Abstract
Treating recurrent prostate cancer poses a great challenge to clinicians. Research efforts in the last decade have shown that adenoviral vector-based gene therapy is a promising approach that could expand the arsenal against prostate cancer. This maturing field is at the stage of being able to translate many preclinical discoveries into clinical practices. At this juncture, it is important to highlight the promising strategies including prostate-targeted gene expression, the use of oncolytic vectors, therapy coupled to reporter gene imaging, and combined treatment modalities. In fact, the early stages of clinical investigation employing combined, multimodal gene therapy focused on loco-regional tumor eradication and showed promising results. Clinicians and scientists should seize the momentum of progress to push forward to improve the therapeutic outcome for the patients.
INTRODUCTION
Like many cancers, prostate cancer is a complex disease, and different kinds of therapeutic strategies will be required in order to demonstrate a benefit in a particular patient cohort. Most of the ~29,000 men who succumb to prostate cancer each year in the United States die of metastatic disease and this highlights the need for better systemic therapies.1 Currently, the best available systemic therapies for prostate cancer, including hormone therapy and the recently approved Taxotere (docetaxel), are not curative, and result only in an extension (sometimes modest) of survival. It is probably for these reasons that vaccine-based gene therapy approaches were the first to be attempted in the clinic and have progressed the furthest. Not only are these approaches seductive because of their potential to impact metastatic disease, they also have scientific merit and have produced provocative results in Phase II trials. What is needed, in the long-term, are therapies that will work towards the eradication of both local and systemic disease, ultimately resulting in a reduction in the annual number of prostate cancer deaths.
As a therapeutic tactic, gene therapy offers the opportunity to target diverse molecular pathways in cancer cells. According to the Genetic Modification Clinical Research Information System,2 72 gene therapy protocols targeting prostate cancer have been submitted to National Institutes of Health’s Recombinant DNA and Advisory Committee for review. However, only a fraction of these have progressed to the stage of clinical trials and even fewer have published results. Besides the logistical issues (i.e., lack of resources, regulatory barriers, etc.), what are the reasons for this meager progress? From the investigations that have taken place during the past decade it is clear that we need to overcome several limitations in order to foster the future success of prostate cancer gene therapy. How can we improve upon the inefficient in vivo gene transduction? Can gene therapy be directed specifically at the cancerous prostate gland without damaging normal tissues? Can the process of in vivo gene transfer and therapy be visualized or monitored non-invasively? How can we augment the efficacy of current therapeutic approaches? We will discuss specifically: how we can harness the potency of viral replicative power to promote the killing of cancerous prostate cells; how we can build upon current prostate-specific transcriptional amplification systems for improving gene expression efficiency; how we can integrate multiple therapeutic modalities and/or molecular imaging to achieve higher efficacy of gene therapy; and how we can acquire the ability to monitor the efficacy of gene therapy in preclinical applications with a view to ultimately translating developed approaches to the clinic. In this report we will give an overview of several therapeutic approaches that are currently being developed and appear to be promising in pre-clinical settings. We will also address the technical advancements that could guide continual progress in this field. The intent of this review is to focus on the potential of transcriptionally-targeted gene therapy and to place it within the framework of newly-developing strategies that are available to cancer therapists, so that it may one day earn a place among the effective treatment modalities for prostate cancer.
TECHNICAL DEVELOPMENTS FOR ADVANCING PROSTATE-TARGETED THERAPY
Prostate-targeted gene expression and amplification strategies
A major limitation of human cancer gene therapy is a lack of significant efficacy. Systemic delivery, specific introduction, and specific expression of the target gene are the major issues to be managed in order to establish a clinically relevant treatment strategy. Although non-specific constitutive viral promoters (cytomegalovirus (CMV), respiratory syncytial virus) have been utilized with success in clinical applications in situ, the use of prostate-specific transcriptional activity could improve the efficacy of both in situ and systemic gene therapies, and ensure that the latter is safe to administer. Transcriptional targeting refers to the use of a particular cell-specific regulatory element (promoters or promoter/enhancers) to restrict gene expression to a particular tissue or cell type. Many prostate-specific gene regulatory regions are well characterized and several of them have been assessed in pre-clinical and clinical therapeutic studies (Table 1).
Table 1.
Prostate-specific regulatory promoter/enhancers applied in Ad vector-mediated interventions in vivo
| Regulatory element configuration | Gene therapy; summary of results | References |
|---|---|---|
| (a) Native promoters | ||
| PSA (prostate-specific antigen) | • HSV-tk (therapeutic); cell killing in vitro and growth inhibition in tumor model | • Huang5 |
| hK2 (Kallikrein-2, PSA-related) | • E1 protein (oncolytic); expression in PSA+, prostate tumor-selective replication | • Yu9 |
| OC (osteocalcin; prostatic metastasis and bone cell-specific) | • HSV-tk (therapeutic); Phase I clinical trial | • Koeneman30 |
| • E1a and E1b (oncolytic); conditional replication competence in OC-expressing cells, growth inhibition in tumor model | • Hsieh22 | |
| (b) Improved promoters | ||
| PSE (PSA promoter/enhancer) | • Luciferase (detection of metastasis); expression (PSE-BC) higher in AI than in AD tumors and excluded from liver. Non-invasive bioluminescence imaging showed that the adenovirus could locate and illuminate lung and spine metastases. Systemic injection of low Ad doses also illuminated lung metastasis | • Adams146 |
| • Nitroreductase (therapeutic); expression (PSE) in PSA+ cells inducible by androgen and comparable to that of CMV vector | • Latham14 | |
| Rat Probasin (ARRPB2) | • E1a (oncolytic); selective replication in PSA+ cells | • Yu109 |
| • Bad (apoptotic); cell-specific expression and cell death in vitro; tumor size reduction in tumor models | • Zhang147 | |
| • HSV-tk (therapeutic); retinoid inducible; growth suppression of AI tumor model | • Furuhata148 | |
| (c) Chimeric elements | ||
| PSMA/PSES (prostate-specific membrane antigen/artificial chimeric enhancer) | • E1a (oncolytic); replication-competent virus, exclusively in prostatic AI cells, efficient killing of PSMA+ cells in vitro and in tumor model | • Lee35 |
| • E1a and E4 (oncolytic); controlled viral vector replication and specific cell killing in prostate cancer cell lines and tumor model | • Li128 | |
| PPT (PSMA enhancer/PSA enhancer/T-cell receptor gamma-chain alternate reading frame protein) | • Luciferase (reporter gene); high prostate-specificity and expression levels in the presence and absence of testosterone in vitro and in vivo; higher activity and selectivity than the CMV promoter following intravenous virus administration | • Cheng36 |
Abbreviations: AD tumors, androgen-dependent tumors; AI tumors, androgen-independent tumors; CMV, cytomegalovirus; HSV-tk, herpes simplex virus thymidine kinase.
Promoters to direct prostate-specific gene expression
The prostate-specific promoter most extensively studied to date is derived from the prostate-specific antigen (PSA or human glandular kallikrein 3, hK3) gene, which encodes a serine protease.3 Since PSA is expressed in normal prostate as well as at all stages of prostate carcinogenesis, it remains an excellent candidate for directing therapeutic gene expression specifically toward prostate tissue. Putative recognition sites such as for the androgen receptor (AR), activating protein-1, and c-fos transcription factors span the larger enhancer regions. However, a minimal 440 base pair core enhancer region was found to be most crucial in conferring strong activity and cell specificity. This enhancer core contains one high affinity androgen response element4 and at least four non-consensus androgen response elements with variable AR affinity,5 and these elements contribute to the synergistic activation of PSA gene expression. Extensive studies have been performed of methods to boost the inherently weak, though specific, activity of the native PSA promoter and enhancer.6,7 The most effective amplification methods to boost the activity of PSA promoter will be discussed in the next section.
Both PSA (hK3) and the hK2 belong to the same large kallikrein family and share similar gene regulatory elements that confer prostate-restricted gene expression.8–10 hK2 gene expression is increased in prostate carcinoma,11 suggesting that this element also can be used for achieving target gene expression in prostate cancer. Approaches taken to enhance the PSA and the rat probasin promoter,12 such as increasing androgen responsive elements,13 have also been effective in increasing the activity and androgen inducibility of the hK2 promoter.14 In the past, concerns had been raised that these androgen- and AR-regulated promoters (i.e., PSA, hK2, and probasin) might not function in patients with androgen-independent (AI) prostate cancer. However, current findings indicate that the AR remains active in AI or metastatic stage of disease, and therefore mechanisms such as mutation or amplification of AR, enhanced co-activator activity, or modulation by other signaling pathways, such as interleukin-6 (IL-6) and insulin-like growth factors, can all compensate for the absence or low level of the activating ligand dihydrotestosterone.15 Thus, the concept that AR-dependent promoters can have application in gene therapy even in recurrent AI prostate cancer is supported by the current understanding of prostate cancer progression.16
As we have mentioned earlier, several of the most extensively studied prostate-specific promoters are AR-dependent. However, the two notable exceptions are the osteocalcin (OC) promoter17 and the prostate-specific membrane antigen (PSMA).18 Epithelial and stromal interactions contribute to the establishment of prostate cancer bone metastasis.19 It is interesting to note that prostate cancer cells begin to express bone specific proteins such as OC, osteopontin, and bone sialoprotein after metastasizing to bone.20 OC gene expression is controlled by multiple elements, including an osteo-specific element 2, the vitamin D-responsive element, and cyclic adenosine monophosphate response element (CRE).21–23 These osteomimetic properties provide a golden opportunity to target bony metastases of prostate cancer.24–30 In the case of PSMA, it encodes an integral membrane protein that is expressed in primary prostate cancer and lymph node metastases.31 Interestingly, PSMA gene expression is induced by androgen deprivation. Hence, PSMA promoter-driven cytotoxic gene therapy has been developed specifically to eradicate AI prostate cancer in the face of hormone ablation treatment.32 However, recent studies have suggested that the induced gene expression is not completely restricted to the prostate,33 and this leaky property might limit its potential use in clinical applications.
Given the heterogeneity of prostate tumors, a legitimate concern is that the use of a cell-specific promoter could limit transgene expression to a subset of the tumor cells, thereby reducing therapeutic efficacy. To address this issue, investigators have developed chimeric promoters by fusing crucial transcriptional elements from distinct prostate-specific promoters. Combining hK2 and PSA enhancer elements enhanced the promoter activity in AR-positive prostate cancer cells.34 Another interesting approach is to create chimeric fusions from promoters with disparate functional activities. A novel prostate-specific enhances PSES chimeric promoter, composed of regulatory elements of PSA and PSMA genes, remained silent in non-prostate cell lines, but mediated high levels of expression in PSA- and PSMA-expressing prostate cancer cell lines in the presence and absence of androgen.35 Thus, the PSES element exhibits promising activity in targeting AI prostate cancer. The most recently described chimeric promoter construct (PPT) is the most complex, combining the T-cell-receptor gamma-chain alternate reading frame protein promoter, the PSMA enhancer, and the PSA enhancer.36 It was reported to be highly active in testosterone-deprived prostate cancer cells (PC-346C tumor model). An advantage of the PSES and the PPT promoters is their ability to remain active both in the presence and in the absence of androgen.36
Despite the high specificity of many prostate-specific regulatory regions developed to date, the magnitude of expression directed by these promoters may not be sufficient to mediate robust expression in vector-based in vivo gene therapy applications. To compensate for the low gene transfer efficiency, it is critically important to incorporate the highest gene expression potency into each vector construct. Several successful approaches have been developed to amplify transcription from cell-specific promoters,37 and the ones examined in prostate cancer applications will be discussed in the next section.
