Abstract
2-Pyridin-2-yl-1H-benzoimidazole L3 is presented as a new, efficient, and versatile bidentate N-donor ligand suitable for the copper-catalyzed formation of vinyl C-N and C-O bonds. This inexpensive and easily prepared ligand facilitates copper-catalyzed cross-coupling reactions of alkenyl bromides and iodides with N-heterocycles and phenols to afford the desired cross-coupled products in good to excellent yields with full retention of stereochemistry. This method is particularly noteworthy given its efficiency i.e., mild reaction conditions, low catalyst loading, simplicity, versatility, and exceptional level of functional group tolerance.
In the past few years significant progress has been made on the development of copper-catalyzed Ullmann-type coupling reactions for the synthesis of aryl amines, aryl ethers, aryl thioethers, and vinyl amides.1 In contrast, only a few methods have appeared for the copper-catalyzed synthesis of vinyl amines and vinyl ethers of synthetic, polymeric, and biological importance.2
The vinyl group itself is of fundamental importance in organic chemistry. N-Vinyl amines are widely used in the preparation of polymeric dyes, catalysis, and ion-exchange resins; while aryl vinyl ethers are useful intermediates in a wide variety of reactions (e.g., cycloaddition, cyclopropanation, and metathesis processes),2 as well as in the synthesis of polymers.2
In addition, the vinyl group can act as an efficient protecting group of N-heterocycles and phenol derivatives.2 Many natural products and compounds which exhibit interesting biological activity contain the N-vinyl heterocycle and aryl vinyl ether moieties.2
Recent studies in our laboratory indicated that ligands such as N-vinyl heterocycles, aryl vinyl ethers, and aryl vinyl thioethers are active against drug resistant strains of tuberculosis, anthrax, and many other strains of drug-resistant Gram-positive bacteria.4 Many of these active compounds require electron donating groups (EDG) in both halves of the molecule.4d One portion of these ligands must contain the structural framework A illustrated in Scheme 1. This substitution pattern was required for potent activity of this new class of antibacterials.4c Due to the importance of these N-vinyl heterocycles and vinyl ether moieties, there have been a number of methods reported regarding their syntheses.5 More often than not, all of these methods require harsh reaction conditions and are complicated by substrate limitations.5 Furthermore, the practical application of these methods often leads to a mixture of Z and E isomers.5
Scheme 1.
Generalized Cu-Catalyzed Coupling Reaction with Electron Rich Systems.
The most efficient methods currently for the synthesis of N-vinyl heterocycles and aryl vinyl ethers are based upon transition-metal-mediated coupling reactions of N-heterocycles and phenols with different vinyl sources. Hg6 and Pd-mediated7 preparations of vinyl azoles and vinyl ethers are notable in this case. The toxicity of mercury and the high cost of Pd, as well as the latter’s air sensitivity, potentially limit their use for many industrial applications.
A few reports on the Cu-catalyzed vinylation of N-heterocyclces, phenols, and thiols have appeared in the last few years. Some of these vinylations require either a stoichiometric Cu-source3a–c or high catalyst loading, as well as harsh reaction conditions.3d–g Although a few of these vinylations require moderate reaction conditions (60–110 °C),2a,b one of their major drawbacks is longer reaction times (24–36 h) and poor applicability for triazole and tetrazole nucleophiles. For example, this longer reaction time, in many cases, is a major problem with highly electron rich substrates, such as those of interest in the structural framework A illustrated in Scheme 1. Earlier attempts to synthesize these electron rich substrates using moderate reaction conditions2a,b and longer reaction times routinely gave a considerable amount of aryl acetylene byproducts (35–65%) and consequently, low yields of the desired product. During the execution of these studies a relatively shorter process (4–10 h) and a more moderate temperature [(82 °C) Cu-catalyzed vinylation] has appeared in the literature.2d In our studies, we recently reported a Cu(I) catalytic system in combination with the ligand cis-1,2-hexanediol which employed moderate reaction conditions and shorter reaction times for the vinylation of thiols.4a Unfortunately, this system proved ineffective for N- and O- vinylations.
A potential solution was envisioned by use of an alternative Cu(I) catalytic system which would work for substrates related to that of A in Scheme 1 under mild conditions. Herein, we report the development of a new Cu(I) catalytic system and its application as a versatile, rapid, efficient, and stereospecific method for the synthesis of N-vinyl heterocycles and aryl vinyl ethers.
