Abstract
Background & Aims
HIV infection increases the rate of liver disease progression in patients infected with hepatitis C virus (HCV) and could shorten survival times of those awaiting liver transplants. The model for end-stage liver disease (MELD) predicts mortality in HIV-negative transplant candidates, but its reliability in predicting mortality of HIV-positive candidates has not been established.
Methods
We evaluated predictors of pre-transplant mortality in HIV-positive liver transplant candidates enrolled in the Solid Organ Multi-Site Transplant Study (HIVTR), matched 1:5 by age, sex, race, and HCV infection with HIV-negative controls from the United Network of Organ Sharing.
Results
Of 167 HIVTR candidates, 24 died (14.4%); this mortality rate was similar to that of controls 88/792 (11.1%, p=0.30) with no significant difference in causes of mortality. A significantly lower proportion of HIVTR candidates (34.7%) underwent liver transplantation, compared with controls (47.6%, p=0.003). In the multivariate model for the combined cohort, baseline MELD predicted pre-transplant mortality (hazard ratio [HR] 1.27, p<0.0001), whereas HIV infection was not significantly associated with increased risk of pre-transplant mortality (HR=1.69, p=0.20). After controlling for pre-transplant CD4+ cell count and HIV RNA levels, the only significant predictor of mortality in the HIV-infected subjects was pre-transplant MELD (HR 1.2, p<0.0001).
Conclusions
Pre-transplant mortality characteristics are similar between HIV-positive and HIV-negative candidates. Although lower CD4+ cell counts and detectable levels of HIV RNA might be associated with a higher rate of pre-transplant mortality, the baseline MELD score was the only significant independent predictor of pre-transplant mortality in HIV-infected liver transplant candidates.
Keywords: AIDS, cirrhosis, liver transplantation, hepatitis C, HIV/HCV co-infection
Introduction
Among individuals with HIV-infection, liver-related death is the most frequent cause of non-AIDS related death (1,2) primarily related to complications of chronic hepatitis B virus (HBV) or hepatitis C virus (HCV) co-infection, as well as hepatoxicity associated with antiretroviral therapy and alcohol use (3–5). Given the improved survival now possible with use of highly active anti-retroviral therapy (HAART), many long-term HIV-infected individuals are developing end-stage liver disease (ESLD) and increasingly being considered for liver transplantation.
Transplant programs have adopted the model for end-stage liver disease (MELD), which was established in HIV-uninfected patients and incorporates creatinine, bilirubin, and INR, to estimate prognosis and determine the medical urgency for liver transplantation, ensuring the appropriate organ allocation to those with the highest risk of death (6). While MELD has been validated as a predictor of survival in a wide variety of patients with ESLD (6,7), no data exist regarding its use in HIV-infected patients with ESLD in whom other factors (e.g., HIV disease stage, CD4 cell count and antiretroviral therapy) may be important predictors of mortality. This is of concern, as HIV-infected patients with ESLD have a shortened survival compared to HIV negative patients with similar MELD scores (8) Previous studies have suggested, in fact, that predictors of mortality used for organ allocation in HIV(−) liver transplant candidates may not be valid in their HIV(+) counterparts (8–11). Thus, it is timely to define predictors of mortality in HIV(+) transplant candidates to optimize organ allocation and clinical outcomes in this group.
The objectives of this study were to determine the incidence of, time to, causes of, and risk factors for pre-transplant mortality, including pre-transplant MELD, CD4+ count, HIV viral load (VL), HCV co-infection, and HAART therapy, in HIV-infected liver transplant candidates and compare these with matched HIV(−) controls.
Patients and Methods
Study Subjects
The HIV in Solid Organ Transplantation Multisite Study (HIVTR) enrolled patients at 20 transplant centers in the US between February 2003 and September 2007 with HIV infection and ESLD who were candidates for liver transplantation [clinicaltrials.gov, NCT00074386]. Patients were required to meet standard site criteria for placement on the liver transplant wait list as well as HIV-specific study inclusion criteria, which included CD4+ cells >100/ul, or >200/ul if there was a history of prior opportunistic infection, HIV-1 RNA <50 copies/mL, or >50 copies/ml in those with hepatotoxicity or HAART intolerance in whom HIV suppression was predicted post-transplant. Subjects with a history of progressive multifocal leukoencephalopathy, chronic intestinal cryptosporidiosis, primary CNS lymphoma, multidrug resistant fungal infections, or significant wasting were excluded from this study. Patients with hepatocellular carcinoma were enrolled in the HIVTR protocol, although excluded from this analysis, since they are typically assigned a higher priority for liver transplantation regardless of MELD.