Transcriptional amplification strategies for increasing prostate-specific gene expression
The ubiquitously active strong viral promoters (e.g., cytomegalovirus CMV enhancer/promoter or SV40 promoter) have been the benchmark promoters used in gene therapy. Hence, a goal in augmenting the activity of tissue-specific promoters is to achieve levels comparable to the CMV promoter, while retaining cell discriminatory activity. A useful two-step transcriptional amplification system has been developed to boost the activity of the weak but tissue-specific chimeric PSA promoter/enhancer (PSE-BC).7 This two-tiered amplification methodology has been extensively tested and validated in amplifying expression from the PSA promoter or its variants,7,16,38–42 and also for non prostate-specific promoters such as vascular endothelial growth factor, carcinoembryonic antigen and Mucin-1.38,43,44 In the two-step transcriptional amplification system, a cell-specific promoter controls the expression of a potent transcriptional activator, which acts on a responsive construct to activate expression of a reporter/therapeutic gene (Figure 1a). The potent activator is the GAL4-VP16 fusion protein, comprising the DNA binding domain from the yeast transcription activator GAL4 and the transactivation domain from the herpes simplex virus 1 (HSV1) viral protein 16 (VP16). This GAL4-VP16 fusion protein binds specifically to multiple repeats of GAL4-binding sites upstream of the gene of interest. This transcriptional amplification system presents an extensive dynamic range that can be modulated by increasing the number of VP16 activator domains expressed in the fusion protein, and/or the number of GAL4 binding sites in the reporter/therapeutic construct. For instance, increasing the activator binding sites from one to five amplified the activity of the system more than 200-fold.7 Several effective adenoviral vectors were generated, containing all the two-step transcriptional amplification components in a contiguous DNA insert and, in prostate tumors, they displayed activity levels higher than the CMV promoter.7,16,38–42 Additionally, the heightened activity of two-step transcriptional amplification vectors enables sufficient expression of gene products to mediate robust positron emission tomography (PET) signals in the prostate tumor (Figure 2).
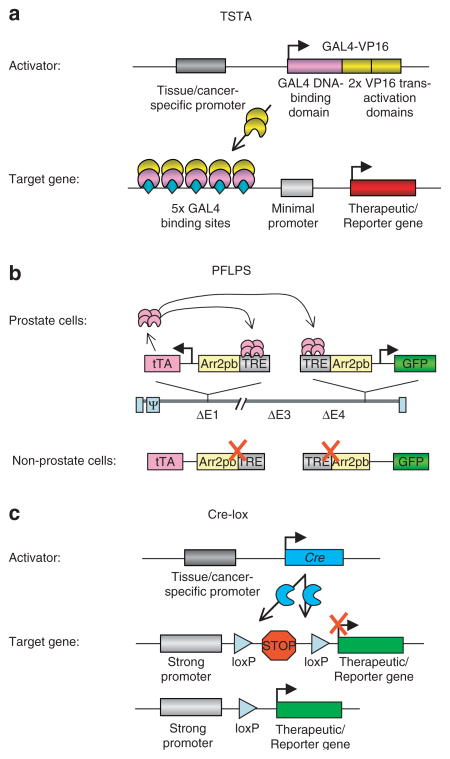
Figure 1. Transcription amplification targeting systems.
(a) The two-step transcriptional amplification (TSTA) system consists of a two-step transcriptional activation process. In the first step, the artificial GAL4-VP16 activator is expressed in a tissue-specific manner by virtue of regulation by a tissue- or cancer-specific promoter (e.g., prostate-specific antigen promoter). In the second step, the GAL4-VP16 protein binds to five GAL4 DNA binding sites upstream of a minimal promoter, and activates expression of a reporter gene or a therapeutic gene. (b) A new amplification system, a novel positive feedback loop with prostate specificity (PFLPS).45 Both tet-transactivator (tTA) and green fluorescent protein (GFP) genes are expressed in a tissue-specific manner by ARR2PB promoters. Binding of tTA protein to the tet-responsive element (TRE) sequences upstream of the ARR2PB promoters can induce GFP and tTA expression further, initiating a positive feedback loop. In non-prostate cells, where the ARR2PB promoter is inactive, gene expression would not be induced. (c) Cre-lox system-mediated activation of gene expression. Cre recombinase expression is regulated by a tissue- or cancer-specific promoter. Activation of transgene expression is induced by removal of the translational inhibition sequence by a Cre-specific recombination between the two loxP sites.
Figure 2. Utility of coupling in vivo imaging to prostate-targeted gene therapy.
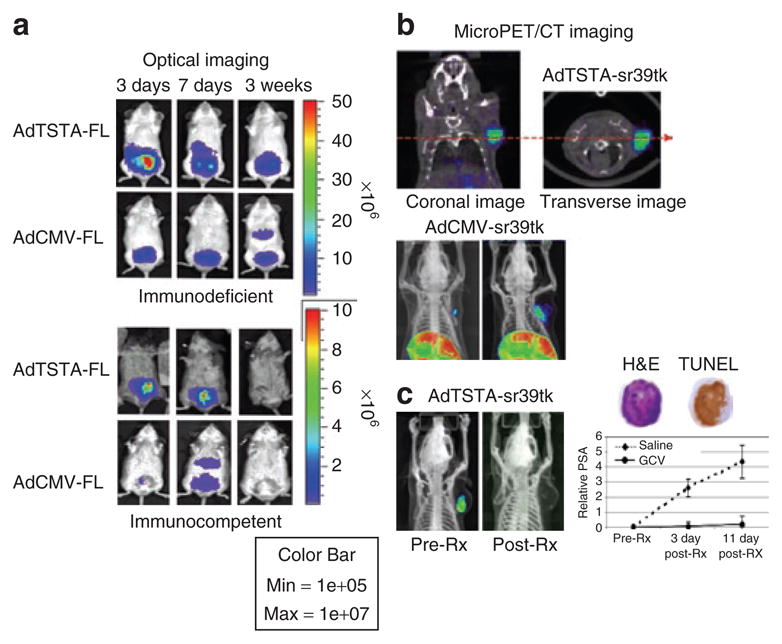
(a) Location and duration of gene expression as followed by optical imaging for firefly luciferase (FL) activity. After intraprostatic administration of 1 × 107 infectious plaque forming units (pfu) of AdTSTA-FL or AdCMV-FL into immune competent (Balb/c × 129) and severe combined immune deficient mice, FL expression directed by the prostate-specific AdTSTA-FL was localized to the prostate gland, whereas AdCMV-FL produced a detectable level of expression in the liver. Trans-gene expression mediated by each of the vectors was higher and more persistent in immune-deficient than in the immune-competent mice. Liver and prostate signals were confirmed by ex vivo organ imaging (data not shown). (b) Magnitude and location of gene expression by vector mediated [18F]-9-(4-fluoro-3-hydroxymethylbutyl)guanine positron emission tomography (FHBG-PET). Robust HSV-sr39tk-dependent PET signal was detected in the LAPC-4 prostate tumor 8 days after intratumoral injection of 4 × 109 pfu of AdTSTA-sr39tk. The coronal and transverse images of a PET/computed tomography (CT) study are represented. The lower panel refers to two animals, both of which received similar 4 × 109 pfu of AdCMV-sr39tk injections directly into the tumor. However, their levels of transgene expression on day 8, reflected by FHBG-PET signals, were different. Unexpectedly, strong PET signals were observed in the livers of both animals. This finding is attributed to spillage of AdCMV-sr39tk into systemic circulation despite the tumor-directed viral administration.41 (c) Monitor suicide gene therapy by PET. The paired images are the PET/CT of one representative animal injected with AdTSTA-sr39tk, pre- and post-treatment with ganciclovir (GCV). The tumor-localized PET signal declined post GCV treatment. The graph on the right shows that the serum prostate-specific antigen (PSA) of the control group (saline treatment) continued to rise, while the serum PSA of GCV-treated cohort showed no increase, thereby indicating that tumor growth was halted in this group. Immunohistochemical analysis of the treated tumor showed substantial apoptosis, indicated by TUNEL-positive staining.41 Ad, adenoviral; CMV, cytomegalovirus; H&E, hematoxylin and eosin; TSTA, two-step transcriptional amplification; TUNEL, terminal deoxynucleotidyl transferase-mediated biotinylated deoxyuridine triphosphate nick end labelling.
A novel regulation system involves a positive feedback loop with prostate specificity (Figure 1b). This systems incorporates a tet-responsive element upstream of the prostate-specific ARR2PB promoter driving a tet-transactivator to enhance its activity with tet regulation in vitro.45 The expressions of both green fluorescent protein (GFP) and tet-transactivator were placed under the control of these TRE-ARR2PB promoters, and a positive feedback loop was demonstrated specifically in prostate cells. The innovation of this design lies in the combination of drug-inducible and tissue-selective transcriptional control to achieve even greater regulation of expression levels in prostate cancer cells.
A different conditional approach to enhancing tissue-specific promoter activity involves the bacteriophage P1-derived Cre-lox system (Figure 1c), a powerful and versatile tool for in vivo DNA recombination. When Cre recombinase expression is targeted using a tissue-specific promoter, conditional “knockout” of a target gene can be achieved in a particular tissue or cell type. This system can also be applied for enhancing tissue- or tumor-specific promoter activities in cancer gene therapy. Target cells are transduced with a vector harboring the therapeutic transgene separated from a strong constitutive promoter by a translational stop cassette flanked by two loxP sites. Co-transduction with a vector expressing Cre from a tissue-specific promoter results in excision of the stop cassette and expression of the transgene in a highly tissue-specific manner.46 The Cre-lox system has been applied to enhance the activity of prostate-specific promoters such as PSA and PSMA.47–49 In this approach, the Ad vector expressing Cre driven by the PSA promoter-enhancer serves to activate the expression of the secondary CMV-loxP-stop-luciferase vector. As expected, this PSA promoter-based system was regulated by androgen, and its use in a cytosine deaminase (CD) suicide gene therapy inhibited LNCaP tumor growth in nude mice.47 The efficiency of this binary system might be limited by the need for two vectors to transduce the same cell in order to achieve activation. As a result, the combined PSA promoter/enhancer and Cre-loxP system exhibited modest increases in activity (3× stronger) when compared with a direct PSA promoter/enhancer-driven vector. When a liposome-mediated gene transfer was used, the combination of the Cre/lox system with the PSMA promoter/enhancer (PEPM) greatly enhanced the efficacy of HSV-tk suicide gene therapy in PSMA+ cells, as compared with the performance of the PEPM promoter alone.48 Because the PSMA promoter is induced by androgen depletion, the PEPM-Cre/CMV-lox system also exhibited a stronger inhibitory effect on tumor growth in castrated mice as compared with intact non-castrated mice. These findings indicate that the PEPM- and Cre-lox mediated cytotoxic therapy may be applicable to patients under androgen deprivation therapy.49 Another interesting strategy involved an altered form of Cre recombinase (CRE-M) that displays an androgen-induced activity. CRE-M was generated by fusion of Cre to the AR ligand binding domain, and three different forms of ligand binding domain (wild-type, non-ligand binding, and Thr → Ala mutant) were examined.50 The activity of CRE-M-LBDwt incorporated in adenovirus was stimulated tenfold by the addition of androgen. Interestingly, CRE-M-LBD mutant activity was inducible by both androgen and the anti-androgen flutamide, suggesting that the CRE-M-AR-LBD system can provide an additional level of regulation to Cre/loxP-mediated prostate gene therapy applications.50
The utility and potential benefits of transcriptional-targeted expression and amplification strategies have been demonstrated largely in preclinical animal models. The incorporation of transductional-targeted strategies will likely enhance the efficacy of gene therapy modalities for the treatment of prostate cancer. Transductional-targeting approaches have shown great potential for systemic delivery in treating metastastic disease. These approaches have included redirecting Ad tropism to prostate cancer cells via alpha6beta1 integrins,51 epidermal growth factor receptor,52 and PSMA,53 either incorporated into the Ad vector or coupled to a polymer coating. Clearly, the efficacy of these newer transcriptionally and transductionally targeted approaches in clinical settings will need to be assessed in comparison with traditional vectors in future clinical trials.
Coupling molecular imaging with gene therapy
The basis of “molecular” imaging is to exploit specific molecular probes to achieve image contrast in order to differentiate target tissue from normal or background tissue in the subject.54 Molecular imaging is particularly beneficial in cancer gene therapy,55 because it can answer many hitherto unanswered questions about the efficacy of gene delivery and expression in vivo. Has the gene reached its target? Is the gene product being expressed in the target, and if yes, what is the magnitude? More importantly, how long does the gene expression persist in vivo? Figure 2 is a synopsis of using in vivo imaging to assess the location, duration, and magnitude of gene transfer, as well as therapeutic activity.
