Abstract
Known as an essential component of the translational apparatus, the aminoacyl-tRNA synthetase family catalyzes the first step reaction in protein synthesis, that is, to specifically attach each amino acid to its cognate tRNA. While preserving this essential role, tRNA synthetases developed other roles during evolution. Human tRNA synthetases, in particular, have diverse functions in different pathways involving angiogenesis, inflammation and apoptosis. The functional diversity is further illustrated in the association with various diseases through genetic mutations that do not affect aminoacylation or protein synthesis. Here we review the accumulated knowledge on how human tRNA synthetases used structural inventions to achieve functional expansions.
Keywords: Aminoacyl-tRNA synthetase, non-canonical function, human, structure, multisynthetase complex, disease
Introduction
When sequencing of the human genome was completed in 2004, it was a surprise to many of us that the number of protein-coding genes in human was not much more than that in the simple roundworm C. elegans [1,2]. The shock was followed by the realization that the complexity of higher organisms is not necessarily reflected by a larger number of protein-coding genes [3]. We now understand that alternative splicing occurs on a wide scale, so that one gene can give rise to many different gene products [4]. In addition, individual proteins have expanded uses, and are regulated at multiple levels to support and to integrate diverse biological systems in humans.
Because of their essential role in protein synthesis, genes encoding aminoacyl-tRNA synthetases (AARS) appeared at the onset of evolution of life. As a family of 20 enzymes in general (one for each amino acid), AARSs activate amino acids for protein synthesis, through specific ligation of each amino acid to its cognate tRNA. While constrained by evolutionary pressure to preserve this essential activity, AARSs still managed to develop additional functions during evolution (reviewed in [5–8]). Interestingly, in higher eukaryotic cells, AARSs are organized into a high molecular mass multisynthetase complex (MSC), where nine AARSs and three accessory proteins are bound together [9]. This complex may promote a more organized protein synthesis and is also thought to serve as a reservoir of regulation molecules for functions beyond aminoacylation [10,11]. (Expanded functions and higher order interactions of human AARSs are summarized in Table 1.)
Table 1.
Mechanism of expanded function and higher order interactions of human aminoacyl-tRNA synthetases and their associated factors
| AspRS | Component of MSC via interaction with MSC p38 | [75] |
| Nonspecific tRNA binding using the appended N-terminal helix | [34,38,39] | |
| Charged tRNA transfer to eEF1γ facilitated by the N-helix | [26] | |
| LysRS | Component of MSC via p38 interaction using adapted core enzyme | [75,88] |
| Transcription activation of MITF and USF2 via direct interaction | [103,107] | |
| Interaction with Gag through homodimer interface for tRNALys incorporation into HIV | [96,97] | |
| Proinflammatory response | [108] | |
| AsnRS | Autoimmune response via interaction with chemokine receptor CCR3 | [109] |
| Anti-apoptosis | [110] | |
| ArgRS | Component of MSC via interaction using appended leucine zipper | [10,30,75] |
| Providing Arg-tRNAArg for posttranslational arginylation | [78,111,112] | |
| ValRS | Complex with eEF1H using the appended GST domain | [28,29,69] |
| CysRS | Complex with eEF1γ using the appended GST domain | [70] |
| Association with diabetic nephropathy | [113] | |
| MetRS | Component of MSC via interaction using appended GST domain | [30,31] |
| rRNA transcription | [114] | |
| GluProRS | Component of MSC via interaction using appended WHEP domains | [55,79,102] |
| Transcription repression by forming WHEP domain-dependent GAIT complex | [64,65] | |
| GlyRS | Causal link to neuropathy via mutations may or may not affect aminoacylation | [16,115–118] |
| Autoimmune response | [119] | |
| HisRS | Autoimmune response provoked by the appended WHEP domain | [120] |
| TrpRS | Angiostasis by inhibiting VE-cadherin using the active site | [92,94,101] |
| TyrRS | (mini-TyrRS) Angiogenesis through ELR motif invention on the catalytic domain | [51,83,84] |
| (C-TyrRS) EMAP II-like Cytokine | [121] | |
| Causal link to neuropathy via mutations may or may not affect aminoacylation | [122,123] | |
| SerRS | Vascular development | [122,123] |
| ThrRS | Autoimmune response | [124] |
| PheRS | Erythroleukemia cell differentiation | [125] |
| LeuRS | Component of MSC via interaction using appended domain | [80] |
| Complex with eEF1γ | [27] | |
| GlnRS | Component of MSC via interaction using adapted catalytic domain | [89] |
| Anti-apoptosis via interaction with ASK1 | [126] | |
| IleRS | Component of MSC via interaction using appended domain | [30,55] |
| Autoimmune response | [124] | |
| AlaRS | Autoimmune response | [127] |
| MSC p43 | Proliferation, angiostasis, and pro-apoptosis (Leucine zipper-containing N-half) | [128–130] |
| Angiostasis via interactions with ATP synthase, integrin α5β1 and PSMA7 (EMAP II) | [131,132] | |
| Glucagon-like hormone | [133] | |
| Autoimmune regulation by retrieving gp96 in endoplasmic reticulum | [134] | |
| Axonal development via interaction with neurofilament light subunit | [135] | |
| TGFβ down-regulation via interaction with Smurf2 | [136] | |
| MSC p38 | Regulate lung cell differentiation via FBP interaction and degradation | [137] |
| Involved in neurodegeneration as a substrate of parkin. | [138] | |
| Pro-apoptosis via interaction with p53 to prevent its degradation | [139] | |
| MSC p18 | Tumor suppressor via interaction with ATM and ATR | [68,71] |
While early work in bacteria and lower eukaryotes pointed to alternative functions for some AARSs [12–14], a rich and diverse set of expanded functions was found in higher eukaryotes, especially in human tRNA synthetases. Some of these expanded functions were inferred by connections, including causal associations, of specific diseases with tRNA synthetases [8,15]. Others were demonstrated by direct experiments showing a role in pathways ranging from inflammation, angiogenesis, angiostasis, apoptosis and autoimmunity. Adding further weight to the concept of expanded functions was the discovery of mutations in genes for tRNA synthetases that are causally linked to disease and, at the same time, do not affect aminoacylation activity or protein synthesis [16–18]. Thus, the concept of alternative functions for tRNA synthetases is now well established.
However, the understanding of how these alternative functions were developed is at its infancy. This understanding has to accommodate the observation that, throughout evolution, the 20 synthetases were constructed on the foundation of one of two basic architectures, which are referred to as class I or class II. The ten class I enzymes have a catalytic domain based on a Rossmann nucleotide binding fold, while the catalytic domain of the ten class II enzymes is a 7-stranded β-sheet with flanking α-helices [19]. These two architectures appeared at the base of the tree of life and, with rare exception [20], the class to which a synthetase is assigned is fixed throughout evolution. Thus, expanded functions for each synthetase were developed in the context of a fixed architecture for the catalytic unit, and functional expansion took place without disturbing the essential catalytic activity.
As described here, nature used structural inventions that, as higher organisms appeared, built upon and expanded the sizes of tRNA synthetases, and also invaded the catalytic unit itself to make subtle changes that did not perturb the canonical aminoacylation activity. In addition, inventions were made to regulate the elucidation of alternative activities, and even to switch the activity of a synthetase from a ‘pure’ aminoacylation function to an alternative function in cell signaling pathways.