Data Collection
Clinical and laboratory data were collected locally at screening, enrollment (time of placement on the transplant waiting list), and every 3 months until transplantation or death, and entered into an online data collection system at each of 20 participating sites. Clinical variables included age, gender, race, liver disease etiology, antiretroviral medications, body mass index (BMI), and cause of death (when appropriate). Laboratory tests included CD4+ cell count, HIV RNA PCR, and serum chemistries for calculating MELD scores, as follows: MELD = [0.957 × Ln (creatinine mg/dL, maximum 4.0) + 0.378 × Ln (bilirubin mg/dL) + 1.120 Ln (INR) + 0.643] × 10.
Control Subjects
Controls were HIV(−) liver transplant candidates enrolled in the United Network for Organ Sharing (UNOS)-administered Organ Procurement and Transplantation Network (OPTN) database during the same time frame as the HIV(+) study subjects from the HIVTR study. Each case subject was matched to up to five controls by age, gender, race, and HCV infection status, as defined by HCV antibody positivity. Controls with hepatocellular cancer were excluded. Other variables collected from the HIV(−) controls included MELD scores and time to transplant, death, or current wait listing.
Analytical Methods
Frequencies and percentages were calculated for categorical variables, and medians and interquartile ranges (IQR) for continuous variables. Fisher's exact test was used to analyze categorical data, and Wilcoxon rank-sum test was used to analyze continuous data. Trajectories for time-to-death, time-to-transplant, and time-to- MELD≥25 were calculated from date of placement on the transplant waiting list. Time-to-event distributions were estimated by the product-limit estimate method, and survival distributions were compared by log-rank test.
Univariate and multivariate proportional hazards (PH) models were developed to examine predictors of pre-transplant mortality. Models were developed for the overall cohort, the HCV-infected subgroup, and the HIV-infected subjects alone. The multivariate PH models for pre-transplant mortality included variables that were significant in the univariate analyses as well as important potential confounders. MELD was examined as a dichotomous, categorical, and a continuous variable. Respective hazard ratios (HR) were determined with 95% confidence intervals (CI). The statistical analysis was carried out using SAS version 9.2, Cary, NC.
Human Subjects Research
Written informed consent was obtained from all study subjects prior to enrollment in the HIV transplant protocol. The study protocol conformed to the ethical guidelines of the Declaration of Helsinki and was reviewed by each site's Institutional Review Board. No donor organs were obtained from executed prisoners or other institutionalized persons.
Results
Baseline characteristics of subjects and controls
Of 196 HIV(+) liver transplant candidates enrolled during the study period, 167 (85.2%) were evaluable and 29 (15%) with hepatocellular cancer (HCC) were excluded from analysis. The 167 subjects were matched with 792 HIV(−) liver transplant candidate controls without HCC from the UNOS database. The median (IQR) follow up time for HIV(+) subjects was similar to that of UNOS controls, 166 days (10–526) vs. 194 days (35–598), p=0.09. An additional 136 HIV(+) subjects with advanced liver disease who were screened did not become transplant candidates in this study, among whom 31 (22.8%) died.
Of the HIV(+) subjects, 24/167 (14.4%) died prior to transplantation, similar to the mortality rate in the HIV(−) UNOS controls, 88/792 (11.1%), p=0.30. Limiting the comparison to HCV(+) subjects only, the mortality rate was similar between the HIV(+) subjects,18/125 (14.4%), and the UNOS controls, 62/592 (10.5%), p=0.28. By contrast, although 75% of both groups were HCV(+), a significantly lower proportion of HIV(+) transplant candidates, 58/167 (34.7%) underwent liver transplantation, as compared with the HIV(−) UNOS controls, 377/792 (47.6%) during the study period, p=0.003.
