been found to increase operant responses supported by reinforcing stimuli including conditioned reinforcers (Hill 1970; Robbins 1976; Robbins and Koob 1978; Phillips and Fibiger 1990; Cunningham and Kelley 1992; Harmer and Phillips 1998; Taylor and Horger 1999; Taylor and Jentsch 2001; Chaudhri et al. 2006). Comparing the reinforcement-enhancing effect across these psychoactive substances would warrant future studies.
The first significant finding of this study was the dose dependency of the reinforcement-enhancing effect of nicotine. Nicotine at doses from 0.06 to 0.48 mg kg−11 h−1 systemically increased responses for the VS, although at the lowest dose (0.06 mg kg−1 h−1), this effect did not reach statistical significance. Moreover, the dose dependency was also reflected by the convergence of response rates of different groups when they were all switched to a single dose of nicotine (0.24 mg kg−1 h−1). Rats in groups with previously lower nicotine doses (0.06 and 0.12 mg kg−1 h−1) substantially increased lever-pressing responses after nicotine dose was switched and reached level similar to that of the 0.24 mg kg−1 h−1 group by the second session. Rats in the highest dose group (0.48 mg/kg−1 h−1) decreased the rate of responding to the level of that of the 0.24 mg kg−1 h−1 group in three sessions. It is noted that the linear dose–response curve describing the reinforcement-enhancing effect of nicotine in the present study differs from the inverted “U”-shaped curve typically reported for i.v. self-administration of nicotine, which presents the maximum effect produced by 0.03 mg/kg per infusion (Shoaib et al. 1997; Donny et al. 1999; Watkins et al. 1999). This could be attributable to two facts. First, if both the nicotine doses expressed as 0.0075–0.06 mg/kg per infusion and the limited, predetermined eight infusions were taken into account, nicotine doses used in this study did not go beyond the ascending limb of a typical dose–response curve obtained from nicotine self-administration paradigms, where there was no limitation on the number of self-administration of nicotine (Shoaib et al. 1997; Donny et al. 1999; Watkins et al. 1999). In this regard, even compared to one of our previous study (Chaudhri et al. 2007) where the noncontingent nicotine injections were yoked to other self-administering rats rather than predetermined, the present study not only supports the finding in that study that the reinforcement-enhancing effect of nicotine can be observed within a wide range of nicotine doses but also for the first time describes the linear dose–response function of the reinforcement-enhancing effect of nicotine. Second, nicotine probably has aversive effects at higher doses, which would reduce responding when both nicotine and the stimulus are contingent on the same operant response, thus leading to the descending limb of the dose–response curve in the standard self-administration paradigm. The argument has gained support from our recent studies. When responding for the VS is dissociated with responses for the nicotine infusion, rats respond at one lever for nicotine injections at a much lower level than that obtained from standard self-administration paradigms, while responses at another lever for the VS presentations reaches levels similar to that of standard self-administration paradigm (Palmatier et al. 2006).