Functional expansion through acquisition of extra domains
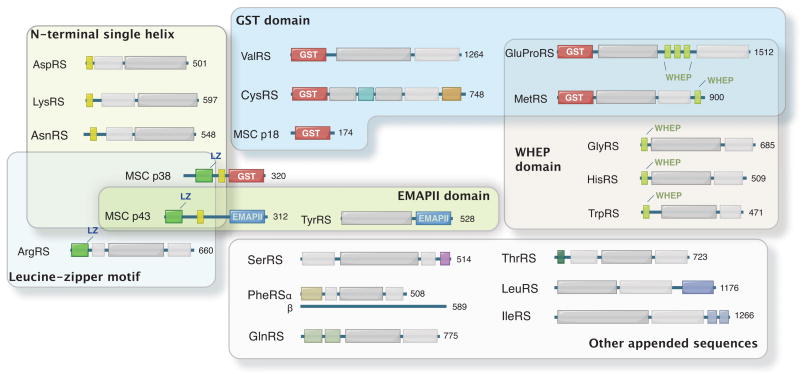
The fusion of a new domain to an existing protein is a straightforward way to introduce a new function. Indeed, compared with their prokaryotic or lower eukaryotic counterparts, all human AARS (except for AlaRS) have extra domains or motifs at either the N- or C-terminus [5,21,22] (Figure 1). Although some of those domains are thought to promote tRNA interactions [23,24] or to facilitate product transfer to the elongation factor [25–29], these domains in most cases are dispensable for aminoacylation. For those AARSs that are components of the MSC, the appended domains are involved in the formation of the complex [30,31]. Supporting this idea, some of the appended domains needed for MSC formation emerged concurrently with the appearance of the MSC in Drosophila [32]. However, about half of the twenty synthetases are not components of the MSC, and yet the frequency of appearance of appended domains in those free tRNA synthetases is similar to that of the bound members of the MSC. Thus, while the need for appended domains to form the MSC seems clear, the appended domains in non-MSC enzymes suggest that other interactions also occur. Indeed, the interactions of the appended domains with partners, such as cell surface receptors, cytoplasmic and nuclear proteins, or nucleic acids, facilitate new functions.
Figure 1.
Appended domain/motifs in human AARSs and MSC-associated factors. Except for human AlaRS, all AARSs have appended domains compared to their bacterial or archaeal homologues. The prokaryotic catalytic domains and tRNA recognition domains are shown as white and gray boxes. N-terminal single helices are present in three class IIb AARSs (yellow boxes). Homologous GST domains are shown in the N-terminal regions of MetRS, GluProRS, ValRS and CysRS, as well as in the C-terminal regions of MSC p18 and p38. MSC p38 also contains a leucine zipper motif (LZ) (green box) and is involved in interactions with leucine zippers of MSC p43 and ArgRS.(Min—in the figure, replace p38/AIMP2 with MSC p38, and same for p43.) The EMAPII domains (blue boxes) in MSC p43 and TyrRS are related. Homologous WHEP domains are shared by five human AARSs. Many other appended domains in AARSs are idiosyncratic to each enzyme and remain to be further characterized.
N-terminal amphiphilic helix — a motif originated from tRNA binding
An N-terminal extension that is absent in their prokaryotic orthologs is found in human LysRS, AspRS and AsnRS. These three class II synthetases are more closely related to each other than to the other members of the class, and were grouped together into a separate subclass designated as IIb [33](Figure 1). According to secondary structure predictions, each N-extension contains a helix of variable length ranging from 20 to 40 amino acids. A NMR structure determination of the N-terminal extension of human AspRS confirmed it has a helical conformation [34]. Those helices, in general, are amphiphilic with charged residues on one side, and hydrophobic residues on the other (Figure 2).
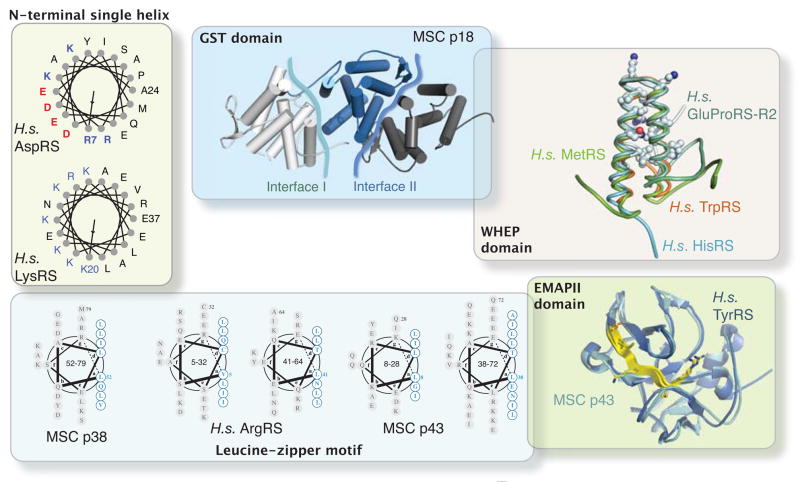
Figure 2.
Structures of appended domain/motifs in human AARSs and associated factors. These appended structures provide extra interfaces for protein-protein and protein-nucleic acid interactions, and are critical for the expanded functions of human AARSs. N-terminal single helices of human AspRS and LysRS are shown to illustrate the amphiphilic nature of those helices. Two protein-protein interaction interfaces are shown as irregular blue lines on MSC p18 (PDB 2uz8). Conserved residues among WHEP domains in different AARSs are shown as sticks (PDB 1x59, 16t, 2djv for human HisRS, TrpRS and MetRS, respectively, and PDB 1r1b for the second WHEP domain (R2) in GluProRS,). The putative cytokine motifs on EMAP II structures of human TyrRS (PDB 1ntg) and MSC p43 (PDB 1fl0) are shown in yellow.
The hydrophilic side of the N-terminal helix of human LysRS is dominated by positively charged residues--ideal for binding negatively charged nucleic acids (Figure 2). Indeed, the N-terminal extension of human LysRS binds to the Tψ C stem-loop region of tRNALys and thereby increases the affinity of the synthetase for its tRNA [24,35]. The role of LysRS in HIV packaging depends on the N-terminal extension, presumably because of its tRNA interaction [36](Table 1). In this role, LysRS interacts with tRNALys3 to deliver it to the virion to function as a primer for reverse transcription [37]. Similar to human LysRS, the amphiphilic N-terminal helices of yeast AspRS and of yeast AsnRS are also positively charged, with tRNA binding properties demonstrated for the N-extension of AspRS [38]. When appended to a catalytic domain, these N-terminal helices appended to tRNA synthetases can enhance tRNA binding affinity [23,35,39,40].
However, because of the non-specific nature of electrostatic interactions, these helices unsurprisingly bind to idiosyncratic sites on a single tRNA, and bind nonspecifically with different tRNAs [24,38]. Furthermore, similar helical motifs occur in a series of double-stranded RNA-recognizing proteins [41,42], suggesting that the N-terminal helix motif may be used broadly to function in associating with other types of RNA in the cellular environment.
Interestingly, compared to the yeast enzymes, human AspRS lacks many of the positively charged residues located in the N-terminal helix (Figure 2). Thus, the amphiphilic helices may have evolved from facilitating tRNA binding to facilitating other interactions in higher eukaryotes [43]. This paradigm, of starting in lower organisms with an appended domain that has nucleic acid binding properties and evolving towards another function in higher organisms, is seen in other examples, such as the EMAP II domain.
EMAP II domain — a second motif originated from tRNA binding
The C-terminal portion of human TyrRS contains an extra domain that is absent in its yeast ortholog (Figure 2). This domain is highly homologous to a known cytokine-- endothelial monocyte-activating polypeptide II (EMAP II) [44], which is a C-terminal proteolytic fragment of a component of the MSC known as p43 [45](Figure 1). The EMAP II domain is homologous to the homodimeric tRNA binding protein Trbp111[46]. The crystal structures of EMAP II and the C-domain of human TyrRS were solved [47–49], and the highly homologous structures were seen to each contain an oligonucleotide-binding (OB) fold β-barrel, while EMAP II has an additional motif that mimics the dimer interface in Trbp111 [48](Figure 2). As a result of the new internal dimer-like interface, the EMAP II domains are monomers [48]. Interestingly, EMAP II-like domains are found only in tRNA synthetases, including those appended to the C-terminus of nematode and plant MetRS, or in tRNA synthetase-associated proteins, including p43 and yeast Arc1p [50]. (In yeast, there is a smaller synthetase complex formed by MetRS, GluRS and a noncatalytic protein called Arc1p [51].) As a trans-acting factor, the EMAP II domain in Arc1p enhances catalytic activities of the associated tRNA synthetases and maintains their cytosolic localization [52,53]. Same functions could be achieved in cis, when the EMAP II domain was directly grafted into the catalytic core of MetRS [54]. Although retaining some of the tRNA binding properties of Trbp111 [50], the EMAP II domain in human TyrRS is dispensable for aminoacylation [55]. Similarly, the association of MSC p43 with human ArgRS does not improve the tRNA aminoacylation properties [56]. Instead, the EMAP II domains are associated with cytokine activities (Table 1) and are bound to specific receptors [57,58]. Thus, while the EMAP II domains may have originally developed for RNA binding, throughout evolution, they apparently gained cytokine functions by accumulating mutations.