The HIV(+) HIVTR and HIV(−) control subjects did not differ overall in most of the baseline characteristics (Tables 1,2). The two groups were similar in age, gender, and ethnicity. The proportion with private medical insurance coverage was significantly lower in HIV(+) than in HIV(−) control subjects, p<0.0001. Another difference was the median baseline body-mass-index (BMI), significantly lower in HIV(+) than in HIV(−) control subjects, p<0.0001. The geographic regions of the transplant centers also varied between both groups (p<0.0001), with the majority of HIV(+) cases receiving a transplant in New England, New York, the mid-Atlantic states, and the Southwestern US.
Table 1.
Characteristics of HIV(+) and UNOS HIV(−) Liver Transplant Candidates
| HIV (+) (N=167) | UNOS (N=792) | p value | |
|---|---|---|---|
| Median (IQR) Age (yr) | 47 (42–52) | 49 (43–53) | 0.06 |
| No (%) Male Gender | 136 (81%) | 642 (81%) | 0.83 |
| Race: Caucasian | 126 (75%) | 579 (73%) | 0.97 |
| African-American | 25 (15%) | 127 (16%) | |
| Other | 17 (10%) | 87 (11%) | |
| Median (IQR) Body-Mass Index | 24 (22–28) | 28 (25–32) | <.0001 |
| Health Care Coverage* | <.0001 | ||
| Private Insurance | 71 (43%) | 464 (64%) | |
| Medicare | 47 (28%) | 71 (10%) | |
| Medicaid | 34 (20%) | 153 (21%) | |
| Other | 15 (9%) | 42 (6%) | |
| UNOS/OPTN region | <.0001 | ||
| 1 (New England) | 28 (17%) | 43 (5%) | |
| 2 (Mid Atlantic) | 50 (30%) | 111 (14%) | |
| 5 (Southwest) | 47 (28%) | 121 (15%) | |
| 9 (New York) | 22 (13%) | 63 (8%) | |
| Other | 20 (12%) | 454 (58%) |
Not reported for 62 (8%) of the UNOS controls.
Table 2.
Characteristics of Liver Transplant Candidates by Transplant Status
| Transplanted | Not Transplanted | Died | P-value* | |
|---|---|---|---|---|
| No. Enrolled | ||||
| HIVTR | 58 (35%) | 85 (51%) | 24 (14%) | N/A |
| UNOS | 377 (48%) | 327 (41%) | 88 (11%) | N/A |
| Median (IQR) Age (yr) | ||||
| HIVTR | 47 (42–52) | 47 (43–51) | 50 (41–55) | 0.85 |
| UNOS | 48 (43–52) | 50 (44–54) | 49 (43–54) | 0.09 |
| Race (Caucasian) | ||||
| HIVTR | 41 (71%) | 68 (80%) | 16 (67%) | 0.34 |
| UNOS | 275 (73%) | 239 (73%) | 69 (78%) | 0.80 |
| Sex Male | ||||
| HIVTR | 44 (76%) | 71 (84%) | 21 (88%) | 0.39 |
| UNOS | 309 (82%) | 254 (78%) | 75 (85%) | 0.19 |
| Median (IQR) Baseline CD4 | ||||
| HIVTR | 315 (229–437) | 264 (189–399) | 237 (146–348) | 0.03 |
| UNOS | - | - | - | |
| No (%) Detectable HIV RNA | ||||
| HIVTR | 9 (16%) | 6 (7%) | 5 (21%) | 0.09 |
| UNOS | - | - | - | |
| No. (%)HCV infection | ||||
| HIVTR | 47 (81%) | 60 (71%) | 18 (75%) | 0.37 |
| UNOS | 277 (73%) | 253 (77%) | 62 (70%) | 0.30 |
For tests comparing Transplanted, Not Transplanted and Died groups within cohort.
The primary causes of pre-transplant death, sepsis and multiple organ system failure, did not differ between groups (Table 3). No patients with HIV infection were reported to have died of opportunistic infections. A total of 34% (29/85) of HIV+ subjects not transplanted were either removed early from the study or temporarily inactive on the liver transplant waiting list due primarily to active illicit drug use or no longer meeting HIV-specific inclusion criteria. On review of the UNOS cohort group, 20% had a status of temporarily inactive on the liver transplant wait list as of the data cutoff date (p=0.01).
Table 3.