The second purpose of this study was to determine whether the reinforcement-enhancing effect of nicotine is sensitive to pharmacological antagonism of nicotinic neurotransmission. Pretreatment with a nonselective nicotinic antagonist, mecamylamine (0.5, 1, 2 mg/kg) significantly attenuated lever-pressing for the VS, when compared to vehicle, in rats that received scheduled nicotine infusions. One may speculate that this agent would produce nonspecific impairment of general locomotor activity because systemic administered mecamylamine might also produce peripheral effects such as ganglionic blockade and hypotension besides its antagonizing action at nicotinic receptors located in brain (Varanda et al. 1985; Loiacono et al. 1993; Eissenberg et al. 1996). However, this possibility can be readily ruled out based on the facts that this agent did not change responses in rats receiving scheduled saline infusions and attenuated responses at only the active but not the inactive lever in nicotine rats and that it produced no depression on operant responses reinforced by other reinforcers such as food (Liu et al. 2007), water (Glick et al. 2002), or stimulus conditioned to food reinforcement (Liu et al. 2007). Another speculation is that the inhibitory effect of mecamylamine might be a result of its attenuating the reinforcing value of the VS per se. However, two facts should be noted: First, mecamylamine only abolished the enhancement of lever pressing produced by nicotine; even at the highest dose of mecamylamine, response rates for the VS were comparable to those of animals received saline infusions (without the reinforcement-enhancing effect of nicotine), and second, in rats that received infusions of saline rather than nicotine, mecamylamine pretreatment had no effect on responding for the VS. Therefore, the inhibitory effect of mecamylamine in nicotine rats was not due to interference with the reinforcing value of the VS and instead resulted from its attenuation of the reinforcement-enhancing effect of nicotine. Our results are in agreement with other findings that mecamylamine reversed nicotine-enhanced lever pressing for a conditioned reinforcer (Olausson et al. 2004) and that in brain self-stimulation studies this agent effectively blocked nicotine-induced decrease in electrical self-stimulation threshold (Huston-Lyons and Kornetsky 1992; Ivanova and Greenshaw 1997). Taken together, these data extend the role of nicotinic neurotransmission in mediating the direct positive reinforcing actions of nicotine (Watkins et al. 1999; Mathieu-Kia et al. 2002; Wonnacott et al. 2005) to the involvement in the reinforcement-enhancing effect of nicotine.
Significantly, the present study further determined involvement of subtypes of the nAChRs. Antagonists selective for the two most characterized subtypes of the nAChRs, α4β2 and α7 (Lena and Changeux 1997; Lukas et al. 1999; Gotti et al. 2006), were tested. DHβE, an α4β2-selective antagonist, effectively decreased the nicotine-enhanced lever responses. This finding is in line with previous studies showing that knockout of β2-subunit of the nAChRs cancelled the ability of nicotine to enhance responding for a food-conditioned reinforcement (Brunzell et al. 2006) and that DHβE reversed nicotine-induced potentiation of the electrical stimulation reward (Harrison et al. 2002; Kenny and Markou 2006). However, the α7-selective antagonist MLA did not change magnitude of nicotine-enhanced responding. Because the dose of MLA used in this study was the highest one or higher than the effective dose ever reported in literature (Gommans et al. 2000; Grottick et al. 2000; Markou and Paterson 2001), this single dose should not give rise to doubt on the lack of effect of this agent. Together, these data suggest that the nAChRs containing α4β2- but not α7-subunits may play an important role in mediating the reinforcement-enhancing effect of nicotine. In addition, the report that MLA also has antagonistic actions on the α3/α6-containing nAChRs (Mogg et al. 2002) suggests exclusion of these nAChR subtypes in the mediation of the reinforcement-enhancing effect of nicotine.
Mesolimbic dopamine circuitry has been implicated in mediating reinforcing properties of pharmacological reinforcers and natural rewards as well (Koob 1987; Robinson and Berridge 1993; Di Chiara 2000; Salamone et al. 2003). There is evidence showing that nicotine modulates dopaminergic neurotransmission. For instance, nicotine increases burst firing of dopamine neurons in the ventral tegmental area and dopamine release in the nucleus accumbens (Nisell et al. 1994; Nisell et al. 1996; Marshall et al. 1997). Recently, Rice and Cragg 2004 found that nicotine selectively enhances dopamine release during phasic but not tonic neuronal firing activity. Because dopamine neurons respond to salient stimuli such as primary or conditioned rewards by switching from tonic to phasic firing (Schultz et al. 1997), it has been suggested that amplification of mesolimbic dopaminergic neurotrans-mission in response to reinforcing stimuli might be a mechanism for nicotine to enhance reinforcement. This argument is supported by the finding that nicotine-induced potentiation of brain electrical stimulation reward was blocked by dopamine antagonists (Huston-Lyons et al. 1993; Ivanova and Greenshaw 1997; Harrison et al. 2002). In light of the fact that the β2-containing nAChRs are mostly expressed on dopaminergic neurons in the midbrain (Klink et al. 2001) and activation of these receptors increases dopaminergic firing rates and dopamine release in this brain region (Picciotto et al. 1998; Klink et al. 2001; Marubio et al. 2003; Schilstrom et al. 2003), it is argued that a neural pathway of signal transduction via DHβE-sensitive, β2-containing nAChR–dopamine cascade may underlie the reinforcement-enhancing effect of nicotine.