The most common method to query in vivo transgene expression is to use imaging reporter genes. Optical imaging technologies based on bioluminescent (e.g., luciferase genes) and fluorescent proteins produced in nature have been applied widely as imaging reporters, allowing efficient, non-invasive and rapid assessment of transgene expression in pre-clinical small animal models (Figure 2a). However, the attenuation and scattering of visible light as it traverses through tissue severely hinders the use of optical imaging for applications in humans. The high-energy radionuclide imaging modality such as PET has the distinct advantage of producing 3D localized signals, which can be translated from small animal to human subjects (Figure 2b). Two categories of PET reporter systems have been developed that either utilize intracellular enzymes (e.g., HSV1 thymidine kinase (HSV1-tk)), or cell membrane receptors (e.g., dopamine, somatostatin, transferin, and sodium iodide symporter (NIS)). We will focus our discussion on the HSV1-tk and NIS genes because they have the dual capacity to provide imaging, and to serve a potentially therapeutic function.
The HSV1-tk gene is currently one of the most frequently used PET reporter genes. The enzymatic activity of HSV1-TK is also the cornerstone of anti-viral treatment56 and suicide gene therapy to treat cancer.57 In PET applications, the phosphorylation of radioactive guanine derivatives leads to the trapping and accumulation of the radiolabeled tracer in cells expressing HSV1-TK. Because the tracer level of the radiolabeled probe used in PET is 3–4 orders of magnitude lower than the pharmacological dosage in cytotoxic therapy it causes no harmful effects. Extensive research in chemical synthesis has been devoted to developing the ideal radionuclide PET probe or substrate for HSV1-TK.58–63 Among the fluorinated analogs developed to date, [18F]-9-(4- fluoro-3-hydroxymethylbutyl)guanine exhibits the most favorable characteristics for in vivo applications.64
Improvements were made to the HSV1-tk gene for imaging applications by carrying out genetic engineering on the thymidine kinase (TK) protein active site to increase the affinity towards acycloguanosine analogs while decreasing the affinity for thymidine,65,66 a natural substrate and competitor. A particular mutant, HSV1-sr39tk, exhibited a greater-than-twofold enhancement in imaging sensitivity for [18F]-9-(4-fluoro-3-hydroxymethylbutyl)guanine when compared with the wild type HSV1-tk gene.67 Among the different reporter gene-and-probe combinations studied, HSV1-sr39tk/[18F]-9-(4-fluoro-3-hydroxymethylbutyl)guanine offered the maximum sensitivity for in vivo imaging,68 enabling the real-time quantification of Ad mediated gene transfer to murine liver61 and prostate tumors.41 In suicide gene therapy, the ability to monitor gene transfer to the targeted tumor in living animals provided credence to the therapeutic effects observed (Figure 2b). Importantly, this PET-coupled HSV1-tk-mediated suicide gene therapy has proven to be feasible in the clinical setting for treating patients with hepatic carcinoma.69
The NIS is a good example of a transporter protein that has been exploited for gene therapy imaging by virtue of its ability to specifically incorporate a labeled tracer into the cell (reviewed in ref. 70). Endogenous NIS is a transmembrane protein that is expressed mainly in the thyroid, the stomach, and the salivary glands.71 In the thyroid, the NIS protein transports iodide into the cytoplasm and concentrates it 20- to 40-fold, driven by the sodium gradient across the basal membrane.72 NIS-mediated radioisotope uptake has been used clinically for decades in the diagnosis and therapy of thyroid disease.73 The cloning of its complementary DNA initiated the use of NIS as an imaging reporter gene74–77 and a cancer therapeutic gene,78 including in prostate cancer.79 One distinct advantage of using NIS in the dual role of reporter gene and therapeutic gene is that the biodistribution, metabolism, and toxicity of many radiotracers are well known from decades of clinical experience. Although isotopes of iodide (123/124/131I) are its most common substrates, NIS transports a wide range of other isotopes including 99 mTc Pertechnetate, 188/186Re Perrhenate, and 211At, which may be employed for many diagnostic and therapeutic purposes. Most recently, several studies demonstrated the utility of the NIS gene in modeling gene transfer into the prostate glands of larger animal subjects such as dogs. For instance, Ad vector-mediated NIS expression was successfully imaged by single photon emission computed tomography.80,81 The magnitude of spreading of an oncolytic Ad vector within the dog prostate gland was assessed by NIS-mediated accumulation of radioactive tracer.80 Collectively, the promising activities of HSV-tk and NIS genes suggest that imaging of prostate cancer gene therapy in action might be on the horizon.
THERAPEUTIC STRATEGIES
Immune-based gene therapy approaches
The major focus of this treatment is to modulate the immune system to reject prostate tumor cells. It is a well-tolerated modality, and has progressed the furthest along among the various therapeutic options under clinical investigation.82,83 The three common approaches to generating prostate cancer-targeted immune response are (i) tumor cell vaccines, (ii) vector-based vaccines, and (iii) dendritic cell (DC)-based vaccines.
Early in the development of whole tumor cell vaccines, irradiated autologous tumor cells engineered to express granulocyte-macrophage colony-stimulating factor were used to treat patients who had metastatic prostate cancer at the time of surgery.84 This autologous vaccine strategy was greatly hampered by the laborious process required for cell preparation and the low volume of tumor cells that could be harvested. Therefore the focus of development was shifted to allogeneic vaccines using established prostate cancer cell lines, such as GVAX, a vaccine composed of a mixture of PC3 and LNCaP cells transduced with granulocyte-macrophage colony-stimulating factor.84,85 Another allogeneic whole cell vaccine is Onyvax-P (Onyvax, London, UK), composed of OnyCap23, LNCaP and P4E6 cells, that also exhibited the ability to delay time to progression in a small cohort of patients with AI prostate cancer.86 The advantages of the allogeneic vaccine are its “off the shelf” feature and the option of adding co-stimulatory molecules and cytokines to augment immune responses. On the other hand, the main drawbacks of this approach are the potential interference from the multitude of antigens in the vaccine, and the fact that it is impossible to assess specific immunological responses during the treatment.
The vector-based vaccine strategy involves the use of viral vectors expressing tumor antigen and immune stimulatory molecules. The development of this strategy has been focused on selecting the proper viral vector, co-stimulatory molecules, cytokine cocktails, and immunization regimes for achieving optimal antigen presentation and T-cell activation in vivo. The vaccination strategy against PSA is achieved by priming with live recombinant vaccinia and boosting with recombinant fowl pox and is among the most well-studied approaches.87–89 Although PSA-specific T-cell responses were demonstrated in these studies, significant clinical benefits were not established.87–89 Taken together, the immune-based therapies hold promise in eliciting a targeted response against disseminated disease. But they are unlikely to be effective in eradicating bulky diseases.83 Hence, the future investigations would be to determine the optimal combination of immune-activating approaches and cytotoxic therapies to improve patient outcomes.
Because of their proficiency in antigen presentation, DCs have been widely exploited in cancer vaccine applications, including in prostate cancer.90 To elicit anti-tumor immunity, the autologous DCs isolated from patients were pulsed ex vivo with messenger RNA, DNA or peptides of tumor antigens, and infused back into the patients. Studies with small cohorts of patients have been completed, employing autologous DCs pulsed with PSMA,91,92 PSA,93,94 or recombinant prostatic acid phosphatase fused to granulocyte-macrophage colony-stimulating factor.95,96 Immunological responses against the targeted antigen were demonstrated in these studies, but clinical benefits to patients were difficult to substantiate. The challenges of processing patient-derived DCs and the inherent non-uniformity of the therapeutic agent are the shortcomings of the DC-based vaccination strategy.
Cytotoxic gene therapy approaches
Cytotoxic or cytolytic gene therapy can involve the transfer of drug-susceptible (“suicide”) genes, or proapoptotic genes. The “suicide” gene strategy uses a gene encoding an enzyme that converts a nontoxic prodrug into a cytotoxic form when transfected into tumor cells. Two common suicide gene therapy systems are the classic HSV1-tk, and the Escherichia coli CD genes. HSV1-TK converts nontoxic nucleoside analogs such as ganciclovir into phosphorylated compounds that act as chain terminators of DNA synthesis, while the prodrug for CD is 5-fluorocytosine. Tumor cell killing is achieved by necrosis and apoptosis.
The E. coli nitroreductase (NTR) is another example of a suicide gene which efficiently kills tumor cells upon reduction of a systemically administered prodrug CB1954 [5-(aziridin1-yl)-2,4-dinitrobenzamide] to a DNA-crosslinking derivative at the tumor site. The effect is independent of cell cycle, and a crucial bystander killing effect is observed on untransduced tumor cells. Testing in vivo by Ad-NTR administration in a murine prostate tumor model induced necrosis and upregulation of heat shock hsp25 and hsp70 proteins.97 In the same study, combining NTR with hsp70 expression elicited enhanced protection over NTR alone for both the treated tumor and a subsequent re-challenge via CD4+ and CD8+ T-cell responses. Ad-NTR/hsp70 therefore comprises a promising cytotoxicplus-immunomodulatory gene combination for treating prostate tumors. It should be interesting to see whether Ad-NTR/hsp70 therapy can be further enhanced by cytokines.
In the proapoptotic gene strategy, Ad vectors expressing tumor necrosis factor family genes such as Fas ligand (FasL) and TRAIL (tumor necrosis factor–related apoptosis-inducing ligand) are employed commonly. Two particular issues that need to be addressed in using this therapeutic mechanism are (i) to overcome the resistance that some tumor cells develop, and (ii) to restrict the cytotoxic effects to tumor cells only. Low dose chemotherapeutic agents can induce sensitivity of prostate tumor cells to TRAIL by down regulation of an intrinsic anti-apoptotic pathway98 by downregulation of the intrinsic anti-apoptotic X-linked mammalian inhibitor of apoptosis protein pathway.99 Recently, a histone deacetylase inhibitor (depsipeptide) was also shown to augment Ad-TRAIL cytotoxic activity in human prostate tumor cells by increasing levels of TRAIL-R1 and R2 in membrane lipid rafts.100 Interestingly, depsipeptide treatment also augmented the expression of the coxsackie Ad receptor, which provided an additional mechanism to boost the efficacy of Ad-mediated TRAIL gene therapy. A prostate-directed inducible strategy was developed to restrict the cytotoxic effects of FasL to prostate tumor. In this approach, the mouse Tnfsf6 (FasL)-GFP fusion gene was controlled by a tetracycline-responsive promoter.101 This intricate expression system will be described in more detail in the next section. Systemic delivery of these vectors showed that the inducible FasL-GFP system was better tolerated at doses lethal to Balb/c mice for a CMV-driven vector, suggesting that inducibility plus prostate-restricted transgene expression may improve the safety and efficacy of cytotoxic gene therapy.
A new class of cancer-specific apoptosis-inducing genes is represented by the mda-7/IL-24 (melanoma differentiation associated gene-7) gene. In vitro, Ad-mda7 induces G2/M arrest through the inhibition of cdc25c pathway and apoptosis in a cancer-specific manner, without affecting normal cells.102,103 These effects are mediated through mitochondrial pathways involving bcl-2, and independent of Bax and p53.102 Furthermore, expression of Ad-mda7/IL-24 in normal cells can induce direct anti-tumor and radiation-enhancing effects that are dependent on the presence of receptors for the IL-24 cytokine on tumor cells.104 The combination of secreted mda7/IL-24 and radiation results in a bystander antitumor effect not only in mda7/IL-24 and radiation-sensitive cancer cells, but also in tumor cells overexpressing anti-apoptotic proteins bcl2 or bcl-xL, and in those resistant to either treatment. Another promising gene that exhibited anti-tumor activity in a pre-clinical model of prostate cancer is the mouse RTVP-1 gene (related to testes specific, vespid, and pathogenesis proteins), which is a direct target of p53.105 Administration of the Ad-RTVP1 in an orthotopic metastatic murine prostate model resulted in extension of animal survival by diverse effects such as reduced metastasis to lung, suppression of tumoral angiogenesis, and increased infiltration of macrophages, DCs, and CD8+ T cells into the tumor. The ability to induce a plethora of anti-tumoral effects such as proapoptotic, antiangiogenic, and immunemodulatory functions will be the hallmark of a promising candidate gene, because these are important not only for the eradication of localized tumors but also in the suppression of systemic metastases of prostate cancer, especially in treating high-risk localized disease.
It is intuitively logical to use prostate- or prostatic cancer-specific promoters in directing potent cytolytic and oncolytic gene therapy. Restricting the expression of cytotoxic genes to the targeted tumor cells could potentially improve the dose-limiting toxicity of a therapeutic vector by reducing systemic side effects.
