Abstract
Background
Little is known about the long-term impact of androgen deprivation therapy (ADT) on body composition in men with prostate cancer. We compared body composition parameters in men with non-metastatic prostate cancer on or not on therapy with healthy, age-matched controls at baseline and monitored changes over a 2-year period.
Methods
We measured body fat mass and lean body mass in 81 men with prostate cancer on no ADT, 43 men on acute ADT (less than 6 months), 67 men on chronic ADT (more than 6 months) and 53 age-matched healthy controls. Measurements were performed every 6 months for 2 years.
Results
Men with prostate cancer on acute ADT (mean 3 months) had significant gains in body fat mass [1499.56 ± 322.28 g (mean ± S.E.) after 12 months, 2167.15 ± 676.45 g after 24 months, p < 0.01 for both] and losses in lean body mass (929.74 ± 296.36 g after 12 months, 1785.81 ± 501.31 g after 24 months, p < 0.01 for both) over 2 years. Men on chronic ADT (mean 31 months) had smaller but still significant body composition changes over 24 months. Changes in body composition in men on no ADT were small and healthy controls had no significant changes.
Conclusions
Men with prostate cancer on ADT have significant gains in body fat mass and losses lean body mass over 2 years. These changes are most pronounced with initiation of ADT.
Keywords: Prostate cancer, Androgen deprivation therapy, Body composition, Dual-energy X-ray absorptiometry
1. Introduction
Androgen deprivation therapy (ADT) has been the primary post-surgical treatment for metastatic prostate cancer since the 1940s [15]. Beginning in the early 1990s, men with non-metastatic prostate cancer were placed on ADT as an adjuvant therapy [6]. The use of ADT as a primary or neo-adjuvant therapy in non-metastatic prostate cancer patients has increased since then [1]. Androgens have important roles not only in the maintenance of bone mineral density [9,12], but also lean body mass. Several cross-sectional and retrospective studies demonstrate accelerated loss of lean body mass and gain in body fat in individuals on ADT for up to 1 year [2,3,5,25–28]. One controlled study compared men on ADT with healthy controls over a 6-month period and reported similar findings [4]. The effects of ADT on body composition for more than 1 year remain unknown. Therefore, we compared body composition parameters in men with non-metastatic prostate cancer on or not on ADT with healthy, age-matched controls at baseline and monitored changes over a 2-year period.
2. Materials and methods
2.1. Subjects
Men with non-metastatic prostate cancer who had previous surgery and/or radiation were recruited from the private practices of the University of Pittsburgh Physicians and cancer support groups as part of a study to examine changes in bone mass [13]. Normal healthy controls were recruited through local newspaper advertisements as previously reported [7]. All participants were community-dwelling men aged 50 years and older. Men with prostate cancer were grouped as having no androgen deprivation therapy (ADT), acute ADT (initiating ADT within the last 6 months prior to enrollment), and chronic ADT (treated with ADT for 6 months or longer). ADT constituted either orchiectomy, treatment with gonadotropin-releasing hormone (GnRH) agonists, treatment with anti-androgens, or combinations of these as previously described [13]. Because this study also involved examining bone loss in men on ADT, subjects were excluded if they had a disease or were taking any medication known to affect bone and mineral metabolism [13]. The protocol was approved by the Institutional Review Board at the University of Pittsburgh. Written informed consent was obtained from all subjects before their enrollment.
2.2. Outcome measures
2.2.1. Measures of body habitus
Measures of body habitus included height (cm), weight (kg), and body mass index (BMI, kg/m2). Height was measured to the nearest centimeter using a Harpenden stadiometer (Holtain Ltd., Crymych Dyfed, UK). Weight was measured with a balance beam scale. Height, weight and BMI were measured at baseline, 12 months and 24 months. Percent body fat and lean body mass were measured using body composition software on a Hologic QDR-4500A bone densitometer (Hologic Inc., Bedford, MA). Body composition parameters were measured at each 6-month visit.