The initial ligand screening and optimization studies for the O-vinylation of phenols were conducted on a simpler substrate, a phenyl vinyl iodide, because it was devoid of any electron withdrawing group (EWG) or EDG (see Supporting Information) groups. The 2-isopropylphenol was used as the prototypical nucleophilic substrate for these optimization experiments.8 Ultimately, a new ligand, 2-pyridin-2-yl-1H-benzoimidazole (L3) gave superior results when used in an equimolar concentration with 5 mol % Cu(I), and 2.0 equivalents of Cs2CO3 in reagent grade DMF (without drying or degasing). Moreover, in contrast to DMF the solvents DME, iPrOH and 1,4-dioxane proved to be ineffective. Interestingly, the normally unstable aryl vinyl halides which contain EDG in framework A (Scheme 1) were found to be stable to some extent in DMF. Thus, the above mentioned reaction conditions with CuI and L3 proved to be the best for the vinylation of phenols to furnish the desired aryl vinyl ethers with little or no formation of the aryl acetylene byproduct.
The exceptional activity of CuI with L3 is, presumably, due to the required electron density on Cu provided by ligand L3 for the vinylation of phenols. In addition, the resulting Cu-L3 complex may be conformationally less rigid than the other ligands screened to date (see Supporting Information) and provided, presumably, a more planar coordination of the Cu(I) cation.9
Encouraged by these early results, these reaction conditions were employed for the coupling of various aryl vinyl halides with N-heterocycles including indole, pyrazole, imidazole, indazole, benzotriazole, and tetrazoles (Table 1 and Table 2). Interestingly, (Z)-ethyl-3-iodo-acrylate (Table 2) gave the desired N-vinylations at relatively lower temperatures (40 °C) and shorter reaction times (3–4 h) as compared to the (E)-vinyl halides (Table 1). This may be due to electronic or steric effects which are under study at present. All reactions shown in Table 1 and Table 2 proceeded in good to excellent yields with full retention of stereochemistry. It is noteworthy to mention that the reaction of aryl vinyl bromides also gave full conversions similar to aryl vinyl iodides, although reactions with aryl vinyl bromides required a little longer reaction time for complete consumption of starting material (Table 1, entries 1–3).
Table 1.
Cu-Catalyzed Cross-Coupling of Various (E)-Aryl Alkenyl Iodides with N-Heterocycles.
 | |||
|---|---|---|---|
| entry | alkenyl halide | product | yielda,b (%) |
| 1 |  |
X = I, 90 X = Br, 88c |
|
| 2 | 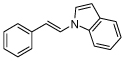 |
X = I, 93 X = Br, 91c |
|
| 3 |  |
X = I, 84 X = Br, 82c |
|
| 4 |  |
 |
86d |
| 5 |  |
 |
86d |
| 6 |  |
 |
84d |
| 7 |  |
 |
86d |
| 8 |  |
 |
95d |
| 9 |  |
93 | |
| 10 |  |
 |
92 |
| 11 |  |
 |
81 |
| 12 |  |
87 | |
Isolated yields, the average of at least two runs.
The starting aryl vinyl halides contained ~ 3–9% Z-isomer; this resulted in ~3–9% of the cis-isomer, reflected in the overall yield.
The reaction was carried out at 80 °C and was stirred for 10 h.
Treatment with TBAF·THF provided the desired N-vinyl heterocycles.
Table 2.
Stereospecific Cu-Catalyzed Cross-Coupling of (Z)-Ethyl 3-Iodo-acrylate with Various N-Heterocycles.
Isolated yields, the average of at least two runs.
The reaction was carried out at 60 °C and was stirred for 6 h.
To demonstrate the effectiveness of the new ligand catalyst system, highly electron rich aryl vinyl iodides which contained a silyl-protected hydroxy group at the 3-position and a methoxy substituent at the 5-position (Table 1, entries 4–8) gave coupling products in excellent yield without the formation of any aryl acetylene byproducts (cf. Scheme 1). This is in contrast to cases which employed the other nitrogen based ligands and catalysts (see Supporting Information) and gave considerable amounts of aryl acetylene byproduct and/or loss of the silyl protecting group before the completion of the coupling due to higher reaction temperatures.