Conditionally replicating oncolytic adenoviruses
From investigations carried out during the past decade it is clear that we need to overcome the inefficient in vivo gene transduction in order to achieve successful cancer gene therapy. Adenovirus is a lytic virus, capable of producing up to 10,000 progeny viruses per host cell. Thus, the rationale for using replication-competent, rather than replication-defective adenoviruses as gene therapy vectors is because: (i) replication-competent adenoviruses are cytolytic and have demonstrated anti-tumor activity in both preclinical tumor models and humans, (ii) replication-competent adenoviruses have the potential to replicate to high copy, thereby resulting in significantly greater therapeutic gene expression in vivo, and (iii) replication-competent adenoviruses have a greater potential to spread locally and therefore could infect a greater number of tumor cells. All of these potential advantages have now been demonstrated in large animal models or humans.106–111 In attempting to harness this potent viral replicative power towards prostate tumor destruction, a key consideration is how to “turn on” this process specifically in prostate tumors. One approach is to restrict viral replication only in prostate tumor cells. This prostate-restricted approach can safeguard against viral replication in normal non-prostate cells in case of viral spillage into systemic circulation during local therapy.9 If proven effective and specific, the prostate-targeted strategy has the potential to be a therapeutic option for metastatic prostate cancer.
The first step in the strategy to develop an oncolytic adenovirus is to take advantage of viral replication pathways that could discriminate between cancer cells and normal ones. It is known that Ad 55 kd E1b protein can inactivate tumor suppressor p53, and p53 is frequently inactivated in tumors. This gave rise to the interesting line of reasoning that a 55 kd-E1b deleted adenovirus called “dl1520” or “ONYX-015” would replicate only in p53-defective tumor cells but not in normal cells.80 This strategy has been intensively investigated, including 16 phase I and II clinical trials for head and neck carcinoma, pancreatic cancer, ovarian cancer, colorectal cancer, hepatobiliary cancer, gastric cancer and gliomas.112–118 In clinical investigations of ONYX-015, modest responses were observed when it was used as a single agent. Recent findings support the view that the replication of ONYX-015 is probably dependent not on p53 inactivation or p19ARF status,119–122 but rather on late viral messenger RNA export.123 Further investigation to determine the underlying targeting mechanism or pathway used by ONYX-015 will enhance its usefulness in cancer therapeutics.
A second strategy involving the use of oncolytic virus that has worked particularly well in prostate cancer is to employ a tissue-specific promoter to control the expression of adenovirus E1A and E1B proteins, which are key regulators of viral life cycle. The E1A proteins are the first viral gene products generated, and they initiate and coordinately regulate the intricate cascade of viral DNA replication and gene expression. The large E1A protein transactivates all other early and late viral genes. The small E1A and E1B gene products modulate the activity of tumor suppressors (e.g., pRB and p53) and apoptotic pathway in the host cell in order to benefit viral propagation. Hence, deletion of E1 genes is the mainstay of creating a replication-defective adenovirus. Conversely, placing E1 gene expression under prostate-specific promoter should restrict virus replication in a tissue-specific manner. As discussed in the previous section, many prostate-specific gene regulatory systems have been developed (Table 1). Among them PSA, PSMA, and OC are biomarkers of prostate cancer, and their promoters are excellent candidates to control prostate-specific Ad replication.
The first prostate-restricted replicative adenovirus was developed by Rodriguez et al.106 Calydon virus (CV706) was engineered by placing the Ad E1A gene under the control of the minimal PSA promoter and enhancer sequences. The E1A expression of CV706 was limited to PSA-positive LNCaP human prostate cancer cells, and anti-tumor activity was demonstrated against LNCaP tumor xenografts.106 When combined with radiation therapy, a significant synergistic effect was demonstrated both in cell culture and xenograft models.107 In a clinical trial, it was found that it is safe to administer CV706 at less than the maximum tolerable dose. A drop in serum PSA greater than 50% was achieved in patients treated with the highest doses of CV706.108 In order to refine the viral oncolytic efficacy in a second generation virus CV787 (also known as CG7870), the viral E3 region was retained to enhance viral spread, and both E1A and E1B genes were driven by prostate-specific promoters; E1A was controlled by the rat probasin promoter and E1B by the human PSA promoter/enhancer. By restricting the expression of both viral early genes (E1A and E1B) to prostate-specific cells, further improvement was achieved in the tissue-selectivity of viral replication as compared with CV706, in which only the E1A expression is restricted.109 CV787 replicated as efficiently as wild-type Ad5 and were successful in eliminating LNCaP xenograft tumors when administered by tail vein injection.109 Further in vivo analysis revealed that the effect of CV787 is synergistically enhanced both with radiation therapy110 and with chemotherapeutic agents such as paclitaxel and docetaxel.111
Improved transcriptional-based strategies have been developed for targeting AI or metastatic prostate cancer cells. In particular, the OC promoter and the novel chimeric PSES promoter have been utilized to drive oncolytic viral replication.124 Matsubara et al. utilized the murine OC promoter to restrict the expression of E1A to prostate epithelia and its supporting bone stroma in osseous metastases of prostate cancer. This virus, named Ad-OC-E1A, appears to be more effective than PSA-controlled virus at killing a broader spectrum of prostate cancer cells, including LNCaP, C4-2, and ARCaP (PSA-positive) as well as PC-3 and DU-145 (PSA-negative). Intratumoral injection of Ad-OC-E1A effectively obliterated subcutaneous AI PC-3 athymic mouse xenograft models. In addition, intraosseous C4-2 prostate cancer xenografts responded very well to the systemic administration of Ad-OC-E1A. One hundred percent of the treated mice responded with a drop in serum PSA below detectable levels. At the conclusion of the study, 40% of the treated mice were cured, with no evidence of prostate cancer cells in the skeleton.125 To improve upon this technology, Hsieh et al. developed Ad-hOC-E1, employing a centrally located human OC promoter to transcript both the E1A and E1B genes.22 Under the control of this vitamin D-responsive promoter, Ad-hOC-E1 induced tenfold higher replication and cytotoxicity than wild-type adenovirus upon administration of vitamin D.22 Vitamin D treatment alone can exert mild anti-proliferative effect on prostate cancer cells.126,127 However, the systemic administration of a combination of vitamin D and Ad-hOC-E1 showed a synergistic effect, with repression of tumor growth being better achieved than with either treatment given alone.22
In applying PSES promoter to oncolytic technology, Li et al. created AdE4PSESE1A with both E1A and E4 genes regulated by the prostate-specific promoter.128 The E4 gene is an essential viral early gene that modulates viral transcription and messenger RNA transport. AdE4PSESE1A replicated and lysed PSA/PSMA-positive tumor cells with the same efficiency as wild-type adenovirus did. As expected, replication of AdE4PSESE1A was severely impaired in PSA/PSMA-negative cells. AdE4PSESE1A effectively suppressed the growth of AI CWR22rv mouse xenograft tumors.128 In order to obtain a better understanding of the kinetics of the oncolytic process, the GFP driven by the CMV promoter was inserted into AdE4PSESE1A, enabling the use of intraviral imaging for monitoring viral replication and spread within the tumor. Three days after AdE4PSESE1A injection, a big boost in GFP expression was observed, indicating rapid replication and viral spreading, decreasing 1 week after injection. Although subsequent rebound in GFP expression indicative of renewed viral amplification was observed, tumor growth and viral oncolysis reached equilibrium at 2 weeks time. After this point, tumor growth exceeded the killing rate.128 Despite the high oncolytic activity of the virus, several studies have reported limited viral spread and incomplete tumor eradication.129,130 What are the mechanisms that hinder the oncolytic process in vivo and how can we improve its efficacy?
One plausible explanation is that conditions within the established tumor become unfavorable for viral replication. As tumor cell lysis occurs, the tumor environment becomes highly necrotic and hypoxic. Several recent studies observed a reduced level of viral replication in hypoxic conditions, attributed to diminished levels of E1A protein expression without concomitant decrease of messenger RNA.131,132 On the basis of these findings, future investigations aimed at understanding the mechanism that modulates E1A protein stability (and how to stabilize E1A in hypoxia) may improve the efficacy of viral oncolytic therapy. Extensive investigation by Wold and colleagues has demonstrated that the 11.6 kd adenovirus death protein encoded in the E3 region enhances viral spreading and cytolytic activity of replication-competent adenovirus in cell culture studies.133,134 The E3 region is involved in modulating the host immune response to Ad infection and is non-essential for in vitro viral replication. Thus, quite often the E3 region is deleted in oncolytic Ad vectors due to space constraints in the E1-containing viral genome. A recent study demonstrated that the incorporation of adenovirus death protein into a multimodality replication-competent adenovirus enhanced the viral spread and the distribution of expressed transgene within the prostate glands of dogs.80 Clearly, replication-restricted viral oncolysis is a promising and emerging technology in treating prostate cancer, but additional modifications and improvements are required for maximum effectiveness. There has been extensive investigation regarding the integration of expression cassettes for therapeutic genes, such as TRAIL, CD/TK, FasL, antiangiogenic factor, and immune modulators into the conditional replication-competent virus in order to enhance therapeutic efficiency.
CONSIDERATIONS IN COMBINED TREATMENT MODALITIES
In cancer therapeutics, and especially in treating advanced stage prostate cancer, rarely is a cure achievable by using only one form of treatment. Due to intratumoral cell heterogeneity, combined modality therapies may achieve additive or synergistic therapeutic activity. As gene therapy matures as a treatment option, an important consideration is about the kinds of rational and beneficial combined treatments that investigators should explore. Combined therapies can take on the form of (i) simultaneous delivery of multiple therapeutic genes, (ii) combining gene therapy and radiation therapy, and (iii) treating with both gene therapy and chemotherapy. In considering simultaneous delivery of multiple gene therapies, let us follow up on the earlier discussion about oncolytic therapy. In the scenario where a hypoxic environment is created by viral therapy within the tumor, the expression of vascular endothelial growth factor A and tumor angiogenesis would be induced, and the resulting neovasculature would promote tumor growth. If this mechanism is in play, then the use of an antiangiogenic factor as an adjuvant to oncolytic therapy should promote tumor eradication. Recently, Jin et al. demonstrated the effectiveness of a combination of Ad-hOC-E1 with Ad-Flk1-Fc, a vector delivering the secreted form of the antiangiogenic factor Flk-1, also known as vascular endothelium growth factor receptor 2. When used as single agents, Ad-hOC-E1 and Ad-Flk1-Fc reduced C4-2 mouse xenograft models by 60 and 40%, respectively. However, when co-infection with both viruses was carried out, the average reduction in tumor size was 90% in 8 weeks, with 30% of the tumors completely regressing by week three.135 Liu et al. combined antiangiogenesis with an E1B-55K-deleted oncolytic virus expressing the apoptosis inducer, TRAIL. In this study, Ad-k5, a replication-deficient Ad delivering the fifth kringle domain of plasminogen, which inhibits angiogenesis by regulating the intrinsic angiogenic factor pathway, was co-injected with ZD55-hTRAIL into colorectal carcinoma mouse xenografts. This combined therapy resulted in the complete eradication of all treated tumors.136 The future designs of replicative adenoviruses could include an antiangiogenic gene engineered into the same vector.
Other multi-gene therapies that have demonstrated enhanced efficacy in comparison with single modality therapies include dual suicide genes for enzyme/prodrug strategies (HSV1-tk and CD),137,138 suicide-plus-apoptotic therapies (HSV1-tk and p53),139 and combined cytotoxic (HSV-tk) and immune-activation (Flt-3).140 Combined administration of paclitaxel and p53 gene revealed therapeutic synergy in prostate DU145 xenografts.141 The combined therapeutic approach using prostate-specific oncolytic Ad CV787 (or CG7870) and Taxotere displayed significant synergistic antitumor activity in mouse tumor models of prostate cancer.110 Because early clinical studies on suicide gene therapies showed low gene transduction rate and limited beneficial results, the multi-gene combined treatment has begun to transition into clinical investigations. Freytag et al. integrated a double suicide gene, a CD/TK fusion gene, into ONYX-015 conditional replication-competent adenovirus (Ad5-CD/TKrep), and further combined gene therapy with radiotherapy.141–143 Following a single intraprostatic adenovirus injection, subjects received 5-fluorocytosine and valganciclovir prodrug therapy and a standard course of conformal radiotherapy.144 The multi-modal treatment was well tolerated, with no dose-limiting toxicities. However this strategy was limited by two factors. First, it was based on ONYX-015 whose replication was neither p53-dependent nor tumor-restricted as discussed earlier. This makes it difficult to predict which of the patients will respond well to the therapy. Second, this strategy could be applied only to treat local disease, because it used a strong universal CMV to control the expression of the CD/TK fusion gene. The use of a prostate-specific promoter, such as OC, PSES and PPT, could broaden its use in treating metastatic lesions. The OC promoter has been applied to control TK gene expression and thereby to inhibit the growth of advanced stage AI prostate cancer cells.125 A Phase I clinical study with Ad-OC-TK demonstrated the safety of the therapy and showed some therapeutic effects in treating bone metastasis.145 In the light of these promising pre-clinical and early clinical findings, clinical trials employing multi-gene therapeutic strategies in combination with standard radiation or chemotherapy will be the logical way forward to improve the management of advanced-stage prostate cancer.