2.2.2. Measures of gonadal status
Total testosterone was measured by competitive immunoassay (ng/dl; Diagnostic Products Corp., Los Angeles, CA) with intra-assay CV of 2.4–8.3%. Free testosterone was measured by tracer equilibrium dialysis (pg/ml; Nichols Institute, San Juan-Capistrano, CA) with an intra-assay CV of 4.2–11.6%. Prostate-specific antigen (PSA) was measured by chemiluminance (ng/ml; Bayer Diagnostics, Tarrytown, NY).
2.3. Statistical analysis
This was a secondary analysis of a study designed to examine changes in bone mass in men with prostate cancer. All data analyses were performed using SAS® software, version 9.1 (SAS Institute, Inc., Cary, NC, USA). Descriptive statistics were used to characterize the four groups in the study sample. Analysis of variance (ANOVA) was used to compare baseline characteristics among the groups. Analysis of covariance (ANCOVA) was used to make adjusted comparisons of both absolute and percent changes in body composition across the groups. These adjusted comparisons were controlled for the baseline value of the change measure and age. In addition, the significance of within-group changes between baseline and follow-up assessments were examined using paired samples t-tests.
3. Results
Of the prostate cancer subjects enrolled, 81 were not on ADT, 43 had been on ADT for less than 6 months (acute ADT, mean duration 3.0 ± 0.3 months), and 67 had been on ADT for over 6 months (chronic ADT, mean duration 30.7 ± 3.8 months). Fifty-three healthy controls also participated in the study (Table 1). Subjects with prostate cancer on ADT were older than those not on ADT or healthy controls (p < 0.0001). Body fat mass and percentage of body fat were lowest in the healthy controls and greater in the prostate cancer patients. Subjects on chronic ADT had the lowest and healthy controls had the highest lean body mass and percentage of lean mass (p = 0.0092 and p < 0.0001 across all groups, respectively). Body mass index (BMI) followed the trends seen in body fat across groups but was not significant across the four groups (p = 0.3352).
Table 1.
Baseline body composition characteristics (mean ± S.E.)
| Parameters | Controls (n = 53) | No ADT (n = 81) | Acute ADT (n = 43) | Chronic ADT (n = 67) | p-Value (across groups) |
|---|---|---|---|---|---|
| Age (years) | 62.55 ± 1.59 | 66.70 ± 0.81 | 70.86 ± 1.19 | 71.07 ± 1.07 | <0.0001 |
| Duration of ADT (months) | 2.95 ± 0.25 | 30.67 ± 3.78 | |||
| Body mass index (kg/m2) | 27.62 ± 0.47 | 28.26 ± 0.41 | 28.43 ± 0.88 | 29.12 ± 0.61 | 0.3352 |
| Body fat (g) | 21629.25 ± 930.57 | 23606.34 ± 895.82 | 23999.39 ± 1240.08 | 27469.28 ± 1129.24 | 0.0013 |
| Body fat % (% of total mass) | 25.14 ± 0.71 | 26.74 ± 0.66 | 27.08 ± 0.83 | 31.43 ± 0.65 | <0.0001 |
| Lean body mass (g) | 60157.05 ± 884.79 | 60238.66 ± 771.04 | 59881.70 ± 1147.27 | 56482.19 ± 1014.13 | 0.0092 |
| Lean body mass % (% of total mass) | 71.62 ± 0.67 | 70.25 ± 0.62 | 69.92 ± 0.79 | 65.86 ± 0.63 | <0.0001 |
| Testosterone (ng/dl) | 421.88 ± 24.64 | 345.5 ± 18.90 | 81.69 ± 30.65 | 34.7 ± 12.87 | <0.0001 |
| Free testosterone (pg/ml) | 66.47 ± 5.00 | 55.4 ± 3.21 | 11.64 ± 6.69 | 2.2 ± 0.38 | <0.0001 |
| PSA (ng/ml) | 1.8 ± 0.2 | 1.6 ± 0.3 | 3.0 ± 1.2 | 6.2 ± 2.1 | 0.0219 |
Abbreviations: ADT, androgen deprivation therapy, PSA, prostate specific antigen.