WHEP domain — a third motif originated from tRNA binding
A helix-turn-helix motif named the WHEP domain is appended to five human tRNA synthetases. These include an N-terminal extension added to TrpRS, HisRS and GlyRS, a C-terminal extension on MetRS, and an insertion that links together the enzymes of the bifunctional GluProRS. In the latter case, three tandem repeats of WHEP domains are used to fuse together the two enzymes [59](Figure 2). (The name of WHEP domain comes from 3 of the 5 WHEP containing proteins: WRS, HRS and EPRS.) Although helix-turn-helix is a common structural motif, the WHEP domains of AARSs have sequence similarities that are not found in any non-AARS. Therefore, the WHEP domain is a true structural invention for AARSs. It has been introduced into AARSs in metazoans, and is entirely absent from all AARSs in lower species [60](Figure 1). Interestingly, removal of the WHEP domain(s) mostly had no effect on enzymatic activities of the WHEP-containing synthetases [61–64] suggesting that the role of the WHEP domain is outside of aminoacylation.
NMR and crystal structure analysis confirmed that WHEP domains have an antiparallel helix-turn-helix conformation, with consensus lysine and arginine residues forming a basic patch on one side of the structure [65,66](Figure 2). Consistently, the WHEP domains, either as isolated domain or as tandem repeats such as those found in GluProRS, are non-specific RNA-binding domain [59,65]. Compared with a single WHEP domain, the binding affinity is largely increased with the three repeated WHEP domains of GluProRS [65]. In addition to RNA, the WHEP domain repeats of GluProRS can bind to DNA [59,65]. However, they can also form protein-protein interactions, within the multi-synthetase complex, with the C-terminal appendix of IleRS, and with the N-terminal extension of ArgRS [59](see below).
Remarkably, the three WHEP domain repeats in GluProRS are essential for the novel role of GluProRS in regulating translation of specific genes associated with the inflammatory response (Table 1) [67,68]. The WHEP domains of GluProRS interact with ribosomal protein L13a, NS1-associated protein-1 (NSAP1), and glyceraldehyde-3-phosphate dehydrogenase to form the “GAIT” (γ-interferon-activated inhibitor of translation) complex. The complex is then brought to the stem-loop structure of the GAIT element in the 3′-UTR of the target mRNAs through WHEP domain-mediated interactions [69]. Therefore, the WHEP domains are involved in both protein-protein and protein-RNA interactions to achieve the novel function of GluProRS in regulating specific gene translation.
GST-like domain—a protein-protein interaction motif
Four class I tRNA synthetases, GluRS (which is linked to ProRS via 3 WHEP domains), MetRS, ValRS and CysRS, and two of the 3 auxiliary factors of the MSC (p38 and p18) contain a glutathione S-transferase (GST)-like domain (Figure 1). This domain is mostly absent from lower eukaryote synthetases and is entirely absent in prokaryotes. GST-like domains, as structural modules, are commonly used for protein assembly and to regulate protein folding [70], and many GST-like domains have no known enzyme activity (such as those in S-crystallins from squid, eukaryotic elongation factors 1-γ (eEF-1γ), 1-β (eEF-1β) and the HSP26 family of stress-related proteins). Interestingly, GST-like domains in tRNA synthetases and their target proteins show higher sequence similarity to each other than to GST-like domains found elsewhere. Thus, like the WHEP domains, the tRNA synthetase-associated GST domains have a common origin.
Not surprisingly, all AARSs containing a GST-like domain are found in complexes with other proteins [5,71]. Human MetRS and GluProRS are part of the MSC, interacting with MSC p18 and p38, respectively [30,72]. Mammalian ValRS forms a complex with eEF-1H (contains α β,γ and σ subunits) [28,29,73], and human CysRS binds to eEF-1γ [74]. All of these the interactions require the GST-like domains embedded in each AARS.
The crystal structure of the dimeric MSC p18 (composed of a single GST-like domain) revealed two potential binding surfaces, including one that resembles the dimeric interface of the GST enzyme, and a second binding surface for interacting with another GST dimer [72](Figure 2). Interestingly, to form the primitive yeast MSC composed of GluRS, MetRS and the yeast auxiliary factor Arc1p, the same two interfaces on the GST-like domain of Arc1p are used to interact with the GST-like domain of GluRS and of MetRS [71]. Those observations show that the GST-like domains mediate higher-order interactions among AARSs, and the interactions are conserved from yeast to human. Furthermore, using one of its two GST binding interfaces, MSC p18 interacts with ATM (a kinase in response to DNA damage and oncogenic stress) to act as a tumor suppressor [75,76]. Therefore, as a protein-protein binding motif, the GST-like domains expand the interaction partners of AARSs both inside and outside of the translational apparatus.
Leucine-zipper—another protein-protein binding motif
Human ArgRS has a domain-extension that is absent in the yeast enzyme (Figure 1). The domain contains two leucine-zipper motifs, which are also found in MSC p43 and p38 (Figure 2). The leucine-zipper is a helical motif that has leucine residues (or other hydrophobic residues) at every 4th position of the heptad repeats, so that the protruding isobutyl side chains are lined up on one side of the helix. This design creates a hydrophobic spine that inter-locks with its partner to form a coiled-coil zipper. Significantly, leucine-zipper motifs are widespread, being predicted in ~ 10 % of all eukaryotic proteins that span a wide range of functions [77,78].
All leucine-zipper containing AARSs are components of the MSC and are associated in the complex, at least in part, through the leucine-zipper motifs. The leucine-zippers embedded in the N-terminal domain of MSC p43 interact with the amphiphilic leucine-zipper helix of p38 [30,79,80]. The association of ArgRS into the MSC is also mediated through its N-terminal leucine-zipper [79]. When the N-terminal leucine-zipper domain of human ArgRS is absent because of alternative translation initiation, the shorter form of ArgRS becomes free in cytoplasm, and no longer is associated with the MSC [10,81,82]. Therefore, leucine-zipper motifs, as well as the GST-like domains, play a key role in regulating the binding and release of proteins from the MSC, which thereby acts as a reservoir for novel functions that get released or silenced on demand [5,11].
Other appended domains and sequences
There are other less characterized extensions in human AARSs. For example, the C-terminal extension of human IleRS contains two repeats of ~90 aa. This extension first appears in C. elegans. These repeats are critical for IleRS to bind to the WHEP domains of GluProRS in the MSC [83]. A similar C-terminal extension of human LeuRS also first appears in C. elegans. The extension was shown to have little effect on enzymatic activity [84]. This extension is involved in binding to the leucine-zipper of ArgRS, and therefore is important for stabilizing ArgRS and LeuRS in the MSC [84].
Other appended sequences of human AARS include the N-terminal extension of ThrRS (~80 aa), and C-terminal extensions of SerRS (~ 20 aa) and LysRS (~ 20 residues and not shown in figures). These are relatively short and without apparent structures. Interestingly, the C-terminal end of LysRS contains a binding site for the PDZ domain-- a specific protein-protein interaction motif found in many proteins involved in signal transduction and intracellular trafficking. PDZ domain-containing proteins TIP-15 and syntenin-1 were found to be associated with LysRS and possibly also with the MSC [85,86].
In summary, appended domains of human AARS can be separated into two groups. The first group of appended domains covers N-terminal amphiphilic helices, and EMAP II and WHEP domains, all of which appear to be evolved from nucleic acid binding motifs, but were expanded to interact with protein partners during evolution. The second group includes GST-like and leucine-zipper motifs that are protein-protein interaction scaffolds found in many protein families. These motifs have no known direct nucleic acid binding interactions. For AARSs, both groups of domains are involved in the organization of the MSC, and have been shown to have, or potentially can have, other interactions once the synthetases are released from the MSC.