In summary, this study characterized the dose dependency of the reinforcement-enhancing effect of nicotine. The finding that pretreatment with mecamylamine attenuated the nicotine-enhanced operant responses but did not change lever responding in saline rats indicates that the blockade of nicotinic neurotransmission produces no influence on the reinforcing properties of the VS but selectively interferes with the reinforcement-enhancing effect of nicotine. The finding that DHβE but not MLA effectively decreased the nicotine-enhanced responses suggests that activation of the α4β2- but not α7-containing nAChRs is implicated in mediating the reinforcement-enhancing effect of nicotine.
Acknowledgments
This work was supported by NIH grant DA17288 (X. Liu) and DA10464 (A.R. Caggiula) from the National Institute on Drug Abuse. The authors would like to thank Sheri Booth, Maysa Gharib, Laure Craven, and Nicole Roehrig for their technical assistance.
References
- Alkondon M, Pereira EF, Wonnacott S, Albuquerque EX. Blockade of nicotinic currents in hippocampal neurons defines methyllycaconitine as a potent and specific receptor antagonist. Mol Pharmacol. 1992;41:802–808. [PubMed] [Google Scholar]
- Brunzell DH, Chang JR, Schneider B, Olausson P, Taylor JR, Picciotto MR. Beta2-subunit-containing nicotinic acetylcholine receptors are involved in nicotine-induced increases in conditioned reinforcement but not progressive ratio responding for food in C57BL/6 mice. Psychopharmacology (Berl) 2006;184:328–338. doi: 10.1007/s00213-005-0099-z. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Cue dependency of nicotine self-administration and smoking. Pharmacol Biochem Behav. 2001;70:515–530. doi: 10.1016/s0091-3057(01)00676-1. [DOI] [PubMed] [Google Scholar]
- Caggiula AR, Donny EC, White AR, Chaudhri N, Booth S, Gharib MA, Hoffman A, Perkins KA, Sved AF. Environmental stimuli promote the acquisition of nicotine self-administration in rats. Psychopharmacology (Berl) 2002;163:230–237. doi: 10.1007/s00213-002-1156-5. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Palmatier MI, Liu X, Sved AF. Complex interactions between nicotine and nonpharmacological stimuli reveal multiple roles for nicotine in reinforcement. Psychopharmacology (Berl) 2006;184:353–366. doi: 10.1007/s00213-005-0178-1. [DOI] [PubMed] [Google Scholar]
- Chaudhri N, Caggiula AR, Donny EC, Booth S, Gharib M, Craven L, Palmatier MI, Liu X, Sved AF. Self-administered and noncontingent nicotine enhance reinforced operant responding in rats: impact of nicotine dose and reinforcement schedule. Psychopharmacology (Berl) 2007;190:353–362. doi: 10.1007/s00213-006-0454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C. Cue reactivity in nicotine and tobacco dependence: a “multiple-action” model of nicotine as a primary reinforcement and as an enhancer of the effects of smoking-associated stimuli. Brain Res Brain Res Rev. 2005;48:74–97. doi: 10.1016/j.brainresrev.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Chiamulera C, Borgo C, Falchetto S, Valerio E, Tessari M. Nicotine reinstatement of nicotine self-administration after long-term extinction. Psychopharmacology (Berl) 1996;127:102–107. doi: 10.1007/BF02805981. [DOI] [PubMed] [Google Scholar]