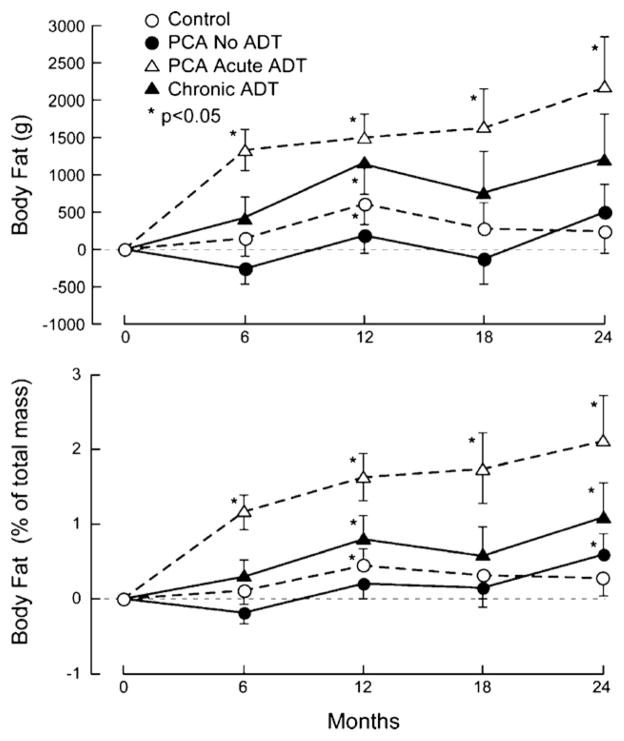
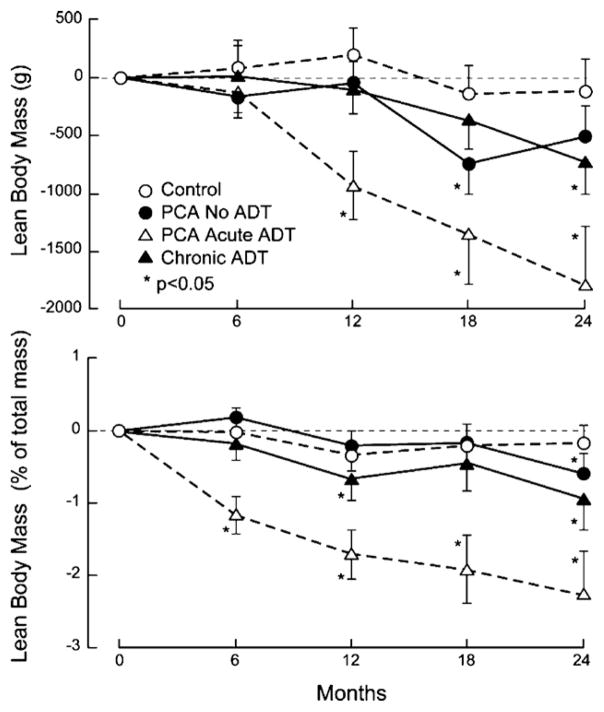
Changes in body composition were observed as early as 6 months in men on acute ADT (Figs. 1 and 2). Subjects on acute ADT at 6 months had significant gains in body fat mass (1332.10 ± 276.69 g (mean ± S.E.M.), p < 0.0001) and percent body fat (1.16% ± 0.23, p < 0.0001) as well as losses in percent lean body mass (1.17 ± 0.26%, p < 0.0001). At 12 months, subjects on acute ADT showed the greatest losses in lean body mass (929.4 ± 296.4 g, p = 0.0035) and percent lean mass from baseline (1.71 ± 0.34%, p < 0.0001) and the highest gains in body fat mass (1499.6 ± 322.3 g, p < 0.0001) and percent body fat (1.63 ± 0.31%, p < 0.0001) from baseline. Subjects on chronic ADT had a smaller but still significant increase in body fat mass (1162.9 ± 423.4 g, p = 0.0088), percent body fat (0.80 ± 0.31%, p = 0.0131) and a decrease in the percent lean body mass (0.67 ± 0.30%, p = 0.0313) compared to baseline. The healthy controls also had small but significant increases in body fat mass (607.77 ± 280.59 g, p = 0.0359) and percent body fat (0.45 ± 0.22%, p = 0.0419). Prostate cancer patients on no ADT had non-significant increases in body fat and decreases in lean body mass parameters over 12 months.