Functional expansion through adaptation of ancient domains
Apart from functional expansions by directly introducing separate domains/motifs into tRNA synthetases, a new function fit for the need of higher organisms can also be acquired through evolving pre-existing domains (e.g., the aminoacylation domain and/or the tRNA anticodon binding domain). The new function can be introduced in two different ways. On the one hand, surfaces not being used for enzymatic functions are obvious candidate sites for developing new interactions that do not disrupt enzyme activity. This scenario illustrates the fine structure inventions that had to occur during evolution. On the other hand, enzymatic functions and their active sites can be redirected to accommodate new functions. In this case, the active site must be switched from one role to another, through a specific structural mechanism.
New adaptation of unused surface in the catalytic domain
An example of a surface adaptation to acquire a cytokine activity on the catalytic domain is seen with human TyrRS. A fragment of human TyrRS--mini-TyrRS--is a product of natural proteolysis. Mini-TyrRS contains the Rossmann-fold catalytic domain and the anticodon recognition domains, but lacks the appended EMAP II-like C-terminal domain. A tripeptide cytokine motif (ELR) that is critical for the function of IL-8-like cytokines is embedded in the Rossmann fold domain of mini-TyrRS. As a result, mini-TyrRS has ELR motif-dependent cytokine activities [55]. Interestingly, yeast TyrRS lacks the EMAP II-like appended domain and therefore is an ortholog of human mini-TyrRS [87]. Yeast TyrRS also lacks the ELR motif and the cytokine activity. When the ELR motif was grafted into yeast TyrRS at a location corresponding to its position in human mini-TyrRS, the resulting chimeric protein had the cytokine activity of the human enzyme [88]. As revealed in the ultra-high resolution crystal structure of mini-TyrRS (1.18 Å resolution), the ELR motif is located on an exposed helix in the Rossmann-fold domain, mostly away from the active site [89]. Importantly, the ELR motif and the active site of mini-TyrRS are functionally independent, so that disruption of one site has no effect on the other [90]. Thus, evolution found a site within the synthetase to place ELR, without disruption of the essential catalytic activity.
Another example of adapting a surface for new interactions is seen with human LysRS. The conserved catalytic and anticodon-binding domains, rather than the appended N-terminal helix motif, are responsible for binding to p38 in the MSC [30]. Consistent with the idea that p38 binding does not affect LysRS in aminoacylation, the p38 binding site on LysRS is separate from the active site and is not shared for tRNA binding [79]. Interestingly, the p38 binding site overlaps with the surface containing two eukaryote-specific sequences, one in the catalytic domain and another in the anticodon-binding domain [91,92]. Therefore, the p38 binding site is created from adaptations to otherwise highly conserved synthetase domains.
Similarly, the association of human GlnRS with MSC is also through the conserved domains [93], and likely uses a surface non-essential for aminoacylation.
Orthogonal use of active site
The most natural way for functional expansion is to utilize what has already been developed — such as the active site. On this point, the angiostatic function of TrpRS is active-site dependent. Along with other angiostatic factors such as IP-10 and MIG, TrpRS expression is highly up-regulated by IFN-γ [94,95]. IFN-γ also induces the secretion of TrpRS to the extracellular space, where the appended WHEP domain is removed by proteolysis to give T2-TrpRS-- a potent angiostatic factor that acts on endothelial cells by interacting with a cell-cell junction protein known as VE-cadherin [96,97]. The ability of VE-cadherin to build junctions depends on two Trp residues near its N-terminus (Trp2 and Trp4). These protruding side chains bind to a hydrophobic pocket of a VE-cadherin partner in a neighbor cell, and vice-versa. Using its tryptophan and AMP binding pockets, respectively, T2-TrpRS binds to Trp2 and Trp4 of VE-cadherin, and thereby inhibits the homophilic interaction of VE-cadherins, breaking up the VE-cadherin-dependent formation of new blood vessels [98]. Strikingly, if the active site is blocked by the Trp-AMP adenylate analog, or disrupted by mutagenesis, T2-TrpRS can no longer inhibit angiogenesis. This example illustrates how the active site of a tRNA synthetase can be used differently to develop new functions, which can be further regulated by the substrates or the products of the mother synthetase.
Dimer interface
Many tRNA synthetases require a homodimeric structure for the aminoacylation function. In those cases, dissociation of the homodimer would inactivate the enzyme, and simultaneously expose the dimer interface for potential new interactions. Interestingly, the dimer interface of LysRS is exploited by the Gag protein of HIV. By interaction with Gag, LysRS is selectively packaged into the HIV-1 virion to facilitate the incorporation of tRNALys, which is used by the virus as a primer for reverse transcription of its RNA genome (see above) [37,99]. The LysRS/Gag-CA complex is a heterodimer [100], and the binding site between LysRS and Gag has been mapped to the dimerization region of each protein [101]. As the dimer interface of human LysRS is highly conserved among LysRSs from different species [92], the interaction apparently was developed through the adaption of the Gag protein of HIV. However, this example suggests that dimer interface of a tRNA synthetase is also a possible binding site for host proteins.
Regulation (activation) of expanded functions
The question of how expanded functions of tRNA synthetases are regulated is under intense investigation. In the case of human TyrRS and TrpRS, switching to a new function is controlled by the removal of an appended domain — the C-terminal EMAP II-like domain of TyrRS or the N-terminal WHEP domain of TrpRS [102]. In these instances, domain removal, from the full-length native tRNA synthetase, unmasks the cytokine function. A detailed study of human TyrRS showed that the EMAP II-like domain shields the essential ELR motif [89]. Using rational site-directed mutagenesis, the full-length enzyme was “opened up” by a single point mutation that released the tether of the EMAP II-like domain, which had shielded the ELR motif in the native protein. The mutant enzyme, designated Y341A TyrRS, had the cytokine activity of mini-TyrRS, which entirely lacks the EMAP II-like domain [103].
In the case of native human TrpRS, the N-terminal WHEP domain sterically hinders binding of VE-cadherin, the extracellular receptor for T2-TrpRS [66,98]. Removal of the WHEP domain, by alternative splicing or proteolysis, provides access for receptor binding [104,105]. Thus, for both TyrRS and TrpRS, an appended domain that appeared only in eukaryotes plays an essential role in controlling the function. For the bifunctional GluProRS, with embedded WHEP domains forming the inter-synthetase linker, the expanded function (of translational repression) is activated by a phosphorylation event that releases the bifunctional synthetase from the MSC and, thereby, promotes the WHEP-domain- dependent interactions in the cytoplasm that form the GAIT complex (vide supra) [69,106]. These examples, among others [107], illustrate two different uses of new domains that were added to eukaryotic tRNA synthetases—as structural elements that control access to a site needed for an expanded function or as motif that has a novel interacting partner in eukaryote cell (such as the interactions of the WHEP domains needed to form the GAIT complex).
Concluding remark
Inspection of sequences of human tRNA synthetases predicts that all AARSs, except for AlaRSs, have expanded domains/motifs that are not found in their respective orthologs in bacteria or archaea. We anticipate that all human tRNA synthetases that have these expansions also have additional functions that are dependent on, or are regulated by, the introduced domains. In the case of AlaRS, extreme selective pressure has been exerted through evolution to develop a framework to prevent serine toxicity from the misactivation of serine, in addition to the misactivation of glycine [108–110]. The exceptional evolutionary constraints placed on AlaRS to ensure its proper function as a synthetase may have limited the ability of this enzyme to incorporate novel motifs.
Acknowledgments
This work was supported by grants GM 15539 and GM 23562 from the National Institutes of Health, by grant CA92577 from the National Cancer Institute and by a fellowship from the National Foundation for Cancer Research.