- Cohen C, Perrault G, Griebel G, Soubrie P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716) Neuropsychopharmacology. 2005;30:145–155. doi: 10.1038/sj.npp.1300541. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berl) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Cunningham ST, Kelley AE. Opiate infusion into nucleus accumbens: contrasting effects on motor activity and responding for conditioned reward. Brain Res. 1992;588:104–114. doi: 10.1016/0006-8993(92)91349-j. [DOI] [PubMed] [Google Scholar]
- Di Chiara G. Role of dopamine in the behavioural actions of nicotine related to addiction. Eur J Pharmacol. 2000;393:295–314. doi: 10.1016/s0014-2999(00)00122-9. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Knopf S, Brown C. Nicotine self-administration in rats. Psychopharmacology (Berl) 1995;122:390–394. doi: 10.1007/BF02246272. [DOI] [PubMed] [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, Gharib MA, Hoffman A, Maldovan V, Shupenko C, McCallum SE. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Donny EC, Chaudhri N, Caggiula AR, Evans-Martin FF, Booth S, Gharib MA, Clements LA, Sved AF. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology (Berl) 2003;169:68–76. doi: 10.1007/s00213-003-1473-3. [DOI] [PubMed] [Google Scholar]
- Egan TM, North RA. Actions of acetylcholine and nicotine on rat locus coeruleus neurons in vitro. Neuroscience. 1986;19:565–571. doi: 10.1016/0306-4522(86)90281-2. [DOI] [PubMed] [Google Scholar]
- Eissenberg T, Griffiths RR, Stitzer ML. Mecamylamine does not precipitate withdrawal in cigarette smokers. Psychopharmacology (Berl) 1996;127:328–336. doi: 10.1007/s002130050094. [DOI] [PubMed] [Google Scholar]
- Fowler H. Implications of sensory reinforcement. The nature of reinforcement. A symposium of the learning research and development center; University of Pittsburgh. R. Glaser. Academic; New York. 1971. pp. 151–196. [Google Scholar]
- Glick SD, Maisonneuve IM, Kitchen BA. Modulation of nicotine self-administration in rats by combination therapy with agents blocking alpha 3 beta 4 nicotinic receptors. Eur J Pharmacol. 2002;448:185–191. doi: 10.1016/s0014-2999(02)01944-1. [DOI] [PubMed] [Google Scholar]
- Goldberg SR, Spealman RD, Goldberg DM. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science. 1981;214:573–575. doi: 10.1126/science.7291998. [DOI] [PubMed] [Google Scholar]
- Gommans J, Stolerman IP, Shoaib M. Antagonism of the discriminative and aversive stimulus properties of nicotine in C57BL/6J mice. Neuropharmacology. 2000;39:2840–2847. doi: 10.1016/s0028-3908(00)00130-1. [DOI] [PubMed] [Google Scholar]
- Gotti C, Zoli M, Clementi F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol Sci. 2006;27:482–491. doi: 10.1016/j.tips.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Trube G, Corrigall WA, Huwyler J, Malherbe P, Wyler R, Higgins GA. Evidence that nicotinic alpha(7) receptors are not involved in the hyperlocomotor and rewarding effects of nicotine. J Pharmacol Exp Ther. 2000;294:1112–1119. [PubMed] [Google Scholar]
- Harmer CJ, Phillips GD. Enhanced appetitive conditioning following repeated pretreatment with d-amphetamine. Behav Pharmacol. 1998;9:299–308. [PubMed] [Google Scholar]
- Harrison AA, Gasparini F, Markou A. Nicotine potentiation of brain stimulation reward reversed by DH beta E and SCH 23390, but not by eticlopride, LY 314582 or MPEP in rats. Psychopharmacology (Berl) 2002;160:56–66. doi: 10.1007/s00213-001-0953-6. [DOI] [PubMed] [Google Scholar]
- Hill RT. Facilitation of conditioned reinforcement as a mechanism for psychomotor stimulation. In: Costa E, Garattini S, editors. Amphtamine and related compound: Proceedings of the Mario Negri Institute of Pharmacological Research. Raven; New York: 1970. pp. 781–795. [Google Scholar]