Causes of Pre-Transplant Mortality in HIV+ and HIV− Candidates
| HIV-TR | UNOS | |
|---|---|---|
| HIV+ Cases | HIV− Controls | |
| No. Deaths (%) | 24/167 (14.4%) | 88/792 (11.1%) |
| Cause of Death* | ||
| Sepsis, Infection | 6/24 (25.0%) | 18/88 (20.4%) |
| Multiple Organ Failure | 4/24 (16.7%) | 23/88 (26.1%) |
| Gastrointestinal Bleed | 3/24 (12.5%) | 5/88 (5.7%) |
| Other Causes | 7/24 (29.2%) | 24/88 (27.3%) |
| Unknown | 4/24 (16.7%) | 18/88 (20.4%) |
p value for comparison between HIVTR and UNOS = 0.67
Time to Elevated MELD Score and Death
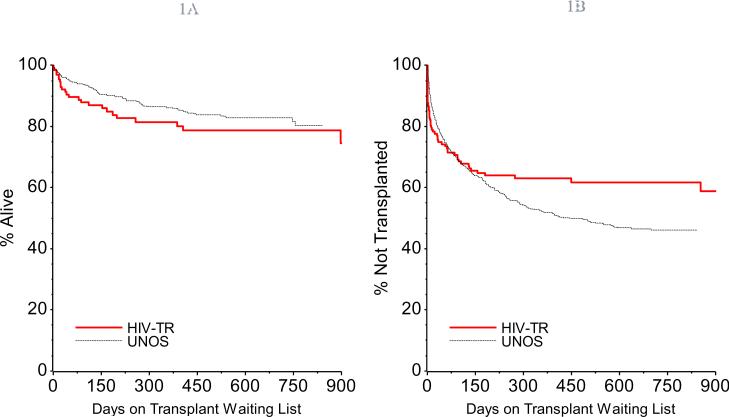
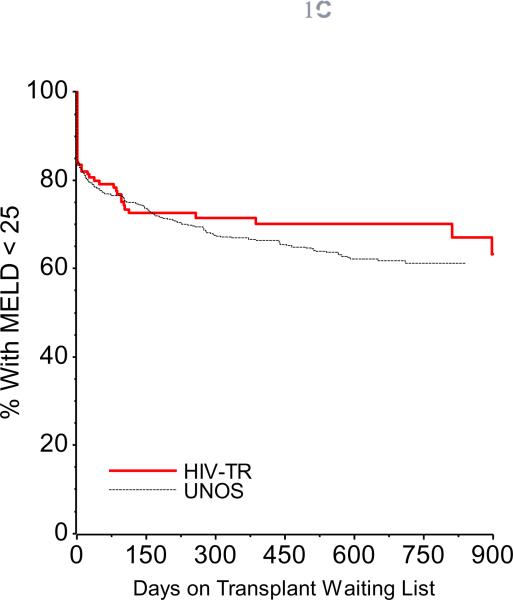
Comparing time-to-event curves for death, transplant, and MELD score ≥25 between HIV(+) subjects and HIV(−) UNOS controls revealed no significant differences (Figure 1), even for the HCV-infected subgroups (data not shown).
Figure 1.
Figure 1a. Time to Death in HIV(+) Transplant Candidates. This Kaplan Meier plot shows that time to death is similar in HIV(+) and HIV(−) transplant candidates., p = 0.18.
Figure 1b. Time to Transplantation in HIV(+) Transplant Candidates. This Kaplan Meier plot shows time to transplantation is similar in HIV(+) and HIV(−) transplant candidates, p = 0.13.
Figure 1c. Time to MELD ≥ 25 in HIV(+) Transplant Candidates. This Kaplan Meier plot shows the time to elevation of MELD to ≥ 25 is similar in HIV(+) and HIV(−) transplant candidates, p = 0.13.
HIV-Related Characteristics
Within the HIV-infected group, HIV-related variables were analyzed by transplant status, as shown in Table 2. HIV(+) subjects who died pre-transplant had a significantly lower median CD4 count at enrollment (237cells/μl) than those who received a transplant (315 cells/μl, p=0.01). No significant differences were observed in nadir CD4 count, proportion with detectable HIV RNA at enrollment, history of AIDS-related opportunistic infections, or use of protease-inhibitor containing initial antiretroviral regimen by transplant status, e.g. expired vs. transplanted. No specific antiretroviral medication or combination of medications was associated with death.