Abbreviations
- MSC
multisynthetase complex
- EMAP II
endothelial monocyte-activating polypeptide II
- GAIT complex
γ-interferon-activated inhibitor of translation complex
- eEF-1
eukaryotic elongation factor 1 (containing α, β, γ, σ subunits)
- HSP26
heat shock protein 26
- ATM kinase
ataxia telangiectasia mutated kinase
- IL-8
interleukin-8
- IFN-γ
γ-interferon
- IP-10
interferon-inducible protein 10
- MIG
monokine induced by γ-interferon
- VE-cadherin
vascular endothelial cadherin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McPherson JD, et al. A physical map of the human genome. Nature. 2001;409:934–41. doi: 10.1038/35057157. [DOI] [PubMed] [Google Scholar]
- 2.Venter JC, et al. The sequence of the human genome. Science. 2001;291:1304–51. doi: 10.1126/science.1058040. [DOI] [PubMed] [Google Scholar]
- 3.Claverie JM. Gene number. What if there are only 30,000 human genes? Science. 2001;291:1255–7. doi: 10.1126/science.1058969. [DOI] [PubMed] [Google Scholar]
- 4.Matlin AJ, Clark F, Smith CW. Understanding alternative splicing: towards a cellular code. Nat Rev Mol Cell Biol. 2005;6:386–98. doi: 10.1038/nrm1645. [DOI] [PubMed] [Google Scholar]
- 5.Lee SW, Cho BH, Park SG, Kim S. Aminoacyl-tRNA synthetase complexes: beyond translation. J Cell Sci. 2004;117:3725–34. doi: 10.1242/jcs.01342. [DOI] [PubMed] [Google Scholar]
- 6.Park SG, Ewalt KL, Kim S. Functional expansion of aminoacyl-tRNA synthetases and their interacting factors: new perspectives on housekeepers. Trends Biochem Sci. 2005;30:569–74. doi: 10.1016/j.tibs.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Hausmann CD, Ibba M. Aminoacyl-tRNA synthetase complexes: molecular multitasking revealed. FEMS Microbiol Rev. 2008;32:705–21. doi: 10.1111/j.1574-6976.2008.00119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Antonellis A, Green ED. The role of aminoacyl-tRNA synthetases in genetic diseases. Annu Rev Genomics Hum Genet. 2008;9:87–107. doi: 10.1146/annurev.genom.9.081307.164204. [DOI] [PubMed] [Google Scholar]
- 9.Mirande M, Cirakoglu B, Waller JP. Macromolecular complexes from sheep and rabbit containing seven aminoacyl-tRNA synthetases. III Assignment of aminoacyl-tRNA synthetase activities to the polypeptide components of the complexes. J Biol Chem. 1982;257:11056–63. [PubMed] [Google Scholar]
- 10.Kyriacou SV, Deutscher MP. An important role for the multienzyme aminoacyl-tRNA synthetase complex in mammalian translation and cell growth. Mol Cell. 2008 doi: 10.1016/j.molcel.2007.11.038. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ray PS, Arif A, Fox PL. Macromolecular complexes as depots for releasable regulatory proteins. Trends Biochem Sci. 2007;32:158–64. doi: 10.1016/j.tibs.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 12.Ryckelynck M, Giege R, Frugier M. tRNAs and tRNA mimics as cornerstones of aminoacyl-tRNA synthetase regulations. Biochimie. 2005;87:835–45. doi: 10.1016/j.biochi.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 13.Paukstelis PJ, Chen JH, Chase E, Lambowitz AM, Golden BL. Structure of a tyrosyl-tRNA synthetase splicing factor bound to a group I intron RNA. Nature. 2008;451:94–7. doi: 10.1038/nature06413. [DOI] [PubMed] [Google Scholar]
- 14.Putney SD, Schimmel P. An aminoacyl tRNA synthetase binds to a specific DNA sequence and regulates its gene transcription. Nature. 1981;291:632–5. doi: 10.1038/291632a0. [DOI] [PubMed] [Google Scholar]
- 15.Park SG, Schimmel P, Kim S. Aminoacyl tRNA synthetases and their connections to disease. Proc Natl Acad Sci USA. 2008;105:11043–9. doi: 10.1073/pnas.0802862105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Seburn KL, Nangle LA, Cox GA, Schimmel P, Burgess RW. An active dominant mutation of glycyl-tRNA synthetase causes neuropathy in a Charcot-Marie-Tooth 2D mouse model. Neuron. 2006;51:715–26. doi: 10.1016/j.neuron.2006.08.027. [DOI] [PubMed] [Google Scholar]
- 17.Nangle LA, Zhang W, Xie W, Yang XL, Schimmel P. Charcot-Marie-Tooth disease-associated mutant tRNA synthetases linked to altered dimer interface and neurite distribution defect. Proc Natl Acad Sci USA. 2007;104:11239–44. doi: 10.1073/pnas.0705055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scheper GC, et al. Mitochondrial aspartyl-tRNA synthetase deficiency causes leukoencephalopathy with brain stem and spinal cord involvement and lactate elevation. Nat Genet. 2007;39:534–9. doi: 10.1038/ng2013. [DOI] [PubMed] [Google Scholar]
- 19.Arnez JG, Moras D. Structural and functional considerations of the aminoacylation reaction. Trends Biochem Sci. 1997;22:211–6. doi: 10.1016/s0968-0004(97)01052-9. [DOI] [PubMed] [Google Scholar]
- 20.Terada T, Nureki O, Ishitani R, Ambrogelly A, Ibba M, Soll D, Yokoyama S. Functional convergence of two lysyl-tRNA synthetases with unrelated topologies. Nat Struct Biol. 2002;9:257–62. doi: 10.1038/nsb777. [DOI] [PubMed] [Google Scholar]
- 21.Jacobo-Molina A, Villa-Garcia M, Chen HC, Yang DC. Proteolytic signal sequences (PEST) in the mammalian aminoacyl-tRNA synthetase complex. FEBS Lett. 1988;232:65–8. doi: 10.1016/0014-5793(88)80387-9. [DOI] [PubMed] [Google Scholar]
- 22.Mirande M. Aminoacyl-tRNA synthetase family from prokaryotes and eukaryotes: structural domains and their implications. Prog Nucleic Acid Res Mol Biol. 1991;40:95–142. doi: 10.1016/s0079-6603(08)60840-5. [DOI] [PubMed] [Google Scholar]
- 23.Wang CC, Schimmel P. Species barrier to RNA recognition overcome with nonspecific RNA binding domains. J Biol Chem. 1999;274:16508–12. doi: 10.1074/jbc.274.23.16508. [DOI] [PubMed] [Google Scholar]
- 24.Francin M, Kaminska M, Kerjan P, Mirande M. The N-terminal domain of mammalian Lysyl-tRNA synthetase is a functional tRNA-binding domain. J Biol Chem. 2002;277:1762–9. doi: 10.1074/jbc.M109759200. [DOI] [PubMed] [Google Scholar]
- 25.Reed VS, Yang DC. Characterization of a novel N-terminal peptide in human aspartyl-tRNA synthetase. Roles in the transfer of aminoacyl-tRNA from aminoacyl-tRNA synthetase to the elongation factor 1 alpha. J Biol Chem. 1994;269:32937–41. [PubMed] [Google Scholar]
- 26.Reed VS, Wastney ME, Yang DC. Mechanisms of the transfer of aminoacyl-tRNA from aminoacyl-tRNA synthetase to the elongation factor 1 alpha. J Biol Chem. 1994;269:32932–6. [PubMed] [Google Scholar]
- 27.Sang Lee J, Gyu Park S, Park H, Seol W, Lee S, Kim S. Interaction network of human aminoacyl-tRNA synthetases and subunits of elongation factor 1 complex. Biochem Biophys Res Commun. 2002;291:158–64. doi: 10.1006/bbrc.2002.6398. [DOI] [PubMed] [Google Scholar]
- 28.Bec G, Kerjan P, Waller JP. Reconstitution in vitro of the valyl-tRNA synthetase-elongation factor (EF) 1 beta gamma delta complex. Essential roles of the NH2-terminal extension of valyl-tRNA synthetase and of the EF-1 delta subunit in complex formation. J Biol Chem. 1994;269:2086–92. [PubMed] [Google Scholar]