This premature loss of the silyl group caused by the harsh reaction conditions noted previously4 made the isolation of coupled products from deprotected aryl vinyl iodide and its eliminated by product very difficult. The presence of the deprotected phenolic vinyl iodide gave more of the acetylene byproduct what was difficult to separate. In contrast, when ligand L3 and the new conditions were employed, which were much milder, the silyl function remained intact to provide the coupled products in high yield. Deprotection was then carried out in the same reaction vessel using TBAF·THF again in high yield, as expected (entries 4–8, Table 1). The scope of this process was extended by using trifluoromethyl-substituted (EWG) aryl vinyl iodides for the N-vinylations and found again to provide excellent yields (entries 9–12, Table 1) of the N-vinyl heterocycles.
The ability to couple E-aryl vinyl halides with phenols was also investigated and CuI/L3/Cs2CO3/DMF proved to be an excellent catalytic system for the vinylation of phenols as well (Table 3), albeit this required somewhat higher temperatures (70–80 °C) and slightly longer reaction times (7–10 h). Vinyl bromides (Table 3, entries 1–3) were found to require longer reaction times (10 h) in comparison to the corresponding vinyl iodides (7 h), as expected.
Table 3.
Cu-Catalyzed Cross-Coupling of Various (E)-Aryl Alkenyl Halides with Phenols.
 | |||
|---|---|---|---|
| entry | alkenyl halide | product | yielda,b (%) |
| 1 |  |
X = I, 95 X = Br, 84c |
|
| 2 |  |
X = I, 93 X = Br, 90c |
|
| 3 |  |
X = I, 95 X = Br, 90c |
|
| 4 |  |
 |
84c |
| 5 |  |
 |
82c |
| 6 |  |
 |
86c |
| 7 |  |
 |
86c,d |
| 8 |  |
 |
84c,d |
| 9 |  |
 |
86c,d |
| 10 |  |
93c | |
| 11 |  |
92 | |
| 12 | 81 | ||
Isolated yields, the average of at least two runs.
The starting aryl vinyl halides contained ~ 3–9% Z-isomer, this led to ~3–9% of the cis-isomer, reflected in the overall yield.
The reaction was carried out at 80 °C and was stirred for 10 h.
Treatment with TBAF·THF provided the desired aryl vinyl ether.
The stereochemistry in all ethers was retained regardless of the (E)- or (Z)-configuration. To ensure the coupling reaction proceeded in a stereospecific fashion, the process was investigated using the (Z)-vinyl iodide, ethyl cis-3-iodo-acrylate, in the presence of various phenols. Interestingly, examination of the results indicated that the reaction of (Z)-vinyl iodides proceeded more rapidly and under milder conditions (i.e., rt in 2–6 h or 40 °C in 30 minutes) than the corresponding (E)-isomers. This functionalized (Z)-vinyl iodide gave excellent yields with complete retention of stereochemistry regardless of whether electron rich or electron poor aryl- and heterocyclic phenols were used (Table 4).
Table 4.
Stereospecific Cu-Catalyzed Cross-Coupling of (Z)-Ethyl 3-Iodo-acrylate with Various Phenols.
 | |||
|---|---|---|---|
| entry | phenol | product | Yielda (%) |
| 1 |  |
 |
98 |
| 2 | 93 | ||
| 3 | 84 | ||
| 4 |  |
 |
95 |
| 5 |  |
 |
96 |
| 6 | 92 | ||
| 7 | 86 | ||
Isolated yields, the average of at least two runs.
In summary, an efficient, stereospecific Cu-mediated catalytic system (CuI/L3) has been developed for the synthesis of N-vinyl heterocycles and aryl vinyl ethers. Ligand L3 was shown to be very effective in these processes. This method is useful because of the mild reaction conditions, broad functional group tolerance, stereospecificity, low catalyst loading, simplicity, and efficiency. Further investigation of the utility of this Cu(I) catalytic system as well as the importance of L3 is currently in progress.
Supplementary Material
Acknowledgment
We thank Dr. Steven. H. Bertz Complexity Study Center, Mendham, New Jersey for helpful discussions, Dr. M. E. Dudley, Marquette University, for technical advice and the NIH as well as The Research Growth Initiative of the University of Wisconsin-Milwaukee for financial support.