Fig. 1.
Changes from baseline in (a) total body fat mass (g) and (b) percent body fat over 24 months. Results are shown as mean ± S.E. (a) p = 0.0024 across all groups at 24 months. (b) p < 0.0001 across all groups at 24 months. Abbreviations: ADT, androgen deprivation therapy.
Fig. 2.
Changes from baseline in (a) total lean body mass (g) and (b) percent lean mass over 24 months. Results are shown as mean ± S.E. (a) p = 0.0630 across all groups at 24 months. (b) p < 0.0001 across all groups at 24 months. Abbreviations: ADT, androgen deprivation therapy.
At 24 months, all men with prostate cancer had lost lean body mass and gained body fat mass. Men on acute ADT had the greatest differences in body fat mass (2167.15 ± 676.45 g, p = 0.0035), percent body fat (2.11 ± 0.62%, p = 0.0021), lean body mass (−1785.81 ± 501.31 g, p = 0.0014), and percent lean body mass (−2.27 ± 0.61%, p = 0.001) from baseline. Men on chronic ADT and no ADT also had smaller but significant increases in the percent body fat mass and decreases in percent lean body mass (all p < 0.05). BMI did not significantly change for all groups at both the 12- and 24-month time points, except in the chronic ADT group at 12 months (0.44 ± 0.18, p = 0.0184).
4. Discussion
Our results demonstrate that body fat mass and percentage of body fat increased and lean body mass and percentage of lean mass decreased in men with prostate cancer on androgen deprivation therapy. Duration of ADT correlated with higher body fat and lower lean body mass parameters. The greatest change in body composition occurred in the first 12 months of therapy in subjects in the acute ADT group. These changes continued after 12 months. Subjects on chronic ADT also had significant changes in body composition after 24 months. Men on no ADT had smaller but significant changes in body fat and lean mass. In comparison, body composition in the control group remained stable over 24 months.
In healthy aging men, declining androgen levels correlate with increasing body fat mass and a decreasing lean body mass [22,29]. The increased adiposity in turn may contribute to the androgen deficiency, primarily due to decreased sex hormone binding globulin levels [8]. Decreased muscle mass leads to diminished strength and a higher risk for frailty endpoints such as falls and fractures [17]. Androgen replacement in men with acquired hypogonadism reverses these changes [16]. Men with actively treated prostate cancer do not have this option. Instead, ADT in this population accelerates these effects on body composition and significantly lowers physical function and general health when compared to healthy, aging men [7].
Our results support the accelerated body composition changes during the first year of ADT reported in previous studies. The mean duration of ADT in the acute group at enrollment to our study was 3 months. One study found significant changes after only 3 months of ADT [6]. Our findings may be an underestimate of actual body composition changes in the first year of therapy.
We also found continued smaller but still significant increases in body fat mass and decreases in lean body mass over 24 months. Support for this continued effect is lacking due to limited ADT-associated outcome data after 1 year. A cross-sectional study in men with prostate cancer up to 5 years ADT had significantly higher weight and body fat percentage than healthy controls [24]. Information on bone health with chronic ADT usage is more robust. In a small, prospective study, lumbar spine and femoral neck bone mineral density (BMD) declined at each 6-month time point up to 18 months [20]. Although the BMD loss at 18 months was significant, the rate of loss between each 6-month time point remained non-significant [20]. In a recent large retrospective analysis of 50,613 men with prostate cancer, 19.4% of surviving patients on ADT had a fracture after 5 years compared to 12.6% of patients not on ADT (p < 0.001) [23].