- Huston-Lyons D, Kornetsky C. Effects of nicotine on the threshold for rewarding brain stimulation in rats. Pharmacol Biochem Behav. 1992;41:755–759. doi: 10.1016/0091-3057(92)90223-3. [DOI] [PubMed] [Google Scholar]
- Huston-Lyons D, Sarkar M, Kornetsky C. Nicotine and brain-stimulation reward: interactions with morphine, amphetamine and pimozide. Pharmacol Biochem Behav. 1993;46:453–457. doi: 10.1016/0091-3057(93)90378-7. [DOI] [PubMed] [Google Scholar]
- Ivanova S, Greenshaw AJ. Nicotine-induced decreases in VTA electrical self-stimulation thresholds: blockade by haloperidol and mecamylamine but not scopolamine or ondansetron. Psychopharmacology (Berl) 1997;134:187–192. doi: 10.1007/s002130050441. [DOI] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology. 2006;31:1203–1211. doi: 10.1038/sj.npp.1300905. [DOI] [PubMed] [Google Scholar]
- Klink R, de Kerchove d'Exaerde A, Zoli M, Changeux JP. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J Neurosci. 2001;21:1452–1463. doi: 10.1523/JNEUROSCI.21-05-01452.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF. Neural substrates of opioid tolerance and dependence. Natl Inst Drug Abuse Res Monogr Ser. 1987;76:46–52. [PubMed] [Google Scholar]
- Lena C, Changeux JP. Pathological mutations of nicotinic receptors and nicotine-based therapies for brain disorders. Curr Opin Neurobiol. 1997;7:674–682. doi: 10.1016/s0959-4388(97)80088-8. [DOI] [PubMed] [Google Scholar]
- Liu X, Caggiula AR, Palmatier MI, Donny EC, Chaudhri N, Sved AF. Reinforcement enhancing effect of nicotine depends on the reinforcement valence of non-drug stimulus. Annual Meeting of Society for Neuroscience. 2005 [Google Scholar]
- Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE. Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology. 2007;32:710–718. doi: 10.1038/sj.npp.1301129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loiacono R, Stephenson J, Stevenson J, Mitchelson F. Multiple binding sites for nicotine receptor antagonists in inhibiting [3H](−)-nicotine binding in rat cortex. Neuropharmacology. 1993;32:847–853. doi: 10.1016/0028-3908(93)90139-t. [DOI] [PubMed] [Google Scholar]
- Lukas RJ, Changeux JP, Le Novere N, Albuquerque EX, Balfour DJ, Berg DK, Bertrand D, Chiappinelli VA, Clarke PB, Collins AC, Dani JA, Grady SR, Kellar KJ, Lindstrom JM, Marks MJ, Quik M, Taylor PW, Wonnacott S. International Union of Pharmacology. XX. Current status of the nomenclature for nicotinic acetylcholine receptors and their subunits. Pharmacol Rev. 1999;51:397–401. [PubMed] [Google Scholar]
- Macallan DR, Lunt GG, Wonnacott S, Swanson KL, Rapoport H, Albuquerque EX. Methyllycaconitine and (+)-anatoxin-a differentiate between nicotinic receptors in vertebrate and invertebrate nervous systems. FEBS Lett. 1988;226:357–363. doi: 10.1016/0014-5793(88)81454-6. [DOI] [PubMed] [Google Scholar]
- Markou A, Paterson NE. The nicotinic antagonist methyllycaconitine has differential effects on nicotine self-administration and nicotine withdrawal in the rat. Nicotine Tob Res. 2001;3:361–373. doi: 10.1080/14622200110073380. [DOI] [PubMed] [Google Scholar]
- Marshall DL, Redfern PH, Wonnacott S. Presynaptic nicotinic modulation of dopamine release in the three ascending pathways studied by in vivo microdialysis: comparison of naive and chronic nicotine-treated rats. J Neurochem. 1997;68:1511–1519. doi: 10.1046/j.1471-4159.1997.68041511.x. [DOI] [PubMed] [Google Scholar]