Footnotes
Supporting Information Available: Detailed experimental procedure, ligand preparation, screening, and optimization and characterization data of each compound. This material is available free of charge via the internet at http:/pubs.acs.org.
References
- 1. Monnier F, Taillefer M. Angew. Chem. Int. Ed. 2009;48:6954. doi: 10.1002/anie.200804497. (Minireview) and references cited therein. Ley SV, Thomas AW. Angew. Chem. Int. Ed. 2003;42:5400. doi: 10.1002/anie.200300594. (Review) and references cited therein. Ley SV, Thomas AW. Angew. Chem. 2003;115:5558. doi: 10.1002/anie.200300594. Kunz K, Scholz U, Ganzer D. Synlett. 2003:2428. Beletskaya IP, Cheprakov AV. Coord. Chem. Rev. 2004;248:2337. Fraln R, Kikeji D. Synthesis. 2006:2271. Corbet J-P, Mignani G. Chem. Rev. 2006;106:2651. doi: 10.1021/cr0505268. Kienle M, Dubakka SR, Brade K, Knochel P. Eur. J. Org. Chem. 2007:4166. Carril M, SanMartin R, Dominguez E. Chem. Soc. Rev. 2008;37:639. doi: 10.1039/b709565c. Shafir A, Lichtor PA, Buchwald SL. J. Am. Chem. Soc. 2007;129:3490. doi: 10.1021/ja068926f. Altman RA, Buchwald SL. Org. Lett. 2007;9:643. doi: 10.1021/ol062904g. Jiang L, Job GE, Klapars A, Buchwald SL. Org. Lett. 2003;5:3667. doi: 10.1021/ol035355c. Gujadhur RK, Bates CG, Venkataraman D. Org. Lett. 2001;3:4315. doi: 10.1021/ol0170105. Harada H, Thalji RK, Bergman RG, Ellman JA. J. Org. Chem. 2008;73:6772. doi: 10.1021/jo801098z. Nordmann G, Buchwald SL. J. Am. Chem. Soc. 2003;125:4978. doi: 10.1021/ja034809y. For intamolecular Ullmann Coupling please see also Chemler SR, Fuller PH. Chem Soc. Rev. 2007;36:1153. doi: 10.1039/b607819m. Evano G, Blanchard N, Toumi M. Chem. Rev. 2008;108:3054. doi: 10.1021/cr8002505. For a highlight on recent major developments in C-C, C-N and C-N coupling please see Monnier F, Taillefer M. Angew. Chem. Int. Ed. 2008;47:3096. doi: 10.1002/anie.200703209. and references cited therein. Chen Y-G, Chen H-H. Org. Lett. 2006;8:5609. doi: 10.1021/ol062339h. For vinylation of amides please see these leading references Pan X, Cai Q, Ma D. Org. Lett. 2004;6:1809. doi: 10.1021/ol049464i. Smith AB, III, Duffey MO, Basu K, Walsh SP, Suennemann HW, Frohn M. J. Am. Chem. Soc. 2008;130:422. doi: 10.1021/ja078293k. Nicolaou KC, Leung GYC, Dethe DH, Guduru R, Sun YP, Lim CS, Chen DY-K. J. Am. Chem. Soc. 2008;130:10019. doi: 10.1021/ja802803e. Rodriguez M, Buchwald SL. Org. Lett. 2007;9:973. doi: 10.1021/ol062978s. Shen R, Lin CT, Bowman EJ, Bowman BJ, Proco JA., Jr J. Am. Chem. Soc. 2003;125:7889. doi: 10.1021/ja0352350. Huang X, Shao N, Huryk R, Palani A, Aslanian R, Seidel-Dugan C. Org. Lett. 2009;11:867. doi: 10.1021/ol802772s. Cesati RR, III, Dwyer G, Jones RC, Hayes MP, Yalamanchili P, Casebier DS. Org. Lett. 2007;9:5617. doi: 10.1021/ol7025729. Other contributors were cited in the aforementioned referenced reviews and minireviews.