We observed that men with prostate cancer on no ADT had small increases in body fat and decreases in lean body mass over 24 months. The reasons for these changes are unclear. Several studies have suggested that obesity and metabolic syndrome are associated with prostate cancer [11,14,18]. However, the relationship is complex and may be related to the grade of prostate cancer. Further studies are needed to clarify this relationship.
Because this was a secondary analysis for a study on skeletal health, subjects with low bone mass or who were using skeletal anti-resorptive agents were not recruited or were discontinued from the study [13]. Men with low BMD and high fracture risk often have reduced muscle mass and higher percentage of body fat [19]. Subjects enrolled in our study may have been healthier than the general population of men with prostate cancer on ADT. Also, free and total testosterone levels were not drawn at the 24-month visit. Therefore, biochemical confirmation of continued chemical castration was not available. However, continued suppression of androgen levels after 2 years of ADT has been demonstrated in another study [21].
Our study has many important strengths. It is the largest study to examine changes in body composition with ADT. It is also the first to prospectively observe body composition changes after 1 year of ADT. Two different control groups (age-matched healthy control subjects and prostate cancer patients not on ADT) were used for comparisons. Finally, we used a single dual-energy X-ray absorptiometry machine for all body composition measurements.
In summary, androgen deprivation therapy for men with prostate cancer has profound effects on body composition. Much of this effect occurs in the first year of therapy. However, significant increases in body fat and decreases in lean body mass continue after 1 year. Interventions such as resistance training have been shown to improve muscle strength and functional performance in older men who have received ADT for prostate cancer [10]. Future studies are needed to determine if these interventions should be considered at initiation of ADT and continued for the duration of therapy.
Acknowledgments
NIH/NIDDKD grant K24 DK062895-03 (PI: Greenspan), NIH/NIA grants T32 AG021885 (PI: Studenski), The University of Pittsburgh Clinical and Translational Research Center (CTRC – Ul1 RR024153), and the CaPCURE foundation.
Biographies
Gijsberta van Londen, M.D., is a senior, clinical geriatric hematology–oncology fellow at the University of Pittsburgh. She is pursuing a Master of Science degree in ‘Clinical Trials of the Aging Population’.
Matthew Levy, M.D., is an endocrinologist in Scranton, Pennsylvania. He has completed a fellowship in endocrinology and has performed research with Dr. Greenspan.
Subashan Perera, Ph.D., is currently an Assistant Professor in Division of Geriatric Medicine and Department of Biostatistics at the University of Pittsburgh, and serves as the Co-Director of the Data Management and Analysis Core of the NIA funded Pittsburgh Claude D. Pepper Older Americans Independence Center.
Joel B. Nelson, M.D., is the Frederic N. Schwentker Professor and Chairman, Department of Urology at the University of Pittsburgh School of Medicine. He has been collaborating with the Principal Investigator on studies involving men with prostate cancer suffering from cancer related bone loss. He has expertise in surgical treatment of prostate cancer and will continue to collaborate with Dr. Greenspan.
Susan L. Greenspan, M.D., Principal Investigator, is board-certified in internal medicine, geriatrics, and endocrinology and metabolism, and currently holds joint appointments in the Division of Endocrinology and Metabolism and the Geriatrics Division of the University of Pittsburgh Medical Center (UPMC). Dr. Greenspan is Professor of Medicine at University of Pittsburgh School of Medicine and Director of the Osteoporosis Prevention and Treatment Center at UPMC. She is also Program Director of UPMC’s Clinical Translational Research Center located in UPMC Braddock Hospital, Pittsburgh, PA. Dr. Greenspan has published numerous papers on topics in bone and mineral metabolism including maintenance of skeletal integrity in elderly men and women, assessments of new techniques and devices for classification of bone status, new biochemical markers of bone turnover for therapeutic assessment, and cancer treatment related bone loss.