- Marubio LM, Gardier AM, Durier S, David D, Klink R, Arroyo-Jimenez MM, McIntosh JM, Rossi F, Champtiaux N, Zoli M, Changeux JP. Effects of nicotine in the dopaminergic system of mice lacking the alpha4 subunit of neuronal nicotinic acetylcholine receptors. Eur J Neurosci. 2003;17:1329–1337. doi: 10.1046/j.1460-9568.2003.02564.x. [DOI] [PubMed] [Google Scholar]
- Mathieu-Kia AM, Kellogg SH, Butelman ER, Kreek MJ. Nicotine addiction: insights from recent animal studies. Psycho-pharmacology (Berl) 2002;162:102–118. doi: 10.1007/s00213-002-1096-0. [DOI] [PubMed] [Google Scholar]
- Mogg AJ, Whiteaker P, Mcintosh JM, Marks K, Collins AC, Wonnacott S. Methyllycaconitine is a potent antagonist of α-conotoxin-MII-sensitive presynaptic nicotinic acetylcholine receptors in rat striatum. J Pharmacol Exp Ther. 2002;302:197–204. doi: 10.1124/jpet.302.1.197. [DOI] [PubMed] [Google Scholar]
- Newman LM. Effects of cholinergic agonists and antagonists on self-stimulation behavior in the rat. J Comp Physiol Psychol. 1972;79:394–413. doi: 10.1037/h0032830. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Svensson TH. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse. 1994;16:36–44. doi: 10.1002/syn.890160105. [DOI] [PubMed] [Google Scholar]
- Nisell M, Nomikos GG, Hertel P, Panagis G, Svensson TH. Condition-independent sensitization of locomotor stimulation and mesocortical dopamine release following chronic nicotine treatment in the rat. Synapse. 1996;22:369–381. doi: 10.1002/(SICI)1098-2396(199604)22:4<369::AID-SYN8>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Olausson P, Jentsch JD, Taylor JR. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology (Berl) 2004;171:173–178. doi: 10.1007/s00213-003-1575-y. [DOI] [PubMed] [Google Scholar]
- Olds ME, Domino EF. Comparison of muscarinic and nicotinic cholinergic agonists on self-stimulation behavior. J Pharmacol Exp Ther. 1969;166:189–204. [PubMed] [Google Scholar]
- Palmatier MI, Evans-Martin FF, Hoffman A, Caggiula AR, Chaudhri N, Donny EC, Liu X, Booth S, Gharib M, Craven L, Sved AF. Dissociating the primary reinforcing and reinforcement-enhancing effects of nicotine using a rat self-administration paradigm with concurrently available drug and environmental reinforcers. Psychopharmacology (Berl) 2006;184:391–400. doi: 10.1007/s00213-005-0183-4. [DOI] [PubMed] [Google Scholar]
- Phillips AG, Fibiger HC. Role of reward and enhancement of conditioned reward in persistence of responding for cocaine. Behav Pharmacol. 1990;1:269–282. doi: 10.1097/00008877-199000140-00002. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Zoli M, Rimondini R, Lena C, Marubio LM, Pich EM, Fuxe K, Changeux JP. Acetylcholine receptors containing the beta2 subunit are involved in the reinforcing properties of nicotine. Nature. 1998;391:173–177. doi: 10.1038/34413. [DOI] [PubMed] [Google Scholar]
- Pradhan SN, Bowling C. Effects of nicotine on self-stimulation in rats. J Pharmacol Exp Ther. 1971;176:229–243. [PubMed] [Google Scholar]
- Rapier C, Lunt GG, Wonnacott S. Nicotinic modulation of [3H] dopamine release from striatal synaptosomes: pharmacological characterisation. J Neurochem. 1990;54:937–945. doi: 10.1111/j.1471-4159.1990.tb02341.x. [DOI] [PubMed] [Google Scholar]
- Rice ME, Cragg SJ. Nicotine amplifies reward-related dopamine signals in striatum. Nat Neurosci. 2004;7:583–584. doi: 10.1038/nn1244. [DOI] [PubMed] [Google Scholar]