- 29.Negrutskii BS, Shalak VF, Kerjan P, El’skaya AV, Mirande M. Functional interaction of mammalian valyl-tRNA synthetase with elongation factor EF-1alpha in the complex with EF-1H. J Biol Chem. 1999;274:4545–50. doi: 10.1074/jbc.274.8.4545. [DOI] [PubMed] [Google Scholar]
- 30.Quevillon S, Robinson JC, Berthonneau E, Siatecka M, Mirande M. Macromolecular assemblage of aminoacyl-tRNA synthetases: identification of protein-protein interactions and characterization of a core protein. J Mol Biol. 1999;285:183–95. doi: 10.1006/jmbi.1998.2316. [DOI] [PubMed] [Google Scholar]
- 31.Rho SB, Kim MJ, Lee JS, Seol W, Motegi H, Kim S, Shiba K. Genetic dissection of protein-protein interactions in multi-tRNA synthetase complex. Proc Natl Acad Sci USA. 1999;96:4488–93. doi: 10.1073/pnas.96.8.4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kerjan P, Cerini C, Semeriva M, Mirande M. The multienzyme complex containing nine aminoacyl-tRNA synthetases is ubiquitous from Drosophila to mammals. Biochim Biophys Acta. 1994;1199:293–7. doi: 10.1016/0304-4165(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 33.Woese CR, Olsen GJ, Ibba M, Söll D. Aminoacyl-tRNA synthetases, the genetic code, and the evolutionary process. Microbiol Mol Biol Rev. 2000;64:202–36. doi: 10.1128/mmbr.64.1.202-236.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheong HK, Park JY, Kim EH, Lee C, Kim S, Kim Y, Choi BS, Cheong C. Structure of the N-terminal extension of human aspartyl-tRNA synthetase: implications for its biological function. Int J Biochem Cell Biol. 2003;35:1548–57. doi: 10.1016/s1357-2725(03)00070-0. [DOI] [PubMed] [Google Scholar]
- 35.Francin M, Mirande M. Functional dissection of the eukaryotic-specific tRNA-interacting factor of lysyl-tRNA synthetase. J Biol Chem. 2003;278:1472–9. doi: 10.1074/jbc.M208802200. [DOI] [PubMed] [Google Scholar]
- 36.Cen S, Javanbakht H, Niu M, Kleiman L. Ability of wild-type and mutant lysyl-tRNA synthetase to facilitate tRNA(Lys) incorporation into human immunodeficiency virus type 1. J Virol. 2004;78:1595–601. doi: 10.1128/JVI.78.3.1595-1601.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kleiman L, Cen S. The tRNALys packaging complex in HIV-1. Int J Biochem Cell Biol. 2004;36:1776–86. doi: 10.1016/j.biocel.2004.02.022. [DOI] [PubMed] [Google Scholar]
- 38.Frugier M, Moulinier L, Giege R. A domain in the N-terminal extension of class IIb eukaryotic aminoacyl-tRNA synthetases is important for tRNA binding. EMBO J. 2000;19:2371–80. doi: 10.1093/emboj/19.10.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryckelynck M, Giege R, Frugier M. Yeast tRNA(Asp) charging accuracy is threatened by the N-terminal extension of aspartyl-tRNA synthetase. J Biol Chem. 2003;278:9683–90. doi: 10.1074/jbc.M211035200. [DOI] [PubMed] [Google Scholar]
- 40.Whelihan EF, Schimmel P. Rescuing an essential enzyme-RNA complex with a non-essential appended domain. EMBO J. 1997;16:2968–74. doi: 10.1093/emboj/16.10.2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krovat BC, Jantsch MF. Comparative mutational analysis of the double-stranded RNA binding domains of Xenopus laevis RNA-binding protein A. J Biol Chem. 1996;271:28112–9. doi: 10.1074/jbc.271.45.28112. [DOI] [PubMed] [Google Scholar]
- 42.Ryter JM, Schultz SC. Molecular basis of double-stranded RNA-protein interactions: structure of a dsRNA-binding domain complexed with dsRNA. EMBO J. 1998;17:7505–13. doi: 10.1093/emboj/17.24.7505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jacobo-Molina A, Peterson R, Yang DC. cDNA sequence, predicted primary structure, and evolving amphiphilic helix of human aspartyl-tRNA synthetase. J Biol Chem. 1989;264:16608–12. [PubMed] [Google Scholar]
- 44.Kao J, et al. Endothelial monocyte-activating polypeptide II. A novel tumor-derived polypeptide that activates host-response mechanisms. J Biol Chem. 1992;267:20239–47. [PubMed] [Google Scholar]
- 45.Quevillon S, Agou F, Robinson JC, Mirande M. The p43 component of the mammalian multi-synthetase complex is likely to be the precursor of the endothelial monocyte-activating polypeptide II cytokine. J Biol Chem. 1997;272:32573–9. doi: 10.1074/jbc.272.51.32573. [DOI] [PubMed] [Google Scholar]
- 46.Swairjo MA, Morales AJ, Wang CC, Ortiz AR, Schimmel P. Crystal structure of trbp111: a structure-specific tRNA-binding protein. EMBO J. 2000;19:6287–98. doi: 10.1093/emboj/19.23.6287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim Y, Shin J, Li R, Cheong C, Kim K, Kim S. A novel anti-tumor cytokine contains an RNA binding motif present in aminoacyl-tRNA synthetases. J Biol Chem. 2000;275:27062–8. doi: 10.1074/jbc.C000216200. [DOI] [PubMed] [Google Scholar]
- 48.Renault L, et al. Structure of the EMAPII domain of human aminoacyl-tRNA synthetase complex reveals evolutionary dimer mimicry. EMBO J. 2001;20:570–8. doi: 10.1093/emboj/20.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang XL, Liu J, Skene RJ, McRee DE, Schimmel P. Crystal structure of an EMAP-II-like cytokine released from a human tRNA synthetase. Helvetica Chimica Acta. 2003;86:1246–1256. [Google Scholar]
- 50.Kaminska M, Deniziak M, Kerjan P, Barciszewski J, Mirande M. A recurrent general RNA binding domain appended to plant methionyl-tRNA synthetase acts as a cis-acting cofactor for aminoacylation. EMBO J. 2000;19:6908–17. doi: 10.1093/emboj/19.24.6908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Simos G, Segref A, Fasiolo F, Hellmuth K, Shevchenko A, Mann M, Hurt EC. The yeast protein Arc1p binds to tRNA and functions as a cofactor for the methionyl- and glutamyl-tRNA synthetases. EMBO J. 1996;15:5437–48. [PMC free article] [PubMed] [Google Scholar]
- 52.Simos G, Sauer A, Fasiolo F, Hurt EC. A conserved domain within Arc1p delivers tRNA to aminoacyl-tRNA synthetases. Mol Cell. 1998;1:235–42. doi: 10.1016/s1097-2765(00)80024-6. [DOI] [PubMed] [Google Scholar]
- 53.Galani K, Grosshans H, Deinert K, Hurt EC, Simos G. The intracellular location of two aminoacyl-tRNA synthetases depends on complex formation with Arc1p. EMBO J. 2001;20:6889–98. doi: 10.1093/emboj/20.23.6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Karanasios E, Boleti H, Simos G. Incorporation of the Arc1p tRNA-binding domain to the catalytic core of MetRS can functionally replace the yeast Arc1p-MetRS complex. J Mol Biol. 2008;381:763–71. doi: 10.1016/j.jmb.2008.06.044. [DOI] [PubMed] [Google Scholar]
- 55.Wakasugi K, Schimmel P. Two distinct cytokines released from a human aminoacyl-tRNA synthetase. Science. 1999;284:147–51. doi: 10.1126/science.284.5411.147. [DOI] [PubMed] [Google Scholar]
- 56.Guigou L, Shalak V, Mirande M. The tRNA-interacting factor p43 associates with mammalian arginyl-tRNA synthetase but does not modify its tRNA aminoacylation properties. Biochemistry. 2004;43:4592–600. doi: 10.1021/bi036150e. [DOI] [PubMed] [Google Scholar]