References
- 1.Alibhai SM, Gogov S, Allibhai Z. Long-term side effects of androgen deprivation therapy in men with non-metastatic prostate cancer: a systematic literature review. Crit Rev Oncol Hematol. 2006;60:201–15. doi: 10.1016/j.critrevonc.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 2.Basaria S, Lieb J, 2nd, Tang AM, et al. Long-term effects of androgen deprivation therapy in prostate cancer patients. Clin Endocrinol (Oxf) 2002;56:779–86. doi: 10.1046/j.1365-2265.2002.01551.x. [DOI] [PubMed] [Google Scholar]
- 3.Berruti A, Dogliotti L, Terrone C, et al. Changes in bone mineral density, lean body mass and fat content as measured by dual energy X-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–7. [discussion: 2367] [PubMed] [Google Scholar]
- 4.Boxer RS, Kenny AM, Dowsett R, Taxel P. The effect of 6 months of androgen deprivation therapy on muscle and fat mass in older men with localized prostate cancer. Aging Male. 2005;8:207–12. doi: 10.1080/13685530500361226. [DOI] [PubMed] [Google Scholar]
- 5.Chen Z, Maricic M, Nguyen P, Ahmann FR, Bruhn R, Dalkin BL. Low bone density and high percentage of body fat among men who were treated with androgen deprivation therapy for prostate carcinoma. Cancer. 2002;95:2136–44. doi: 10.1002/cncr.10967. [DOI] [PubMed] [Google Scholar]
- 6.Cooperberg MR, Grossfeld GD, Lubeck DP, Carroll PR. National practice patterns and time trends in androgen ablation for localized prostate cancer. J Natl Cancer Inst. 2003;95:981–9. doi: 10.1093/jnci/95.13.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dacal K, Sereika SM, Greenspan SL. Quality of life in prostate cancer patients taking androgen deprivation therapy. J Am Geriatr Soc. 2006;54:85–90. doi: 10.1111/j.1532-5415.2005.00567.x. [DOI] [PubMed] [Google Scholar]
- 8.de Moor P, Joossens JV. An inverse relation between body weight and the activity of the steroid binding-globulin in human plasma. Steroidologia. 1970;1:129–36. [PubMed] [Google Scholar]
- 9.Eastham JA. Bone health in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2007;177:17–24. doi: 10.1016/j.juro.2006.08.089. [DOI] [PubMed] [Google Scholar]
- 10.Galvao DA, Nosaka K, Taaffe DR, et al. Resistance training and reduction of treatment side effects in prostate cancer patients. Med Sci Sports Exerc. 2006;38:2045–52. doi: 10.1249/01.mss.0000233803.48691.8b. [DOI] [PubMed] [Google Scholar]
- 11.Gong Z, Neuhouser ML, Goodman PJ, et al. Obesity, diabetes, and risk of prostate cancer: results from the prostate cancer prevention trial. Cancer Epidemiol Biomarkers Prev. 2006;15:1977–83. doi: 10.1158/1055-9965.EPI-06-0477. [DOI] [PubMed] [Google Scholar]
- 12.Greenspan SL. Approach to the prostate cancer patient with bone disease. J Clin Endocrinol Metab. 2008;93:2–7. doi: 10.1210/jc.2007-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greenspan SL, Coates P, Sereika SM, Nelson JB, Trump DL, Resnick NM. Bone loss after initiation of androgen deprivation therapy in patients with prostate cancer. J Clin Endocrinol Metab. 2005;90:6410–7. doi: 10.1210/jc.2005-0183. [DOI] [PubMed] [Google Scholar]
- 14.Hsing AW, Sakoda LC, Chua S., Jr Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr. 2007;86:s843–57. doi: 10.1093/ajcn/86.3.843S. [DOI] [PubMed] [Google Scholar]