Predictors of Pre-Transplant Mortality for the Overall Cohort
In multivariate PH regression models for the overall cohort and the HCV-infected subgroup, adjusting for the baseline MELD, BMI and region, the hazard of pre-transplant mortality was not significantly higher in HIV(+) subjects HR=1.69 (95% CI: 0.76–3.75, p=0.20) and HR=1.71 (95% CI: 0.67–4.35, p=0.26), respectively, as compared to HIV(−) subjects., An additional interaction term between HIV status and baseline MELD was also non-significant (p=0.33), i.e., HIV status did not have a significant impact on the association between baseline MELD and the hazard of death. In both multivariate models the baseline MELD was the only significant predictor of pre-transplant mortality (HR=1.31(95% CI: 1.21–1.41) and 1.36 (95% CI: 1.22–1.51), respectively; p<0.0001).
Predictors of Pre-Transplant Mortality for HIV-Infected Subjects
In univariate proportional hazards models (Table 4) HCV infection, CD4 count at enrollment, and protease inhibitor use were not associated with increased risk of death or MELD elevation of ≥25. Detectable HIV RNA at baseline, however, increased the hazard of death (HR=3.2, 95% CI: 1.2–8.6, p=0.02) and rate of progression to MELD≥25 (HR=2.79, 95% CI 1.4–5.7, p=0.005). Not surprisingly, high baseline MELD (≥25) was also associated with greater hazard of death (HR=15.0, 95% CI 5.2–43.3, p<0.0001).
Table 4.
Risk Factors for Pre-Transplant Mortality in HIV+ Transplant Candidates
| Univariate Analysis | ||||
|---|---|---|---|---|
| Risk Factor at Enrollment | Hazard for Death | Hazard for MELD >25 | ||
| MELD > 25 | HR = 15.0 | p<0.0001 | - | |
| HCV Co-Infection | HR = 1.0 | p = 0.97 | HR = 0.8 | p = 0.52 |
| Protease Inhibitor Regimen | HR = 1.5 | p = 0.37 | HR = 1.2 | p = 0.59 |
| CD4 < 200/μl | HR = 1.1 | p = 0.74 | HR = 1.0 | p = 0.94 |
| Detectable HIV RNA | HR = 3.2 | p = 0.02 | HR = 2.79 | p = 0.005 |
| Multivariate Analysis | ||
|---|---|---|
| Risk Factor at Enrollment | Hazard for Death | |
| MELD 15–19 | HR = 5.7 | p = 0.005 |
| MELD 20–24 | HR = 21.4 | p < 0.0001 |
| MELD ≥ 25 | HR = 101.1 | p < 0.0001 |
| CD4 < 200/μl | HR = 2.4 | p = 0.07 |
| Detectable HIV RNA | HR = 1.0 | p = 0.98 |
In multivariate proportional hazards models for death, after controlling for CD4 count and detectable HIV RNA, the only significant predictor of mortality was pre-transplant MELD. This was true for MELD as a dichotomous variable <25 and ≥25 (HR=19.5, 95% CI: 5.8–66.0, p<0.001), and as a four-level categorical variable (Table 4). When MELD <15 was used as the reference category, relative hazard increased as MELD score increased. When baseline MELD score was examined as a linear variable, after adjusting for both CD4 count <200/μl and detectable baseline HIV RNA, each unit increase in MELD score was associated with a 20% increase in the risk of pre-transplant death (p<0.0001).
Discussion
End-stage liver disease (ESLD) in patients co-infected with human immunodeficiency virus (HIV) and hepatitis C virus (HCV) has only recently become an indication for liver transplantation (12–14). With the availability of highly active antiretroviral therapies the mortality from HIV is declining. It is projected, however, that mortality from HCV will continue to increase over the next 25 years (15). HIV is known to accelerate the progression of hepatitis C virus-associated liver fibrosis, cirrhosis, hepatic encephalopathy, and death (9,16), while HAART may slow ESLD progression among co-infected individuals (11,17). Unique risk factors for ESLD and liver-related mortality have been identified in HIV/HCV co-infected patients, such as a limited CD4 improvement and failure to maintain an undetectable viral load on antiretroviral therapy (8–10). Despite these data suggesting that HIV disease stage and/or its treatment with antiretroviral therapy may impact mortality in HIV-infected patients considered for liver transplantation, organ allocation in most centers has been based on models derived from HIV-uninfected persons with ESLD. Importantly, our data demonstrate that after controlling for variables such as CD4 count at the time of listing and HIV RNA level, baseline MELD was the only independent predictor of pre-transplant mortality in HIV-infected liver transplant candidates.