- 57.Hou Y, Plett PA, Ingram DA, Rajashekhar G, Orschell CM, Yoder MC, March KL, Clauss M. Endothelial-monocyte-activating polypeptide II induces migration of endothelial progenitor cells via the chemokine receptor CXCR3. Exp Hematol. 2006;34:1125–32. doi: 10.1016/j.exphem.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 58.Awasthi N, Schwarz MA, Verma V, Cappiello C, Schwarz RE. Endothelial monocyte activating polypeptide II interferes with VEGF-induced proangiogenic signaling. Lab Invest. 2009;89:38–46. doi: 10.1038/labinvest.2008.106. [DOI] [PubMed] [Google Scholar]
- 59.Rho SB, Lee JS, Jeong EJ, Kim KS, Kim YG, Kim S. A multifunctional repeated motif is present in human bifunctional tRNA synthetase. J Biol Chem. 1998;273:11267–73. doi: 10.1074/jbc.273.18.11267. [DOI] [PubMed] [Google Scholar]
- 60.Cerini C, Semeriva M, Gratecos D. Evolution of the aminoacyl-tRNA synthetase family and the organization of the Drosophila glutamyl-prolyl-tRNA synthetase gene. Intron/exon structure of the gene, control of expression of the two mRNAs, selective advantage of the multienzyme complex. Eur J Biochem. 1997;244:176–85. doi: 10.1111/j.1432-1033.1997.00176.x. [DOI] [PubMed] [Google Scholar]
- 61.Cerini C, Kerjan P, Astier M, Gratecos D, Mirande M, Semeriva M. A component of the multisynthetase complex is a multifunctional aminoacyl-tRNA synthetase. EMBO J. 1991;10:4267–77. doi: 10.1002/j.1460-2075.1991.tb05005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kerjan P, Triconnet M, Waller JP. Mammalian prolyl-tRNA synthetase corresponds to the approximately 150 kDa subunit of the high-M(r) aminoacyl-tRNA synthetase complex. Biochimie. 1992;74:195–205. doi: 10.1016/0300-9084(92)90046-h. [DOI] [PubMed] [Google Scholar]
- 63.Ting SM, Bogner P, Dignam JD. Isolation of prolyl-tRNA synthetase as a free form and as a form associated with glutamyl-tRNA synthetase. J Biol Chem. 1992;267:17701–9. [PubMed] [Google Scholar]
- 64.Ewalt KL, Yang XL, Otero FJ, Liu J, Slike B, Schimmel P. Variant of human enzyme sequesters reactive intermediate. Biochemistry. 2005;44:4216–21. doi: 10.1021/bi048116l. [DOI] [PubMed] [Google Scholar]
- 65.Cahuzac B, Berthonneau E, Birlirakis N, Guittet E, Mirande M. A recurrent RNA-binding domain is appended to eukaryotic aminoacyl-tRNA synthetases. EMBO J. 2000;19:445–52. doi: 10.1093/emboj/19.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yang XL, Otero FJ, Skene RJ, McRee DE, Schimmel P, Ribas de Pouplana L. Crystal structures that suggest late development of genetic code components for differentiating aromatic side chains. Proc Natl Acad Sci USA. 2003;100:15376–80. doi: 10.1073/pnas.2136794100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mazumder B, Seshadri V, Imataka H, Sonenberg N, Fox PL. Translational silencing of ceruloplasmin requires the essential elements of mRNA circularization: poly(A) tail, poly(A)-binding protein, and eukaryotic translation initiation factor 4G. Mol Cell Biol. 2001;21:6440–9. doi: 10.1128/MCB.21.19.6440-6449.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sampath P, Mazumder B, Seshadri V, Fox PL. Transcript-selective translational silencing by gamma interferon is directed by a novel structural element in the ceruloplasmin mRNA 3′ untranslated region. Mol Cell Biol. 2003;23:1509–19. doi: 10.1128/MCB.23.5.1509-1519.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mukhopadhyay R, Jia J, Arif A, Ray PS, Fox PL. The GAIT system: a gatekeeper of inflammatory gene expression. Trends Biochem Sci. 2009;34:324–31. doi: 10.1016/j.tibs.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Koonin EV, Mushegian AR, Tatusov RL, Altschul SF, Bryant SH, Bork P, Valencia A. Eukaryotic translation elongation factor 1 gamma contains a glutathione transferase domain--study of a diverse, ancient protein superfamily using motif search and structural modeling. Protein Sci. 1994;3:2045–54. doi: 10.1002/pro.5560031117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simader H, Hothorn M, Kohler C, Basquin J, Simos G, Suck D. Structural basis of yeast aminoacyl-tRNA synthetase complex formation revealed by crystal structures of two binary sub-complexes. Nucleic Acids Res. 2006;34:3968–79. doi: 10.1093/nar/gkl560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim KJ, et al. Determination of three-dimensional structure and residues of the novel tumor suppressor AIMP3/p18 required for the interaction with ATM. J Biol Chem. 2008;283:14032–40. doi: 10.1074/jbc.M800859200. [DOI] [PubMed] [Google Scholar]
- 73.Jiang S, Wolfe CL, Warrington JA, Norcum MT. Three-dimensional reconstruction of the valyl-tRNA synthetase/elongation factor-1H complex and localization of the delta subunit. FEBS Lett. 2005;579:6049–54. doi: 10.1016/j.febslet.2005.09.062. [DOI] [PubMed] [Google Scholar]
- 74.Kim JE, Kim KH, Lee SW, Seol W, Shiba K, Kim S. An elongation factor-associating domain is inserted into human cysteinyl-tRNA synthetase by alternative splicing. Nucleic Acids Res. 2000;28:2866–72. doi: 10.1093/nar/28.15.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Park BJ, et al. The haploinsufficient tumor suppressor p18 upregulates p53 via interactions with ATM/ATR. Cell. 2005;120:209–21. doi: 10.1016/j.cell.2004.11.054. [DOI] [PubMed] [Google Scholar]
- 76.Park BJ, Oh YS, Park SY, Choi SJ, Rudolph C, Schlegelberger B, Kim S. AIMP3 haploinsufficiency disrupts oncogene-induced p53 activation and genomic stability. Cancer Res. 2006;66:6913–8. doi: 10.1158/0008-5472.CAN-05-3740. [DOI] [PubMed] [Google Scholar]
- 77.Rose A, Meier I. Scaffolds, levers, rods and springs: diverse cellular functions of long coiled-coil proteins. Cell Mol Life Sci. 2004;61:1996–2009. doi: 10.1007/s00018-004-4039-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rose A, Schraegle SJ, Stahlberg EA, Meier I. Coiled-coil protein composition of 22 proteomes--differences and common themes in subcellular infrastructure and traffic control. BMC Evol Biol. 2005;5:66. doi: 10.1186/1471-2148-5-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Robinson JC, Kerjan P, Mirande M. Macromolecular assemblage of aminoacyl-tRNA synthetases: quantitative analysis of protein-protein interactions and mechanism of complex assembly. J Mol Biol. 2000;304:983–94. doi: 10.1006/jmbi.2000.4242. [DOI] [PubMed] [Google Scholar]
- 80.Ahn HC, Kim S, Lee BJ. Solution structure and p43 binding of the p38 leucine zipper motif: coiled-coil interactions mediate the association between p38 and p43. FEBS Lett. 2003;542:119–24. doi: 10.1016/s0014-5793(03)00362-4. [DOI] [PubMed] [Google Scholar]
- 81.Vellekamp G, Sihag RK, Deutscher MP. Comparison of the complexed and free forms of rat liver arginyl-tRNA synthetase and origin of the free form. J Biol Chem. 1985;260:9843–7. [PubMed] [Google Scholar]
- 82.Zheng YG, Wei H, Ling C, Xu MG, Wang ED. Two forms of human cytoplasmic arginyl-tRNA synthetase produced from two translation initiations by a single mRNA. Biochemistry. 2006;45:1338–44. doi: 10.1021/bi051675n. [DOI] [PubMed] [Google Scholar]