- 15.Huggins C, Hodges CV. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. 1941. J Urol. 2002;168:9–12. doi: 10.1016/s0022-5347(05)64820-3. [DOI] [PubMed] [Google Scholar]
- 16.Katznelson L, Finkelstein JS, Schoenfeld DA, Rosenthal DI, Anderson EJ, Klibanski A. Increase in bone density and lean body mass during testosterone administration in men with acquired hypogonadism. J Clin Endocrinol Metab. 1996;81:4358–65. doi: 10.1210/jcem.81.12.8954042. [DOI] [PubMed] [Google Scholar]
- 17.Larsson L, Grimby G, Karlsson J. Muscle strength and speed of movement in relation to age and muscle morphology. J Appl Physiol. 1979;46:451–6. doi: 10.1152/jappl.1979.46.3.451. [DOI] [PubMed] [Google Scholar]
- 18.Laukkanen JA, Laaksonen DE, Niskanen L, Pukkala E, Hakkarainen A, Salonen JT. Metabolic syndrome and the risk of prostate cancer in Finnish men: a population-based study. Cancer Epidemiol Biomarkers Prev. 2004;13:1646–50. [PubMed] [Google Scholar]
- 19.Luukinen H, Koski K, Laippala P, Kivela SL. Factors predicting fractures during falling impacts among home-dwelling older adults. J Am Geriatr Soc. 1997;45:1302–9. doi: 10.1111/j.1532-5415.1997.tb02928.x. [DOI] [PubMed] [Google Scholar]
- 20.Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–22. [PubMed] [Google Scholar]
- 21.Morote J, Orsola A, Abascal JM, et al. Bone mineral density changes in patients with prostate cancer during the first 2 years of androgen suppression. J Urol. 2006;175:1679–83. doi: 10.1016/S0022-5347(05)00999-7. [discussion: 1683] [DOI] [PubMed] [Google Scholar]
- 22.Seidell JC, Bjorntorp P, Sjostrom L, Kvist H, Sannerstedt R. Visceral fat accumulation in men is positively associated with insulin, glucose, and C-peptide levels, but negatively with testosterone levels. Metabolism. 1990;39:897–901. doi: 10.1016/0026-0495(90)90297-p. [DOI] [PubMed] [Google Scholar]
- 23.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 24.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of the “androgen deprivation syndrome” in men receiving androgen deprivation for prostate cancer. Arch Intern Med. 2006;166:465–71. doi: 10.1001/.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smith JC, Bennett S, Evans LM, et al. The effects of induced hypogonadism on arterial stiffness, body composition, and metabolic parameters in males with prostate cancer. J Clin Endocrinol Metab. 2001;86:4261–7. doi: 10.1210/jcem.86.9.7851. [DOI] [PubMed] [Google Scholar]
- 26.Smith MR. Changes in fat and lean body mass during androgen-deprivation therapy for prostate cancer. Urology. 2004;63:742–5. doi: 10.1016/j.urology.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 27.Smith MR, Finkelstein JS, McGovern FJ, et al. Changes in body composition during androgen deprivation therapy for prostate cancer. J Clin Endocrinol Metab. 2002;87:599–603. doi: 10.1210/jcem.87.2.8299. [DOI] [PubMed] [Google Scholar]
- 28.Stoch SA, Parker RA, Chen L, et al. Bone loss in men with prostate cancer treated with gonadotropin-releasing hormone agonists. J Clin Endocrinol Metab. 2001;86:2787–91. doi: 10.1210/jcem.86.6.7558. [DOI] [PubMed] [Google Scholar]
- 29.Vermeulen A, Goemaere S, Kaufman JM. Testosterone, body composition and aging. J Endocrinol Invest. 1999;22:110–6. [PubMed] [Google Scholar]