CONCLUDING REMARKS
Contrary to public perception, gene therapy has demonstrated an outstanding safety profile in humans. Although it has yet to demonstrate a significant improvement in outcome in a randomized, controlled trial of prostate cancer, the strategy of combining multi-modal gene therapy with conventional radiation or chemotherapy has generated promising results in early stage trials in select patient groups. To achieve the most effective control of the disease, gene therapy should be considered as one arm of a multi-pronged approach to improve the outcome of first-line therapies such as surgery or radiotherapy. Although novel systemic therapies (e.g., GVAX, improved androgen blockage therapy) have showed some promise, the management of metastatic disease still poses great challenges for clinicians. As a collective group of investigators with the common goal of improving the health of prostate cancer patients, we cannot sit idle and rely on yet unproven promises. We need to maintain diligence and continue to improve our technologies. Transcriptional targeting is a means to further improve specificity and efficacy of gene therapy. Amplifying the magnitude of transgene expression, boosting copies of a vector genome in a replication-competent system, and combining multiple therapeutic modalities including gene therapy, are all proven strategies to achieve synergistic augmentation of therapeutic efficacy. Coupling non-invasive imaging to gene therapy will not only provide better transparency of the therapeutic process in vivo but will also provide an early confirmation of gene transfer, which should be predictive of therapeutic efficacy. Clearly, the numerous improvements achieved in the last 5 years in gene therapy for prostate cancer will promote the translation of many promising therapies to the clinics.
Acknowledgments
We deeply appreciate Makoto Sato and Mai Johnson (Department of Urology, University of California, Los Angeles) for having helpful discussions with us and for assisting in the preparation of this manuscript. This work is supported by National Institutes of Health (NIH) R01 CA101904, DAMD17-03-1-0095, Prostate Cancer Foundation and the California Cancer Research Program 3NI0226 (to L.W.), NIH grant CA074042, DOD grant W23RX-3270-N729 (to C.K.), and R25CA098010, K01CA117921 (to M.L.F.).
References
- 1.National Cancer Institute, D. Surveillance Research Program, Cancer Statistics Branch (released April 2005, based on the November 2004 submission) Surveillance, Epidemiology, and End Results (SEER) Program. ( www.seer.cancer.gov) SEER Stat Database: Incidence—SEER 9 Regs Public-Use, Nov 2004 Sub (1973–2002)
- 2.Genetic Modification Clinical Research Information System, M.C.R.I.S. < http://www4.od.nih.gov/oba/RAC/GeMCRIS/GeMCRIS.htm>.
- 3.Aumuller G, Seitz J, Lilja H, Abrahamsson PA, von der Kammer H, Scheit KH. Species- and organ-specificity of secretory proteins derived from human prostate and seminal vesicles. Prostate. 1990;17:31–40. doi: 10.1002/pros.2990170105. [DOI] [PubMed] [Google Scholar]
- 4.Cleutjens KB, van Eekelen CC, van der Korput HA, Brinkmann AO, Trapman J. Two androgen response regions cooperate in steroid hormone regulated activity of the prostate-specific antigen promoter. J Biol Chem. 1996;271:6379–6388. doi: 10.1074/jbc.271.11.6379. [DOI] [PubMed] [Google Scholar]
- 5.Huang W, Shostak Y, Tarr P, Sawyers C, Carey M. Cooperative assembly of androgen receptor into a nucleoprotein complex that regulates the prostate-specific antigen enhancer. J Biol Chem. 1999;274:25756–25768. doi: 10.1074/jbc.274.36.25756. [DOI] [PubMed] [Google Scholar]
- 6.Wu L, Matherly J, Smallwood A, Adams JY, Billick E, Belldegrun A, et al. Chimeric PSA enhancers exhibit augmented activity in prostate cancer gene therapy vectors. Gene Ther. 2001;8:1416–1426. doi: 10.1038/sj.gt.3301549. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L, Adams JY, Billick E, Ilagan R, Iyer M, Le K, et al. Molecular engineering of a two-step transcription amplification (TSTA) system for transgene delivery in prostate cancer. Mol Ther. 2002;5:223–232. doi: 10.1006/mthe.2002.0551. [DOI] [PubMed] [Google Scholar]
- 8.Mitchell SH, Murtha PE, Zhang S, Zhu W, Young CY. An androgen response element mediates LNCaP cell dependent androgen induction of the hK2 gene. Mol Cell Endocrinol. 2000;168:89–99. doi: 10.1016/s0303-7207(00)00319-1. [DOI] [PubMed] [Google Scholar]
- 9.Yu DC, Sakamoto GT, Henderson DR. Identification of the transcriptional regulatory sequences of human kallikrein 2 and their use in the construction of calydon virus 764, an attenuated replication competent adenovirus for prostate cancer therapy. Cancer Res. 1999;59:1498–1504. [PubMed] [Google Scholar]
- 10.Xie X, Zhao X, Liu Y, Young CY, Tindall DJ, Slawin KM, et al. Robust prostate-specific expression for targeted gene therapy based on the human kallikrein 2 promoter. Hum Gene Ther. 2001;12:549–561. doi: 10.1089/104303401300042483. [DOI] [PubMed] [Google Scholar]
- 11.Yousef GM, Diamandis EP. Expanded human tissue kallikrein family—a novel panel of cancer biomarkers. Tumour Biol. 2002;23:185–192. doi: 10.1159/000064027. [DOI] [PubMed] [Google Scholar]
- 12.Kasper S, Rennie PS, Bruchovsky N, Sheppard PC, Cheng H, Lin L, et al. Cooperative binding of androgen receptors to two DNA sequences is required for androgen induction of the probasin gene. J Biol Chem. 1994;269:31763–31769. [PubMed] [Google Scholar]
- 13.Zhang X, Lee C, Ng PY, Rubin M, Shabsigh A, Buttyan R. Prostatic neoplasia in transgenic mice with prostate-directed overexpression of the c-myc oncoprotein. Prostate. 2000;43:278–285. doi: 10.1002/1097-0045(20000601)43:4<278::aid-pros7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 14.Latham JP, Searle PF, Mautner V, James ND. Prostate-specific antigen promoter/enhancer driven gene therapy for prostate cancer: construction and testing of a tissue-specific adenovirus vector. Cancer Res. 2000;60:334–341. [PubMed] [Google Scholar]
- 15.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nat Rev Cancer. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 16.Sato M, Johnson M, Zhang L, Gambhir SS, Carey M, Wu L. Functionality of androgen receptor-based gene expression imaging in hormone refractory prostate cancer. Clin Cancer Res. 2005;11:3743–3749. doi: 10.1158/1078-0432.CCR-04-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yeung F, Law WK, Yeh CH, Westendorf JJ, Zhang Y, Wang R, et al. Regulation of human osteocalcin promoter in hormone-independent human prostate cancer cells. J Biol Chem. 2002;277:2468–2476. doi: 10.1074/jbc.M105947200. [DOI] [PubMed] [Google Scholar]
- 18.Fair WR, Israeli RS, Heston WD. Prostate-specific membrane antigen. Prostate. 1997;32:140–148. doi: 10.1002/(sici)1097-0045(19970701)32:2<140::aid-pros9>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 19.Chung LW, Baseman A, Assikis V, Zhau HE. Molecular insights into prostate cancer progression: the missing link of tumor microenvironment. J Urol. 2005;173:10–20. doi: 10.1097/01.ju.0000141582.15218.10. [DOI] [PubMed] [Google Scholar]
- 20.Koeneman KS, Yeung F, Chung LW. Osteomimetic properties of prostate cancer cells: a hypothesis supporting the predilection of prostate cancer metastasis and growth in the bone environment. Prostate. 1999;39:246–261. doi: 10.1002/(sici)1097-0045(19990601)39:4<246::aid-pros5>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 21.Lian JB, Stein GS, Stein JL, van Wijnen AJ. Regulated expression of the bone-specific osteocalcin gene by vitamins and hormones. Vitam Horm. 1999;55:443–509. doi: 10.1016/s0083-6729(08)60941-3. [DOI] [PubMed] [Google Scholar]
- 22.Hsieh CL, Yang L, Miao L, Yeung F, Kao C, Yang H, et al. A novel targeting modality to enhance adenoviral replication by vitamin D(3) in androgen-independent human prostate cancer cells and tumors. Cancer Res. 2002;62:3084–3092. [PubMed] [Google Scholar]
- 23.Huang WC, Xie Z, Konaka H, Sodek J, Zhau HE, Chung LW. Human osteocalcin and bone sialoprotein mediating osteomimicry of prostate cancer cells: role of cAMP-dependent protein kinase A signaling pathway. Cancer Res. 2005;65:2303–2313. doi: 10.1158/0008-5472.CAN-04-3448. [DOI] [PubMed] [Google Scholar]
- 24.Gleave M, Hsieh JT, Gao CA, von Eschenbach AC, Chung LW. Acceleration of human prostate cancer growth in vivo by factors produced by prostate and bone fibroblasts. Cancer Res. 1991;51:3753–3761. [PubMed] [Google Scholar]
- 25.Festuccia C, Bologna M, Gravina GL, Guerra F, Angelucci A, Villanova I, et al. Osteoblast conditioned media contain TGF-β1 and modulate the migration of prostate tumor cells and their interactions with extracellular matrix components. Int J Cancer. 1999;81:395–403. doi: 10.1002/(sici)1097-0215(19990505)81:3<395::aid-ijc13>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 26.Wu HC, Hsieh JT, Gleave ME, Brown NM, Pathak S, Chung LW. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406–412. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 27.Thalmann GN, Anezinis PE, Chang SM, Zhau HE, Kim EE, Hopwood VL, et al. Androgen-independent cancer progression and bone metastasis in the LNCaP model of human prostate cancer. Cancer Res. 1994;54:2577–2581. [PubMed] [Google Scholar]
- 28.Chung LW. The role of stromal-epithelial interaction in normal and malignant growth. Cancer Surv. 1995;23:33–42. [PubMed] [Google Scholar]
- 29.Rhee HW, Zhau HE, Pathak S, Multani AS, Pennanen S, Visakorpi T, et al. Permanent phenotypic and genotypic changes of prostate cancer cells cultured in a three-dimensional rotating-wall vessel. In Vitro Cell Dev Biol Anim. 2001;37:127–140. doi: 10.1290/1071-2690(2001)037<0127:PPAGCO>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 30.Koeneman KS, Kao C, Ko SC, Yang L, Wada Y, Kallmes DF, et al. Osteocalcin-directed gene therapy for prostate-cancer bone metastasis. World J Urol. 2000;18:102–110. doi: 10.1007/s003450050181. [DOI] [PubMed] [Google Scholar]
- 31.Horoszewicz JS, Kawinski E, Murphy GP. Monoclonal antibodies to a new antigenic marker in epithelial prostatic cells and serum of prostatic cancer patients. Anticancer Res. 1987;7:927–935. [PubMed] [Google Scholar]
- 32.O’Keefe DS, Uchida A, Bacich DJ, Watt FB, Martorana A, Molloy PL, et al. Prostate-specific suicide gene therapy using the prostate-specific membrane antigen promoter and enhancer. Prostate. 2000;45:149–157. doi: 10.1002/1097-0045(20001001)45:2<149::aid-pros9>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 33.Silver DA, Pellicer I, Fair WR, Heston WD, Cordon-Cardo C. Prostate-specific membrane antigen expression in normal and malignant human tissues. Clin Cancer Res. 1997;3:81–85. [PubMed] [Google Scholar]