- 83.Rho SB, Lee KH, Kim JW, Shiba K, Jo YJ, Kim S. Interaction between human tRNA synthetases involves repeated sequence elements. Proc Natl Acad Sci USA. 1996;93:10128–33. doi: 10.1073/pnas.93.19.10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ling C, Yao YN, Zheng YG, Wei H, Wang L, Wu XF, Wang ED. The C-terminal appended domain of human cytosolic leucyl-tRNA synthetase is indispensable in its interaction with arginyl-tRNA synthetase in the multi-tRNA synthetase complex. J Biol Chem. 2005;280:34755–63. doi: 10.1074/jbc.M413511200. [DOI] [PubMed] [Google Scholar]
- 85.Fabre S, Reynaud C, Jalinot P. Identification of functional PDZ domain binding sites in several human proteins. Mol Biol Rep. 2000;27:217–24. doi: 10.1023/a:1011008313677. [DOI] [PubMed] [Google Scholar]
- 86.Meerschaert K, et al. The tandem PDZ protein Syntenin interacts with the aminoacyl tRNA synthetase complex in a lysyl-tRNA synthetase-dependent manner. J Proteome Res. 2008;7:4962–73. doi: 10.1021/pr800325u. [DOI] [PubMed] [Google Scholar]
- 87.Wakasugi K, Slike BM, Hood J, Ewalt KL, Cheresh DA, Schimmel P. Induction of angiogenesis by a fragment of human tyrosyl-tRNA synthetase. J Biol Chem. 2002;277:20124–6. doi: 10.1074/jbc.C200126200. [DOI] [PubMed] [Google Scholar]
- 88.Liu J, Yang XL, Ewalt KL, Schimmel P. Mutational switching of a yeast tRNA synthetase into a mammalian-like synthetase cytokine. Biochemistry. 2002;41:14232–7. doi: 10.1021/bi0205395. [DOI] [PubMed] [Google Scholar]
- 89.Yang XL, Skene RJ, McRee DE, Schimmel P. Crystal structure of a human aminoacyl-tRNA synthetase cytokine. Proc Natl Acad Sci USA. 2002;99:15369–74. doi: 10.1073/pnas.242611799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kapoor M, Otero FJ, Slike BM, Ewalt KL, Yang XL. Mutational separation of aminoacylation and cytokine activities of human tyrosyl-tRNA synthetase. Chem Biol. 2009;16:531–9. doi: 10.1016/j.chembiol.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Halwani R, Cen S, Javanbakht H, Saadatmand J, Kim S, Shiba K, Kleiman L. Cellular distribution of Lysyl-tRNA synthetase and its interaction with Gag during human immunodeficiency virus type 1 assembly. J Virol. 2004;78:7553–64. doi: 10.1128/JVI.78.14.7553-7564.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo M, Ignatov M, Musier-Forsyth K, Schimmel P, Yang XL. Crystal structure of tetrameric form of human lysyl-tRNA synthetase: Implications for multisynthetase complex formation. Proc Natl Acad Sci USA. 2008;105:2331–6. doi: 10.1073/pnas.0712072105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kim T, Park SG, Kim JE, Seol W, Ko YG, Kim S. Catalytic peptide of human glutaminyl-tRNA synthetase is essential for its assembly to the aminoacyl-tRNA synthetase complex. J Biol Chem. 2000;275:21768–72. doi: 10.1074/jbc.M002404200. [DOI] [PubMed] [Google Scholar]
- 94.Frolova LY, Grigorieva AY, Sudomoina MA, Kisselev LL. The human gene encoding tryptophanyl-tRNA synthetase: interferon-response elements and exon-intron organization. Gene. 1993;128:237–45. doi: 10.1016/0378-1119(93)90568-n. [DOI] [PubMed] [Google Scholar]
- 95.Aboagye-Mathiesen G, Ebbesen P, von der Maase H, Celis JE. Interferon gamma regulates a unique set of proteins in fresh human bladder transitional cell carcinomas. Electrophoresis. 1999;20:344–8. doi: 10.1002/(SICI)1522-2683(19990201)20:2<344::AID-ELPS344>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 96.Tzima E, Reader JS, Irani-Tehrani M, Ewalt KL, Schwartz MA, Schimmel P. Biologically active fragment of a human tRNA synthetase inhibits fluid shear stress-activated responses of endothelial cells. Proc Natl Acad Sci USA. 2003;100:14903–7. doi: 10.1073/pnas.2436330100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tzima E, Reader JS, Irani-Tehrani M, Ewalt KL, Schwartz MA, Schimmel P. VE-cadherin links tRNA synthetase cytokine to anti-angiogenic function. J Biol Chem. 2005;280:2405–8. doi: 10.1074/jbc.C400431200. [DOI] [PubMed] [Google Scholar]
- 98.Zhou Q, et al. Orthogonal use of a human tRNA synthetase active site to achieve multi-functionality. Nat Struct Mol Biol. 2009 doi: 10.1038/nsmb.1706. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Barat C, et al. HIV-1 reverse transcriptase specifically interacts with the anticodon domain of its cognate primer tRNA. EMBO J. 1989;8:3279–85. doi: 10.1002/j.1460-2075.1989.tb08488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kovaleski BJ, Kennedy R, Hong MK, Datta SA, Kleiman L, Rein A, Musier-Forsyth K. In vitro characterization of the interaction between HIV-1 Gag and human lysyl-tRNA synthetase. J Biol Chem. 2006;281:19449–56. doi: 10.1074/jbc.M601189200. [DOI] [PubMed] [Google Scholar]
- 101.Guo F, Gabor J, Cen S, Hu K, Mouland AJ, Kleiman L. Inhibition of cellular HIV-1 protease activity by lysyl-tRNA synthetase. J Biol Chem. 2005;280:26018–23. doi: 10.1074/jbc.M502454200. [DOI] [PubMed] [Google Scholar]
- 102.Yang XL, Schimmel P, Ewalt KL. Relationship of two human tRNA synthetases used in cell signaling. Trends Biochem Sci. 2004;29:250–6. doi: 10.1016/j.tibs.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 103.Yang XL, et al. Gain-of-function mutational activation of human tRNA synthetase procytokine. Chem Biol. 2007;14:1323–33. doi: 10.1016/j.chembiol.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Otani A, et al. A fragment of human TrpRS as a potent antagonist of ocular angiogenesis. Proc Natl Acad Sci USA. 2002;99:178–83. doi: 10.1073/pnas.012601899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wakasugi K, Slike BM, Hood J, Otani A, Ewalt KL, Friedlander M, Cheresh DA, Schimmel P. A human aminoacyl-tRNA synthetase as a regulator of angiogenesis. Proc Natl Acad Sci USA. 2002;99:173–7. doi: 10.1073/pnas.012602099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Arif A, Jia J, Mukhopadhyay R, Willard B, Kinter M, Fox PL. Two-site phosphorylation of EPRS coordinates multimodal regulation of noncanonical translational control activity. Mol Cell. 2009;35:164–80. doi: 10.1016/j.molcel.2009.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Yannay-Cohen N, et al. LysRS serves as a key signaling molecule in the immune response by regulating gene expression. Mol Cell. 2009;34:603–11. doi: 10.1016/j.molcel.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 108.Guo M, Chong YE, Beebe K, Shapiro R, Yang XL, Schimmel P. The C-Ala domain brings together editing and aminoacylation functions on one tRNA. Science. 2009;325:744–7. doi: 10.1126/science.1174343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Naganuma M, Sekine S, Fukunaga R, Yokoyama S. Unique protein architecture of alanyl-tRNA synthetase for aminoacylation, editing, and dimerization. Proc Natl Acad Sci USA. 2009;106:8489–94. doi: 10.1073/pnas.0901572106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sokabe M, Ose T, Nakamura A, Tokunaga K, Nureki O, Yao M, Tanaka I. The structure of alanyl-tRNA synthetase with editing domain. Proc Natl Acad Sci USA. 2009;106:11028–33. doi: 10.1073/pnas.0904645106. [DOI] [PMC free article] [PubMed] [Google Scholar]