- 2.(a) Taillefer M, Ouali A, Renard B, Spindler J-F. Chem. Eur. J. 2006;12:5301. doi: 10.1002/chem.200501411. [DOI] [PubMed] [Google Scholar]; (b) Bao W, Xin Lv YL. Synthesis. 2008;12:1911. [Google Scholar]; (c) Ouali A, Laurent R, Caminade A-M, Majoral J-P, Taillefer M. J. Am. Chem. Soc. 2006;128:15990. doi: 10.1021/ja066505s. [DOI] [PubMed] [Google Scholar]; (d) Kaddouri H, Vicente V, Ouali A, Ouazzani F, Taillefer M. Angew. Chem. Int. Ed. 2009;48:333. doi: 10.1002/anie.200800688. [DOI] [PubMed] [Google Scholar]
- 3.(a) Lam PYS, Vincent G, Bonne D, Clark CG, Deudon S. Tetrahedron Lett. 2003;44:4927. [Google Scholar]; (b) Lam PYS, Vincent G, Clark CG, Deudon S, Lam PYS, Vincent G, Jadhav PK. Tetrahedron Lett. 2001;42:3415. [Google Scholar]; (c) Blouin M, Frenette R. J. Org. Chem. 2001;66:9043. doi: 10.1021/jo016037z. [DOI] [PubMed] [Google Scholar]; (d) Wan Z, Jones CD, Koenig TM, Pu YJ, Mitchell D. Tetrahedron Lett. 2003;44:8257. [Google Scholar]; (e) Ma D, Cai Q, Xie X. Synlett. 2005;11:1767. [Google Scholar]; (f) Shen G, Lv X, Qian W, Bao W. Tetrahedron Lett. 2008;49:4556. [Google Scholar]; (g) Wang Z, Bao W, Jiang Y. Chem. Commun. 2005:2849. doi: 10.1039/b501628b. [DOI] [PubMed] [Google Scholar]; (h) Mao J, Hua Q, Guo J, Shi D, Ji S. Synlett. 2008;13:2011. [Google Scholar]
- 4.(a) Kabir MS, Van Linn ML, Monte A, Cook JM. Org. Lett. 2008;10:3363. doi: 10.1021/ol801149n. [DOI] [PubMed] [Google Scholar]; (b) Kabir MS, Monte A, Cook JM. Tetrahedron Lett. 2007;48:7269. [Google Scholar]; (c) Kabir MS, Kathleen E, Rebecca P, Krueger SM, Ignasiak R, Rott M, Schwan WR, Stemper ME, Reed KD, Sherman D, Cook JM, Monte A. Bioorg. Med. Chem. Lett. 2008;18:5745. doi: 10.1016/j.bmcl.2008.09.085. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Monte A, Kabir MS, Cook JM, Rott M, Schwan WR, Defoe L. U.S. Pat. Appl. Publ. 2007:37.
- 5.(a) Reference 2a and references cited therein. Okimoto Y, Sakaguchi S, Ishii Y. J. Am. Chem. Soc. 2002;124:1590. doi: 10.1021/ja0173932. Crivello JV, Kong S. J. Org. Chem. 1998;63:6745. Suprenant S, Chan WY, Berthelette C. Org. Lett. 2003;5:4851. doi: 10.1021/ol035918k.
- (a) Kizhnyaev VN, Pokatilov FA, Tsypina NA, Ratovskii GV, Vereshchagin LI, Smirnov AI. Russ. J. Org. Chem. 2002;38:1056. [Google Scholar]; (b) Chen YL, Hedberg KG, Guarino KJ. Tetrahedron Lett. 1989;30:1067. [Google Scholar]
- 7.(a) Lebedev AY, Izmer VV, Kazyul’kin DN, Beletskaya IP, Voskoboynikov AZ. Org. Lett. 2002;4:623–626. doi: 10.1021/ol0172370. [DOI] [PubMed] [Google Scholar]; (b) Willis MC, Taylor D, Gillmore AT. Chem. Commun. 2003:2222. [PubMed] [Google Scholar]
- 8.Optimization studies of the reaction conditions were accomplished with nitrogen and oxygen nucleophiles in parallel experiments which led to the same results in terms of yields. For reasons of chemical economy, more conditions were screened in the cases of oxygen nucleophiles (see Suppoting Information) and the best results extended to the nitrogen nucleophiles
- 9.Zeng M-H, Shen X-C, Ng SW. Acta Cryst. 2006;E62:2194. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.