- 34.Tsui KH, Wu L, Chang PL, Hsieh ML, Juang HH. Identifying the combination of the transcriptional regulatory sequences on prostate specific antigen and human glandular kallikrein genes. J Urol. 2004;172:2029–2034. doi: 10.1097/01.ju.0000141147.96640.76. [DOI] [PubMed] [Google Scholar]
- 35.Lee SJ, Zhang Y, Lee SD, Jung C, Li X, Kim HS, et al. Targeting prostate cancer with conditionally replicative adenovirus using PSMA enhancer. Mol Ther. 2004;10:1051–1058. doi: 10.1016/j.ymthe.2004.08.028. [DOI] [PubMed] [Google Scholar]
- 36.Cheng WS, Kraaij R, Nilsson B, van der Weel L, de Ridder CM, Totterman TH, et al. A novel TARP-promoter-based adenovirus against hormone-dependent and hormone-refractory prostate cancer. Mol Ther. 2004;10:355–364. doi: 10.1016/j.ymthe.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 37.Nettelbeck DM, Jerome V, Muller R. Gene therapy: designer promoters for tumour targeting. Trends Genet. 2000;16:174–181. doi: 10.1016/s0168-9525(99)01950-2. [DOI] [PubMed] [Google Scholar]
- 38.Qiao J, Doubrovin M, Sauter BV, Huang Y, Guo ZS, Balatoni J, et al. Tumor-specific transcriptional targeting of suicide gene therapy. Gene Ther. 2002;9:168–175. doi: 10.1038/sj.gt.3301618. [DOI] [PubMed] [Google Scholar]
- 39.Sato M, Johnson M, Zhang L, Zhang B, Le K, Gambhir SS, et al. Optimization of adenoviral vectors to direct highly amplified prostate-specific expression for imaging and gene therapy. Mol Ther. 2003;8:726–737. doi: 10.1016/j.ymthe.2003.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segawa T, Takebayashi H, Kakehi Y, Yoshida O, Narumiya S, Kakizuka A. Prostate-specific amplification of expanded polyglutamine expression: a novel approach for cancer gene therapy. Cancer Res. 1998;58:2282–2287. [PubMed] [Google Scholar]
- 41.Johnson M, Sato M, Burton J, Gambhir SS, Carey M, Wu L. Micro-PET/CT monitoring of herpes thymidine kinase suicide gene therapy in a prostate cancer xenograft: the advantage of a cell-specific transcriptional targeting approach. Mol Imaging. 2005;4:463–472. doi: 10.2310/7290.2005.05154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ilagan R, Zhang LJ, Pottratz J, Le K, Salas S, Iyer M, et al. Imaging androgen receptor function during flutamide treatment in the LAPC9 xenograft model. Mol Cancer Ther. 2005;4:1662–1669. doi: 10.1158/1535-7163.MCT-05-0197. [DOI] [PubMed] [Google Scholar]
- 43.Block A, Milasinovic D, Mueller J, Schaefer P, Schaefer H, Greten H. Amplified Muc1-specific gene expression in colon cancer cells utilizing a binary system in adenoviral vectors. Anticancer Res. 2002;22:3285–3292. [PubMed] [Google Scholar]
- 44.Wang Y, Iyer M, Annala A, Wu L, Carey M, Gambhir SS. Noninvasive indirect imaging of vascular endothelial growth factor gene expression using bioluminescence imaging in living transgenic mice. Physiol Genomics. 2006;24:173–180. doi: 10.1152/physiolgenomics.00308.2004. [DOI] [PubMed] [Google Scholar]
- 45.Woraratanadharm J, Rubinchik S, Yu H, Fan F, Morrow SM, Dong JY. Highly specific transgene expression mediated by a complex adenovirus vector incorporating a prostate-specific amplification feedback loop. Gene Ther. 2004;11:1399–1407. doi: 10.1038/sj.gt.3302307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sato Y, Tanaka K, Lee G, Kanegae Y, Sakai Y, Kaneko S, et al. Enhanced and specific gene expression via tissue-specific production of Cre recombinase using adenovirus vector. Biochem Biophys Res Commun. 1998;244:455–462. doi: 10.1006/bbrc.1997.8087. [DOI] [PubMed] [Google Scholar]
- 47.Yoshimura I, Ikegami S, Suzuki S, Tadakuma T, Hayakawa M. Adenovirus mediated prostate specific enzyme prodrug gene therapy using prostate specific antigen promoter enhanced by the Cre-loxP system. J Urol. 2002;168:2659–2664. doi: 10.1016/S0022-5347(05)64239-5. [DOI] [PubMed] [Google Scholar]
- 48.Ikegami S, Tadakuma T, Suzuki S, Yoshimura I, Asano T, Hayakawa M. Development of gene therapy using prostate-specific membrane antigen promoter/enhancer with Cre Recombinase/LoxP system for prostate cancer cells under androgen ablation condition. Jpn J Cancer Res. 2002;93:1154–1163. doi: 10.1111/j.1349-7006.2002.tb01218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ikegami S, Tadakuma T, Ono T, Suzuki S, Yoshimura I, Asano T, et al. Treatment efficiency of a suicide gene therapy using prostate-specific membrane antigen promoter/enhancer in a castrated mouse model of prostate cancer. Cancer Sci. 2004;95:367–370. doi: 10.1111/j.1349-7006.2004.tb03217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kaczmarczyk SJ, Green JE. Induction of cre recombinase activity using modified androgen receptor ligand binding domains: a sensitive assay for ligand-receptor interactions. Nucleic Acids Res. 2003;31:e86. doi: 10.1093/nar/gng087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevenson M, Hale AB, Hale SJ, Green NK, Black G, Fisher KD, et al. Incorporation of a laminin-derived peptide (SIKVAV) on polymer-modified adenovirus permits tumor-specific targeting via alpha6-integrins. Cancer Gene Ther. 2007;14:335–345. doi: 10.1038/sj.cgt.7701022. [DOI] [PubMed] [Google Scholar]
- 52.Bonsted A, Engesaeter BO, Hogset A, Maelandsmo GM, Prasmickaite L, D’Oliveira C, et al. Photochemically enhanced transduction of polymer-complexed adenovirus targeted to the epidermal growth factor receptor. J Gene Med. 2006;8:286–297. doi: 10.1002/jgm.853. [DOI] [PubMed] [Google Scholar]
- 53.Kraaij R, van Rijswijk AL, Oomen MH, Haisma HJ, Bangma CH. Prostate specific membrane antigen (PSMA) is a tissue-specific target for adenoviral transduction of prostate cancer in vitro. Prostate. 2005;62:253–259. doi: 10.1002/pros.20150. [DOI] [PubMed] [Google Scholar]
- 54.Massoud TF, Gambhir SS. Molecular imaging in living subjects: seeing fundamental biological processes in a new light. Genes Dev. 2003;17:545–580. doi: 10.1101/gad.1047403. [DOI] [PubMed] [Google Scholar]
- 55.Iyer M, Sato M, Johnson M, Gambhir SS, Wu L. Applications of molecular imaging in cancer gene therapy. Curr Gene Ther. 2005;5:607–618. doi: 10.2174/156652305774964695. [DOI] [PubMed] [Google Scholar]
- 56.Oliver S, Bubley G, Crumpacker C. Inhibition of HSV-transformed murine cells by nucleoside analogs, 2′-NDG and 2′-nor-cGMP: mechanisms of inhibition and reversal by exogenous nucleosides. Virology. 1985;145:84–93. doi: 10.1016/0042-6822(85)90203-x. [DOI] [PubMed] [Google Scholar]
- 57.Freeman SM, Whartenby KA, Freeman JL, Abboud CN, Marrogi AJ. In situ use of suicide genes for cancer therapy. Semin Oncol. 1996;23:31–45. [PubMed] [Google Scholar]
- 58.Tjuvajev JG, Finn R, Watanabe K, Joshi R, Oku T, Kennedy J, et al. Noninvasive imaging of herpes virus thymidine kinase gene transfer and expression: a potential method for monitoring clinical gene therapy. Cancer Res. 1996;56:4087–4095. [PubMed] [Google Scholar]
- 59.Tjuvajev JG, Avril N, Oku T, Sasajima T, Miyagawa T, Joshi R, et al. Imaging herpes virus thymidine kinase gene transfer and expression by positron emission tomography. Cancer Res. 1998;58:4333–4341. [PubMed] [Google Scholar]
- 60.Tjuvajev JG, Chen SH, Joshi A, Joshi R, Guo ZS, Balatoni J, et al. Imaging adenoviral-mediated herpes virus thymidine kinase gene transfer and expression in vivo. Cancer Res. 1999;59:5186–5193. [PubMed] [Google Scholar]
- 61.Gambhir SS, Barrio JR, Wu L, Iyer M, Namavari M, Satyamurthy N, et al. Imaging of adenoviral-directed herpes simplex virus type 1 thymidine kinase reporter gene expression in mice with radiolabeled ganciclovir. J Nucl Med. 1998;39:2003–2011. [PubMed] [Google Scholar]
- 62.Gambhir SS, Barrio JR, Herschman HR, Phelps ME. Assays for noninvasive imaging of reporter gene expression. Nucl Med Biol. 1999;26:481–490. doi: 10.1016/s0969-8051(99)00021-9. [DOI] [PubMed] [Google Scholar]
- 63.Gambhir SS, Herschman HR, Cherry SR, Barrio JR, Satyamurthy N, Toyokuni T, et al. Imaging transgene expression with radionuclide imaging technologies. Neoplasia. 2000;2:118–138. doi: 10.1038/sj.neo.7900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Iyer M, Wu L, Carey M, Wang Y, Smallwood A, Gambhir SS. Two-step transcriptional amplification as a method for imaging reporter gene expression using weak promoters. Proc Natl Acad Sci USA. 2001;98:14595–14600. doi: 10.1073/pnas.251551098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kokoris MS, Black ME. Characterization of herpes simplex virus type 1 thymidine kinase mutants engineered for improved ganciclovir or acyclovir activity. Protein Sci. 2002;11:2267–2272. doi: 10.1110/ps.2460102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kokoris MS, Sabo P, Black ME. In vitro evaluation of mutant HSV-1 thymidine kinases for suicide gene therapy. Anticancer Res. 2000;20:959–963. [PubMed] [Google Scholar]
- 67.Gambhir SS, Bauer E, Black ME, Liang Q, Kokoris MS, Barrio JR, et al. A mutant herpes simplex virus type 1 thymidine kinase reporter gene shows improved sensitivity for imaging reporter gene expression with positron emission tomography. Proc Natl Acad Sci USA. 2000;97:2785–2790. doi: 10.1073/pnas.97.6.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Min JJ, Iyer M, Gambhir SS. Comparison of [18F]FHBG and [14C]FIAU for imaging of HSV1-tk reporter gene expression: adenoviral infection vs stable transfection. Eur J Nucl Med Mol Imaging. 2003;30:1547–1560. doi: 10.1007/s00259-003-1238-6. [DOI] [PubMed] [Google Scholar]
- 69.Penuelas I, Mazzolini G, Boan JF, Sangro B, Marti-Climent J, Ruiz M, et al. Positron emission tomography imaging of adenoviral-mediated transgene expression in liver cancer patients. Gastroenterology. 2005;128:1787–1795. doi: 10.1053/j.gastro.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 70.Chung JK. Sodium iodide symporter: its role in nuclear medicine. J Nucl Med. 2002;43:1188–1200. [PubMed] [Google Scholar]
- 71.Dai G, Levy O, Carrasco N. Cloning and characterization of the thyroid iodide transporter. Nature. 1996;379:458–460. doi: 10.1038/379458a0. [DOI] [PubMed] [Google Scholar]
- 72.Filetti S, Bidart JM, Arturi F, Caillou B, Russo D, Schlumberger M. Sodium/iodide symporter: a key transport system in thyroid cancer cell metabolism. Eur J Endocrinol. 1999;141:443–457. doi: 10.1530/eje.0.1410443. [DOI] [PubMed] [Google Scholar]
- 73.Shimura H, Haraguchi K, Miyazaki A, Endo T, Onaya T. Iodide uptake and experimental 131I therapy in transplanted undifferentiated thyroid cancer cells expressing the Na+/I− symporter gene. Endocrinology. 1997;138:4493–4496. doi: 10.1210/endo.138.10.5571. [DOI] [PubMed] [Google Scholar]