- Robbins TW. Relationship between reward-enhancing and stereotypical effects of psychomotor stimulant drugs. Nature. 1976;264:57–59. doi: 10.1038/264057a0. [DOI] [PubMed] [Google Scholar]
- Robbins TW, Koob GF. Pipradrol enhances the reinforcing properties of stimuli paired. Pharmacol Biochem Behav. 1978;8:219–221. doi: 10.1016/0091-3057(78)90308-8. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food–seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Schilstrom B, Rawal N, Mameli-Engvall M, Nomikos GG, Svensson TH. Dual effects of nicotine on dopamine neurons mediated by different nicotinic receptor subtypes. Int J Neuropsychopharmacol. 2003;6:1–11. doi: 10.1017/S1461145702003188. [DOI] [PubMed] [Google Scholar]
- Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997;275:1593–1599. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- Shoaib M, Schindler CW, Goldberg SR. Nicotine self-administration in rats: strain and nicotine pre-exposure effects on acquisition. Psychopharmacology (Berl) 1997;129:35–43. doi: 10.1007/s002130050159. [DOI] [PubMed] [Google Scholar]
- Stewart J. Reinforcing effects of light as a function of intensity and reinforcement schedule. J Comp Physiol Psychol. 1960;53:187–193. doi: 10.1037/h0047315. [DOI] [PubMed] [Google Scholar]
- Takayama H, Majewska MD, London ED. Interactions of noncompetitive inhibitors with nicotinic receptors in the rat brain. J Pharmacol Exp Ther. 1989;251:1083–1089. [PubMed] [Google Scholar]
- Taylor JR, Horger BA. Enhanced responding for conditioned reward produced by intra-accumbens amphetamine is potentiated after cocaine sensitization. Psychopharmacology (Berl) 1999;142:31–40. doi: 10.1007/s002130050859. [DOI] [PubMed] [Google Scholar]
- Taylor JR, Jentsch JD. Repeated intermittent administration of psychomotor stimulant drugs alters the acquisition of Pavlovian approach behavior in rats: differential effects of cocaine, d-amphetamine and 3,4-methylenedioxymethamphetamine (“Ecstasy”) Biol Psychiatry. 2001;50:137–143. doi: 10.1016/s0006-3223(01)01106-4. [DOI] [PubMed] [Google Scholar]
- Varanda WA, Aracava Y, Sherby SM, VanMeter WG, Eldefrawi ME, Albuquerque EX. The acetylcholine receptor of the neuromuscular junction recognizes mecamylamine as a noncompetitive antagonist. Mol Pharmacol. 1985;28:128–137. [PubMed] [Google Scholar]
- Wang YT, Neuman RS, Bieger D. Nicotinic cholinoceptor-mediated excitation in ambigual motoneurons of the rat. Neuroscience. 1991;40:759–767. doi: 10.1016/0306-4522(91)90010-l. [DOI] [PubMed] [Google Scholar]
- Watkins SS, Epping-Jordan MP, Koob GF, Markou A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol Biochem Behav. 1999;62:743–751. doi: 10.1016/s0091-3057(98)00226-3. [DOI] [PubMed] [Google Scholar]
- Williams M, Robinson JL. Binding of the nicotinic cholinergic antagonist, dihydro-beta-erythroidine, to rat brain tissue. J Neurosci. 1984;4:2906–2911. doi: 10.1523/JNEUROSCI.04-12-02906.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R, Marcangione C, Bauco P. Blockade of the reward-potentiating effects of nicotine on lateral hypothalamic brain stimulation by chlorisondamine. Synapse. 1998;29:72–79. doi: 10.1002/(SICI)1098-2396(199805)29:1<72::AID-SYN6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Wonnacott S, Sidhpura N, Balfour DJ. Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol. 2005;5:53–59. doi: 10.1016/j.coph.2004.12.002. [DOI] [PubMed] [Google Scholar]