A lower proportion of the HIV-infected patients was transplanted as compared to the UNOS controls. This may be partly explained by the regional variation of the participating transplant centers or complicating illness precluding transplantation. The majority of HIV-infected patients were located in regions of the country that historically have longer wait times for organs, while the UNOS controls were evenly distributed throughout the US. A higher percentage of HIV-infected subjects were either temporarily inactive status or removed from the wait list as compared to the UNOS controls, which may have led to the differential rates of transplant. There was, however, no difference in cause of death between both groups nor were there any deaths due to opportunistic infections in the HIV-infected patients. This cohort of patients was required to have a fairly robust CD4 count at entry and maintain it above 100 cells/mL in order to remain eligible for transplant. This may explain the lack of opportunistic infections seen.
Interestingly, there was no significant difference in mortality in HIV-infected and uninfected patients waiting for liver transplantation. In contrast, previous reports have found shorter pre-transplant survival in HIV-positive liver transplant candidates, unrelated to severity of liver or HIV disease (8,9). Unlike these studies that included patients irrespective of stage of HIV disease, while patients on our study met specific HIV disease criteria (e.g. adequately controlled HIV replication and CD4 ≥ 100/ul) in addition to standard transplant criteria for other comorbid conditions. Further, they were enrolled only at the time of placement on the transplant wait list, and underwent rigorous routine pre-transplant evaluation, including cardiac stress tests, pulmonary function tests, with optimization of their overall health status by multiple subspecialty care providers. It is possible that lead bias affected our results, for example, if subjects in this study were at an earlier stage of ESLD than HIV-infected patients in the previous studies. Nonetheless, among HIV-infected persons considered for liver transplant, our findings strongly support the use of the MELD score to predict mortality and to guide the allocation of limited organs given that each point increase in MELD was associated with a 20% greater risk of death in our HIV-infected patients.
Patients with hepatocellular carcinoma (HCC) were not included in this cohort as they receive priority for organ allocation and which could introduce bias when evaluating predictors of mortality. A recent case-control study of patients with HCC showed that those with HIV infection were usually younger and more likely symptomatic, but tumor staging and survival appeared to be similar in those with and without HIV-infection (18). It will be important to prospectively study outcomes in HIV-infected patients with HCC.
The data we report have implications for monitoring and management of the HIV/HCV co-infected individual. There may be better predictors of mortality in HIV/HCV co-infection, especially in patients with more advanced HIV disease, such as MELD combined with CD4 count. Further, MELD-Na, which incorporates serum sodium concentrations along with the traditional MELD calculations, may be a more accurate predictor (19). Unfortunately, we had insufficient data to determine if any of these markers would have been better predictors of pre-transplant mortality.
Our study suggests a smaller proportion of co-infected patients come to transplantation as compared to HCV mono-infected patients. Although the reasons for this are not known, it would seem prudent for HIV providers to calculate MELD scores on all HIV/HCV co-infected patients during routine quarterly HIV monitoring to help guide decisions on prompt referral for transplant evaluation. Overall, this study demonstrates that pre-transplant mortality risks in HIV(+) transplant candidates with CD4 > 100, who meet criteria for placement on the liver transplant wait list are similar to HIV(−) candidates. As MELD is an equally reliable predictor in both groups, these data support the incorporation of MELD as part of standard HIV medical management.
Acknowledgments
Grant Support On behalf of HIVTR: Solid Organ Transplantation in HIV Multisite Study Investigators.
This study is sponsored by NIAID Grant U01 A1052748 (PS) and R01 DA016065 (MSS)
List of Abbreviations
- HIV
Human Immunodeficiency Virus
- HCV
Hepatitis C Virus
- HCC
Hepatocellular Cancer
- MELD
Model for End Stage Liver Disease
- UNOS
United Network for Organ Sharing
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: The authors have no conflicts of interest to disclose.