- 74.Haberkorn U, Henze M, Altmann A, Jiang S, Morr I, Mahmut M, et al. Transfer of the human NaI symporter gene enhances iodide uptake in hepatoma cells. J Nucl Med. 2001;42:317–325. [PubMed] [Google Scholar]
- 75.Groot-Wassink T, Aboagye EO, Glaser M, Lemoine NR, Vassaux G. Adenovirus biodistribution and noninvasive imaging of gene expression in vivo by positron emission tomography using human sodium/iodide symporter as reporter gene. Hum Gene Ther. 2002;13:1723–1735. doi: 10.1089/104303402760293565. [DOI] [PubMed] [Google Scholar]
- 76.Groot-Wassink T, Aboagye EO, Wang Y, Lemoine NR, Reader AJ, Vassaux G. Quantitative imaging of Na/I symporter transgene expression using positron emission tomography in the living animal. Mol Ther. 2004;9:436–442. doi: 10.1016/j.ymthe.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 77.Mandell RB, Mandell LZ, Link CJ., Jr Radioisotope concentrator gene therapy using the sodium/iodide symporter gene. Cancer Res. 1999;59:661–668. [PubMed] [Google Scholar]
- 78.Cho JY, Shen DH, Yang W, Williams B, Buckwalter TL, La Perle KM, et al. In vivo imaging and radioiodine therapy following sodium iodide symporter gene transfer in animal model of intracerebral gliomas. Gene Ther. 2002;9:1139–1145. doi: 10.1038/sj.gt.3301787. [DOI] [PubMed] [Google Scholar]
- 79.Spitzweg C, O’Connor MK, Bergert ER, Tindall DJ, Young CY, Morris JC. Treatment of prostate cancer by radioiodine therapy after tissue-specific expression of the sodium iodide symporter. Cancer Res. 2000;60:6526–6530. [PubMed] [Google Scholar]
- 80.Barton KN, Paielli D, Zhang Y, Koul S, Brown SL, Lu M, et al. Second-generation replication-competent oncolytic adenovirus armed with improved suicide genes and ADP gene demonstrates greater efficacy without increased toxicity. Mol Ther. 2006;13:347–356. doi: 10.1016/j.ymthe.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 81.Dwyer RM, Schatz SM, Bergert ER, Myers RM, Harvey ME, Classic KL, et al. A preclinical large animal model of adenovirus-mediated expression of the sodium-iodide symporter for radioiodide imaging and therapy of locally recurrent prostate cancer. Mol Ther. 2005;12:835–841. doi: 10.1016/j.ymthe.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 82.Tarassoff CP, Arlen PM, Gulley JL. Therapeutic vaccines for prostate cancer. Oncologist. 2006;11:451–462. doi: 10.1634/theoncologist.11-5-451. [DOI] [PubMed] [Google Scholar]
- 83.Mabjeesh NJ, Zhong H, Simons JW. Gene therapy of prostate cancer: current and future directions. Endocr Relat Cancer. 2002;9:115–139. doi: 10.1677/erc.0.0090115. [DOI] [PubMed] [Google Scholar]
- 84.Simons JW, Mikhak B, Chang JF, DeMarzo AM, Carducci MA, Lim M, et al. Induction of immunity to prostate cancer antigens: results of a clinical trial of vaccination with irradiated autologous prostate tumor cells engineered to secrete granulocyte-macrophage colony-stimulating factor using ex vivo gene transfer. Cancer Res. 1999;59:5160–5168. [PubMed] [Google Scholar]
- 85.Dranoff G, Jaffee E, Lazenby A, Golumbek P, Levitsky H, Brose K, et al. Vaccination with irradiated tumor cells engineered to secrete murine granulocyte-macrophage colony-stimulating factor stimulates potent, specific, and long-lasting anti-tumor immunity. Proc Natl Acad Sci USA. 1993;90:3539–3543. doi: 10.1073/pnas.90.8.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Michael A, Ball G, Quatan N, Wushishi F, Russell N, Whelan J, et al. Delayed disease progression after allogeneic cell vaccination in hormone-resistant prostate cancer and correlation with immunologic variables. Clin Cancer Res. 2005;11:4469–4478. doi: 10.1158/1078-0432.CCR-04-2337. [DOI] [PubMed] [Google Scholar]
- 87.Arlen PM, Gulley JL, Todd N, Lieberman R, Steinberg SM, Morin S, et al. Antiandrogen, vaccine and combination therapy in patients with nonmetastatic hormone refractory prostate cancer. J Urol. 2005;174:539–546. doi: 10.1097/01.ju.0000165159.33772.5b. [DOI] [PubMed] [Google Scholar]
- 88.Gulley JL, Arlen PM, Bastian A, Morin S, Marte J, Beetham P, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–3362. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 89.Kaufman HL, Wang W, Manola J, DiPaola RS, Ko YJ, Sweeney C, et al. Phase II randomized study of vaccine treatment of advanced prostate cancer (E7897): a trial of the Eastern Cooperative Oncology Group. J Clin Oncol. 2004;22:2122–2132. doi: 10.1200/JCO.2004.08.083. [DOI] [PubMed] [Google Scholar]
- 90.Ragde H, Cavanagh WA, Tjoa BA. Dendritic cell based vaccines: progress in immunotherapy studies for prostate cancer. J Urol. 2004;172:2532–2538. doi: 10.1097/01.ju.0000144211.51111.e4. [DOI] [PubMed] [Google Scholar]
- 91.Murphy GP, Tjoa BA, Simmons SJ, Rogers MK, Kenny GM, Jarisch J. Higher-dose and less frequent dendritic cell infusions with PSMA peptides in hormone-refractory metastatic prostate cancer patients. Prostate. 2000;43:59–62. doi: 10.1002/(sici)1097-0045(20000401)43:1<59::aid-pros8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 92.Lodge PA, Jones LA, Bader RA, Murphy GP, Salgaller ML. Dendritic cell-based immunotherapy of prostate cancer: immune monitoring of a phase II clinical trial. Cancer Res. 2000;60:829–833. [PubMed] [Google Scholar]
- 93.Barrou B, Benoit G, Ouldkaci M, Cussenot O, Salcedo M, Agrawal S, et al. Vaccination of prostatectomized prostate cancer patients in biochemical relapse, with autologous dendritic cells pulsed with recombinant human PSA. Cancer Immunol Immunother. 2004;53:453–460. doi: 10.1007/s00262-003-0451-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Heiser A, Coleman D, Dannull J, Yancey D, Maurice MA, Lallas CD, et al. Autologous dendritic cells transfected with prostate-specific antigen RNA stimulate CTL responses against metastatic prostate tumors. J Clin Invest. 2002;109:409–417. doi: 10.1172/JCI14364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Burch PA, Croghan GA, Gastineau DA, Jones LA, Kaur JS, Kylstra JW, et al. Immunotherapy (APC8015, Provenge) targeting prostatic acid phosphatase can induce durable remission of metastatic androgen-independent prostate cancer: a Phase 2 trial. Prostate. 2004;60:197–204. doi: 10.1002/pros.20040. [DOI] [PubMed] [Google Scholar]
- 96.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, et al. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18:3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 97.Lipinski KS, Pelech S, Mountain A, Irvine AS, Kraaij R, Bangma CH, et al. Nitroreductase-based therapy of prostate cancer, enhanced by raising expression of heat shock protein 70, acts through increased anti-tumour immunity. Cancer Immunol Immunother. 2006;55:347–354. doi: 10.1007/s00262-005-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Voelkel-Johnson C, King DL, Norris JS. Resistance of prostate cancer cells to soluble TNF-related apoptosis-inducing ligand (TRAIL/Apo2L) can be overcome by doxorubicin or adenoviral delivery of full-length TRAIL. Cancer Gene Ther. 2002;9:164–172. doi: 10.1038/sj.cgt.7700420. [DOI] [PubMed] [Google Scholar]
- 99.Ng CP, Zisman A, Bonavida B. Synergy is achieved by complementation with Apo2L/TRAIL and actinomycin D in Apo2L/TRAIL-mediated apoptosis of prostate cancer cells: role of XIAP in resistance. Prostate. 2002;53:286–299. doi: 10.1002/pros.10155. [DOI] [PubMed] [Google Scholar]
- 100.Vanoosten RL, Moore JM, Ludwig AT, Griffith TS. Depsipeptide ( FR901228) enhances the cytotoxic activity of TRAIL by redistributing TRAIL receptor to membrane lipid rafts. Mol Ther. 2005;11:542–552. doi: 10.1016/j.ymthe.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 101.Rubinchik S, Wang D, Yu H, Fan F, Luo M, Norris JS, et al. A complex adenovirus vector that delivers FASL-GFP with combined prostate-specific and tetracycline-regulated expression. Mol Ther. 2001;4:416–426. doi: 10.1006/mthe.2001.0478. [DOI] [PubMed] [Google Scholar]
- 102.Lebedeva IV, Sarkar D, Su ZZ, Kitada S, Dent P, Stein CA, et al. Bcl-2 and Bcl-x(L) differentially protect human prostate cancer cells from induction of apoptosis by melanoma differentiation associated gene-7, mda–7/IL-24. Oncogene. 2003;22:8758–8773. doi: 10.1038/sj.onc.1206891. [DOI] [PubMed] [Google Scholar]
- 103.Saito Y, Miyahara R, Gopalan B, Litvak A, Inoue S, Shanker M, et al. Selective induction of cell cycle arrest and apoptosis in human prostate cancer cells through adenoviral transfer of the melanoma differentiation-associated-7 (mda-7)/interleukin-24 (IL-24) gene. Cancer Gene Ther. 2005;12:238–247. doi: 10.1038/sj.cgt.7700780. [DOI] [PubMed] [Google Scholar]
- 104.Su Z, Emdad L, Sauane M, Lebedeva IV, Sarkar D, Gupta P, et al. Unique aspects of mda-7/IL-24 antitumor bystander activity: establishing a role for secretion of MDA-7/IL-24 protein by normal cells. Oncogene. 2005;24:7552–7566. doi: 10.1038/sj.onc.1208911. [DOI] [PubMed] [Google Scholar]
- 105.Satoh T, Timme TL, Saika T, Ebara S, Yang G, Wang J, et al. Adenoviral vector-mediated mRTVP-1 gene therapy for prostate cancer. Hum Gene Ther. 2003;14:91–101. doi: 10.1089/104303403321070793. [DOI] [PubMed] [Google Scholar]
- 106.Rodriguez R, Schuur ER, Lim HY, Henderson GA, Simons JW, Henderson DR. Prostate attenuated replication competent adenovirus (ARCA) CN706: a selective cytotoxic for prostate-specific antigen-positive prostate cancer cells. Cancer Res. 1997;57:2559–2563. [PubMed] [Google Scholar]
- 107.Chen Y, DeWeese T, Dilley J, Zhang Y, Li Y, Ramesh N, et al. CV706, a prostate cancer-specific adenovirus variant, in combination with radiotherapy produces synergistic antitumor efficacy without increasing toxicity. Cancer Res. 2001;61:5453–5460. [PubMed] [Google Scholar]
- 108.DeWeese TL, van der Poel H, Li S, Mikhak B, Drew R, Goemann M, et al. A phase I trial of CV706, a replication-competent, PSA selective oncolytic adenovirus, for the treatment of locally recurrent prostate cancer following radiation therapy. Cancer Res. 2001;61:7464–7472. [PubMed] [Google Scholar]
- 109.Yu DC, Chen Y, Seng M, Dilley J, Henderson DR. The addition of adenovirus type 5 region E3 enables calydon virus 787 to eliminate distant prostate tumor xenografts. Cancer Res. 1999;59:4200–4203. [PubMed] [Google Scholar]
- 110.Dilley J, Reddy S, Ko D, Nguyen N, Rojas G, Working P, et al. Oncolytic adenovirus CG7870 in combination with radiation demonstrates synergistic enhancements of antitumor efficacy without loss of specificity. Cancer Gene Ther. 2005;12:715–722. doi: 10.1038/sj.cgt.7700835. [DOI] [PubMed] [Google Scholar]
- 111.Yu DC, Chen Y, Dilley J, Li Y, Embry M, Zhang H, et al. Antitumor synergy of CV787, a prostate cancer-specific adenovirus, and paclitaxel and docetaxel. Cancer Res. 2001;61:517–525. [PubMed] [Google Scholar]
- 112.Reid TR, Freeman S, Post L, McCormick F, Sze DY. Effects of Onyx-015 among metastatic colorectal cancer patients that have failed prior treatment with 5-FU/leucovorin. Cancer Gene Ther. 2005;12:673–681. doi: 10.1038/sj.cgt.7700819. [DOI] [PubMed] [Google Scholar]
- 113.Nemunaitis J, Khuri F, Ganly I, Arseneau J, Posner M, Vokes E, et al. Phase II trial of intratumoral administration of ONYX-015, a replication-selective adenovirus, in patients with refractory head and neck cancer. J Clin Oncol. 2001;19:289–298. doi: 10.1200/JCO.2001.19.2.289. [DOI] [PubMed] [Google Scholar]
- 114.Mulvihill S, Warren R, Venook A, Adler A, Randlev B, Heise C, et al. Safety and feasibility of injection with an E1B-55 kda gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: a phase I trial. Gene Ther. 2001;8:308–315. doi: 10.1038/sj.gt.3301398. [DOI] [PubMed] [Google Scholar]
- 115.Morley S, MacDonald G, Kirn D, Kaye S, Brown R, Soutar D. The dl1520 virus is found preferentially in tumor tissue after direct intratumoral injection in oral carcinoma. Clin Cancer Res. 2004;10:4357–4362. doi: 10.1158/1078-0432.CCR-03-0443. [DOI] [PubMed] [Google Scholar]
- 116.Khuri FR, Nemunaitis J, Ganly I, Arseneau J, Tannock IF, Romel L, et al. A controlled trial of intratumoral ONYX-015, a selectively-replicating adenovirus, in combination with cisplatin and 5-fluorouracil in patients with recurrent head and neck cancer. Nat Med. 2000;6:879–885. doi: 10.1038/78638. [DOI] [PubMed] [Google Scholar]
- 117.Hecht JR, Bedford R, Abbruzzese JL, Lahoti S, Reid TR, Soetikno RM, et al. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 118.Ganly I, Kirn D, Eckhardt G, Rodriguez GI, Soutar DS, Otto R, et al. A phase I study of Onyx-015, an E1B attenuated adenovirus, administered intratumorally to patients with recurrent head and neck cancer. Clin Cancer Res. 2000;6:798–806. [PubMed] [Google Scholar]
- 119.Harada JN, Berk AJ. p53-Independent and -dependent requirements for E1B-55K in adenovirus type 5 replication. J Virol. 1999;73:5333–5344. doi: 10.1128/jvi.73.7.5333-5344.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Goodrum FD, Ornelles DA. p53 status does not determine outcome of E1B 55-kilodalton mutant adenovirus lytic infection. J Virol. 1998;72:9479–9490. doi: 10.1128/jvi.72.12.9479-9490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Edwards SJ, Dix BR, Myers CJ, Dobson-Le D, Huschtscha L, Hibma M, et al. Evidence that replication of the antitumor adenovirus ONYX-015 is not controlled by the p53 and p14(ARF) tumor suppressor genes. J Virol. 2002;76:12483–12490. doi: 10.1128/JVI.76.24.12483-12490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rothmann T, Hengstermann A, Whitaker NJ, Scheffner M, zur Hausen H. Replication of ONYX-015, a potential anticancer adenovirus, is independent of p53 status in tumor cells. J Virol. 1998;72:9470–9478. doi: 10.1128/jvi.72.12.9470-9478.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Shea CC, Johnson L, Bagus B, Choi S, Nicholas C, Shen A, et al. Late viral RNA export, rather than p53 inactivation, determines ONYX-015 tumor selectivity. Cancer Cell. 2004;6:611–623. doi: 10.1016/j.ccr.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 124.Lee SJ, Kim HS, Yu R, Lee K, Gardner TA, Jung C, et al. Novel prostate-specific promoter derived from PSA and PSMA enhancers. Mol Ther. 2002;6:415–421. doi: 10.1006/mthe.2002.0682. [DOI] [PubMed] [Google Scholar]
- 125.Matsubara S, Wada Y, Gardner TA, Egawa M, Park MS, Hsieh CL, et al. A conditional replication-competent adenoviral vector, Ad-OC-E1a, to cotarget prostate cancer and bone stroma in an experimental model of androgen-independent prostate cancer bone metastasis. Cancer Res. 2001;61:6012–6019. [PubMed] [Google Scholar]
- 126.Zhao XY, Feldman D. The role of vitamin D in prostate cancer. Steroids. 2001;66:293–300. doi: 10.1016/s0039-128x(00)00164-1. [DOI] [PubMed] [Google Scholar]
- 127.Getzenberg RH, Light BW, Lapco PE, Konety BR, Nangia AK, Acierno JS, et al. Vitamin D inhibition of prostate adenocarcinoma growth and metastasis in the Dunning rat prostate model system. Urology. 1997;50:999–1006. doi: 10.1016/S0090-4295(97)00408-1. [DOI] [PubMed] [Google Scholar]
- 128.Li X, Zhang YP, Kim HS, Bae KH, Stantz KM, Lee SJ, et al. Gene therapy for prostate cancer by controlling adenovirus E1a and E4 gene expression with PSES enhancer. Cancer Res. 2005;65:1941–1951. doi: 10.1158/0008-5472.CAN-04-3666. [DOI] [PubMed] [Google Scholar]
- 129.Sauthoff H, Hu J, Maca C, Goldman M, Heitner S, Yee H, et al. Intratumoral spread of wild-type adenovirus is limited after local injection of human xenograft tumors: virus persists and spreads systemically at late time points. Hum Gene Ther. 2003;14:425–433. doi: 10.1089/104303403321467199. [DOI] [PubMed] [Google Scholar]
- 130.Harrison D, Sauthoff H, Heitner S, Jagirdar J, Rom WN, Hay JG. Wild-type adenovirus decreases tumor xenograft growth, but despite viral persistence complete tumor responses are rarely achieved–deletion of the viral E1b-19-kD gene increases the viral oncolytic effect. Hum Gene Ther. 2001;12:1323–1332. doi: 10.1089/104303401750270977. [DOI] [PubMed] [Google Scholar]
- 131.Shen BH, Hermiston TW. Effect of hypoxia on Ad5 infection, transgene expression and replication. Gene Ther. 2005;12:902–910. doi: 10.1038/sj.gt.3302448. [DOI] [PubMed] [Google Scholar]
- 132.Pipiya T, Sauthoff H, Huang YQ, Chang B, Cheng J, Heitner S, et al. Hypoxia reduces adenoviral replication in cancer cells by downregulation of viral protein expression. Gene Ther. 2005;12:911–917. doi: 10.1038/sj.gt.3302459. [DOI] [PubMed] [Google Scholar]
- 133.Tollefson AE, Ryerse JS, Scaria A, Hermiston TW, Wold WS. The E3–11.6-kDa adenovirus death protein (ADP) is required for efficient cell death: characterization of cells infected with adp mutants. Virology. 1996;220:152–162. doi: 10.1006/viro.1996.0295. [DOI] [PubMed] [Google Scholar]
- 134.Doronin K, Kuppuswamy M, Toth K, Tollefson AE, Krajcsi P, Krougliak V, et al. Tissue-specific, tumor-selective, replication-competent adenovirus vector for cancer gene therapy. J Virol. 2001;75:3314–3324. doi: 10.1128/JVI.75.7.3314-3324.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jin F, Xie Z, Kuo CJ, Chung LW, Hsieh CL. Cotargeting tumor and tumor endothelium effectively inhibits the growth of human prostate cancer in adenovirus-mediated antiangiogenesis and oncolysis combination therapy. Cancer Gene Ther. 2005;12:257–267. doi: 10.1038/sj.cgt.7700790. [DOI] [PubMed] [Google Scholar]
- 136.Liu XY, Qiu SB, Zou WG, Pei ZF, Gu JF, Luo CX, et al. Effective gene-virotherapy for complete eradication of tumor mediated by the combination of hTRAIL (TNFSF10) and plasminogen k5. Mol Ther. 2005;11:531–541. doi: 10.1016/j.ymthe.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 137.Uckert W, Kammertons T, Haack K, Qin Z, Gebert J, Schendel DJ, et al. Double suicide gene (cytosine deaminase and herpes simplex virus thymidine kinase) but not single gene transfer allows reliable elimination of tumor cells in vivo. Hum Gene Ther. 1998;9:855–865. doi: 10.1089/hum.1998.9.6-855. [DOI] [PubMed] [Google Scholar]
- 138.Freytag SO, Paielli D, Wing M, Rogulski K, Brown S, Kolozsvary A, et al. Efficacy and toxicity of replication-competent adenovirus-mediated double suicide gene therapy in combination with radiation therapy in an orthotopic mouse prostate cancer model. Int J Radiat Oncol Biol Phys. 2002;54:873–885. doi: 10.1016/s0360-3016(02)03005-5. [DOI] [PubMed] [Google Scholar]
- 139.Xie Y, Gilbert JD, Kim JH, Freytag SO. Efficacy of adenovirus-mediated CD/5-FC and HSV-1 thymidine kinase/ganciclovir suicide gene therapies concomitant with p53 gene therapy. Clin Cancer Res. 1999;5:4224–4232. [PubMed] [Google Scholar]
- 140.Ali S, King GD, Curtin JF, Candolfi M, Xiong W, Liu C, et al. Combined immunostimulation and conditional cytotoxic gene therapy provide long-term survival in a large glioma model. Cancer Res. 2005;65:7194–7204. doi: 10.1158/0008-5472.CAN-04-3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Freytag SO, Rogulski KR, Paielli DL, Gilbert JD, Kim JH. A novel three-pronged approach to kill cancer cells selectively: concomitant viral, double suicide gene, and radiotherapy. Hum Gene Ther. 1998;9:1323–1333. doi: 10.1089/hum.1998.9.9-1323. [DOI] [PubMed] [Google Scholar]
- 142.Kim JH, Kim SH, Brown SL, Freytag SO. Selective enhancement by an antiviral agent of the radiation-induced cell killing of human glioma cells transduced with HSV-tk gene. Cancer Res. 1994;54:6053–6056. [PubMed] [Google Scholar]
- 143.Khil MS, Kim JH, Mullen CA, Kim SH, Freytag SO. Radiosensitization by 5-fluorocytosine of human colorectal carcinoma cells in culture transduced with cytosine deaminase gene. Clin Cancer Res. 1996;2:53–57. [PubMed] [Google Scholar]
- 144.Freytag SO, Stricker H, Pegg J, Paielli D, Pradhan DG, Peabody J, et al. Phase I study of replication-competent adenovirus-mediated double-suicide gene therapy in combination with conventional-dose three-dimensional conformal radiation therapy for the treatment of newly diagnosed, intermediate- to high-risk prostate cancer. Cancer Res. 2003;63:7497–7506. [PubMed] [Google Scholar]
- 145.Kubo H, Gardner TA, Wada Y, Koeneman KS, Gotoh A, Yang L, et al. Phase I dose escalation clinical trial of adenovirus vector carrying osteocalcin promoter-driven herpes simplex virus thymidine kinase in localized and metastatic hormone-refractory prostate cancer. Hum Gene Ther. 2003;14:227–241. doi: 10.1089/10430340360535788. [DOI] [PubMed] [Google Scholar]
- 146.Adams JY, Johnson M, Sato M, Berger F, Gambhir SS, Carey M, et al. Visualization of advanced human prostate cancer lesions in living mice by a targeted gene transfer vector and optical imaging. Nat Med. 2002;8:891–897. doi: 10.1038/nm743. [DOI] [PubMed] [Google Scholar]
- 147.Zhang Y, Yu J, Unni E, Shao TC, Nan B, Snabboon T, et al. Monogene and polygene therapy for the treatment of experimental prostate cancers by use of apoptotic genes bax and bad driven by the prostate-specific promoter ARR(2)PB. Hum Gene Ther. 2002;13:2051–2064. doi: 10.1089/10430340260395901. [DOI] [PubMed] [Google Scholar]
- 148.Furuhata S, Ide H, Miura Y, Yoshida T, Aoki K. Development of a prostate-specific promoter for gene therapy against androgen-independent prostate cancer. Mol Ther. 2003;7:366–374. doi: 10.1016/s1525-0016(02)00059-x. [DOI] [PubMed] [Google Scholar]