References
- 1.Mocroft A, Soriano V, Rockstroh J, et al. Is there evidence for an increase in the death rate from liver-related disease in patients with HIV? AIDS. 2005;19:2117–2125. doi: 10.1097/01.aids.0000194799.43799.ea. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Sabin CA, Friis-Møller N, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 3.Koziel MJ, Peters MG. Viral hepatitis and HIV infection. N Engl J Med. 2007;356:1445–1454. doi: 10.1056/NEJMra065142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sulkowski MS, Thomas DL, Chaisson RE, et al. Hepatotoxicity associated with antiretroviral therapy in adults infected with human immunodeficiency virus and the role of hepatitis C or B virus infection. JAMA. 2000;283:74–80. doi: 10.1001/jama.283.1.74. [DOI] [PubMed] [Google Scholar]
- 5.Sulkowski MS. Viral hepatitis and HIV coinfection. J Hepatol. 2008;48:353–367. doi: 10.1016/j.jhep.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464–470. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 7.Kamath PS, Kim WR. The model for end-stage liver disease (MELD) Hepatology. 2007;45:797–805. doi: 10.1002/hep.21563. [DOI] [PubMed] [Google Scholar]
- 8.Ragni MV, Eghtesad B, Schlesinger KW, et al. Pre-transplant survival is shorter in HIV-positive than HIV-negative subjects with end-stage liver disease. Liver Transpl. 2005;11:1425–1430. doi: 10.1002/lt.20534. [DOI] [PubMed] [Google Scholar]
- 9.Pineda JA, Romero-Gómez M, Díaz-García F, et al. HIV co-infection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41:779–789. doi: 10.1002/hep.20626. [DOI] [PubMed] [Google Scholar]
- 10.Stock PG. Rapid deterioration of HIV co-infected patients waiting for liver transplantation is not predicted by MELD. Liver Transpl. 2005;11:1315–1317. doi: 10.1002/lt.20539. [DOI] [PubMed] [Google Scholar]
- 11.Pineda JA, Garcia-Garcia JA, Aguilar-Guisado M, et al. Clinical progression of Hepatitis C Virus-related chronic liver disease in Human Immunodeficiency Virus-infected patients undergoing highly active antiretroviral therapy. Hepatology. 2007;46:622–630. doi: 10.1002/hep.21757. [DOI] [PubMed] [Google Scholar]
- 12.Ragni MV, Belle SH, Im K, et al. Survival in HIV-infected liver transplant recipients. J Infect Dis. 2003;188:1412–1420. doi: 10.1086/379254. [DOI] [PubMed] [Google Scholar]
- 13.Fung JJ, Eghtesad B, Patel TK, et al. Liver transplantation in patients with HIV infection. Liver Transplantation. 2004;10(Suppl 2):S39–53. doi: 10.1002/lt.20261. [DOI] [PubMed] [Google Scholar]
- 14.Soriano V, Puoti M, Sulkowski M, et al. Care of patients co-infected with HIV and hepatitis C virus: 2007 updated recommendations from the HCV-HIV International Panel. AIDS. 2007;21:1073–1089. doi: 10.1097/QAD.0b013e3281084e4d. [DOI] [PubMed] [Google Scholar]
- 15.Deuffic-Burban S, Poynard T, Sulkowski MS, et al. Estimating the future health burden of chronic hepatitis C and human immunodeficiency virus infections in the United States. J Viral Hepatol. 2007;14:107–115. doi: 10.1111/j.1365-2893.2006.00785.x. [DOI] [PubMed] [Google Scholar]
- 16.Ragni MV, Belle SH. Impact of human immunodeficiency virus (HIV) on progression to end-stage liver disease in individuals with hemophilia and hepatitis C. J Infect Dis. 2001;183:1112–1115. doi: 10.1086/319273. [DOI] [PubMed] [Google Scholar]
- 17.Ragni MV, Schillo R, Nalesnik MA, Dang Q. HAART improves ESLD-free survival in HIV-HCV co-infection. Haemophilia. 2009;15:552–558. doi: 10.1111/j.1365-2516.2008.01935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bräu N, Fox RK, Xiao P, et al. Presentation and outcome of hepatocellular carcinoma in HIV-infected patients: a U.S.-Canadian multicenter study. J Hepatol. 2007;47:527–537. doi: 10.1016/j.jhep.2007.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Biggins SW, Kim RW, Terrault NA, et al. Evidence-based Incorporation of Serum Sodium Concentration into MELD. Gastroenterology. 2006;130:1652–1660. doi: 10.1053/j.gastro.2006.02.010. [DOI] [PubMed] [Google Scholar]