Abstract
Angiotensin II maintains renal cortical blood flow and renal oxygenation in the clipped kidney of early two-kidney, one clip Goldblatt hypertensive rats (2K,1C). The involvement of Ang II is believed to decline whereas oxidative stress increases during progression of 2K,1C hypertension. We investigated the hypothesis that the acute administration of drugs to inhibit reactive oxygen species (tempol) or angiotensin II type 1 receptors (candesartan) or angiotensin converting enzyme (enalaprilat), lower mean arterial pressure, and increase kidney blood flow and oxygenation in the clipped kidney of chronic 2K,1C rats in contrast to Sham controls. Twelve months after left renal artery clipping or Sham, mean arterial pressure, renal cortical blood flow and renal cortical and medullary oxygen tension were measured after acute administration of tempol followed by enalaprilat or candesartan followed by enalaprilat
The mean arterial pressure of the 2K,1C was reduced by candesartan (−9%), and more effectively by tempol (−35%). All applied treatments had similar blood pressure lowering effect in sham (average −21%). Only tempol increased cortical blood flow (+35%) and cortical and medullary oxygen tensions (+17% and +94%, respectively) in clipped kidneys of 2K,1C.
Administration of enalaprilat had no additional effect, except for a modest reduction in cortical blood flow in the clipped kidney of 2K,1C when co-administered with candesartan (−10%).
In conclusion, acute administration of tempol is more effective than candesartan in reducing the mean arterial blood pressure and improving renal blood perfusion and oxygenation in the clipped kidney of chronic 2K,1C rats.
Keywords: Goldblatt hypertension, renal oxygen tension, renal blood flow, tempol, angiotensin receptor blockers, angiotensin converting enzyme inhibitors
Introduction
A reduced renal perfusion pressure following the clipping of a renal artery increases angiotensin II (Ang II) concentrations in both kidneys.1 There is an early development of Ang II-dependent hypertension.2–4 Ang II acting on Ang II type 1 receptors (AT1-Rs) results in activation of superoxide (O2.−) production by the nicotinamide adenine dinucleotide phosphate (NADPH) oxidase,5 which is abundantly expressed in the kidney.6 The involvement of oxidative stress in early two-kidney, one-clip (2K,1C) Goldblatt hypertensive rats has been demonstrated by prolonged administration of tempol to reduce reactive oxygen species (ROS) which reduced the mean arterial pressure (MAP), and improved the renal blood flow and glomerular filtration rate and oxygen tension (pO2) of the clipped kidney.7 In contrast, the administration of an AT1-R blocker (ARB) indeed reduced the MAP both during the early7 and the chronic phase4 in 2K,1C hypertension, but failed to improve either the renal hemodynamics or oxygenation during the early phase.7 Therefore, therapeutic options for correcting hypertension and renal ischemia in the chronic phase of 2K,1C renovascular hypertension are presently limited.8 Nevertheless, prolonged Ang II infusion reduces renal tissue pO2,7,9,10 which is ascribed to excessive formation of ROS. Several of the conditions commonly associated with increased oxidative stress display reduced kidney pO2, including diabetes,11–13 lipopolysaccharide (LPS)-induced sepsis14 and ischemia-reperfusion injury.15 Therefore, we investigated the role of ROS in renal vasoconstriction and oxygenation in chronic 2K, 1C hypertension.
Anderson et al proposed that increased Ang II in the early 2K,1C model are a homeostatic modification to provide sufficient glomerular capillary pressure to sustain the glomerular filtration rate (GFR).16 Indeed, we have reported that oxygen availability in clipped kidneys of early (3 weeks) 2K,1C is maintained by Ang II acting on Ang II type 2 receptors (AT2-Rs) resulting in nitric oxide (NO) release.17 This may be of importance since chronic renal hypoxia, and repeated episodes of renal ischemia, may contribute to hypertension10 and progressive kidney disease.18,19 However, the long-term consequences of increased levels of Ang II and subsequent oxidative stress for the function of the clipped kidney of the 2K,1C model of renovascular hypertension is currently not well understood. The role of Ang II evolves during 2K,1C hypertension.4,20 Therefore, the present work was designed to investigate the role of ROS and/or Ang II acting on AT1-Rs in the regulation of MAP, cortical renal blood flow and tissue oxygen availability in the clipped kidney of chronic 2K,1C rats.
Material and Methods
These studies were performed under guidelines recommended by the National Institutes of Health and approved by the Georgetown University Animal Care and Use Committee. As described in detail previously,7,17 young male Sprague-Dawley rats (80–100 g) were anesthetized with isoflurane (0.5–1.5%). A silver clip (0.2 mm) was placed around the left renal artery (2K,1C). Age-matched rats were used as controls (Sham). All rats received hydralazine + hydrochlorothiazide + reserpine (HHR; 30 + 10 + 0.2 mg · kg−1 · day−1) in the drinking water as previously described9 in order to maximize survival of the clipped rats. The HHR treatment was discontinued 14 days prior to the acute experiments.
Twelve months after clipping, all rats were anesthetized with Inactin (100 mg · kg−1 i.p.; Sigma-Aldrich, St. Louis, MO), and an endotracheal tube was inserted for spontaneous respiration. Rats were prepared, and followed a similar protocol, as those with acute 2K,1C hypertension described in detail previously.17 Briefly, the left femoral artery was catheterized for monitoring MAP and the left femoral vein for infusion of saline (5 ml · kg bw−1 · h−1). The left kidney was immobilized in a plastic cup while renal cortical pO2 and cortical blood flow (CBF) were measured with oxygen microelectrodes (Unisense, Aarhus, Denmark) and laser-Doppler needle probes (Transonic Systems Inc, Ithaca, NY) as described previously.12,17,21 The location of each measurement was visually verified at the end of the experiments by dissecting the kidneys under a microscope. Measurements were made before and after the administrations of candesartan (1 mg · kg bw−1 bolus + 1 mg · kg bw−1 · h−1; kind gift from Astra Zeneca, Södertälje Sweden; manufacturer recommended dose for maximal inhibition of AT1-Rs in vivo; Sham n=7 and 2K,1C n=8)17 followed after 30 minutes by enalaprilat (0.3 mg · kg bw−1·h−1; Novaplus 1.25 mg · ml−1, Baxter Healthcare Corporation, Deerfield, IL; fully effective antihypertensive dose in acute 2K,1C rats),17, or tempol (174 μmol · kg bw−1 bolus + 174 μmol · kg bw−1 · h−1; Sigma Aldrich; fully effective antihypertensive dose in spontaneously hypertensive rats; Sham n=7 and 2K,1C n=7)7 followed after 30 minutes by enalaprilat (0.3 mg · kg bw−1·h−1).
We selected this protocol, using candesartan followed by enalaprilat, for comparison with a prior series in acute 2K,1C rats17 where an increase in blood pressure or renal vascular resistance with enalaprilat in rats pretreated with candesartan was an indication of an AT2-Rs mediated change. Since enalaprilat reduced CBF modestly after candesartan (Fig 2), this suggested some role for AT2-Rs in maintaining CBF even in the chronic model. Therefore, we undertook a limited study of 2K,1C rats (n=7) given the AT2-R antagonist PD-123,319 (1 mg · kg bw−1 bolus + 1 mg · kg bw−1 · h−1; Sigma Aldrich; fully effective dose in acute 2K,1C rats)17 followed 30 minutes later by enalaprilat (0.3 mg · kg bw−1 bolus + 0.3 mg · kg bw−1 · h−1) to test this hypothesis more directly.
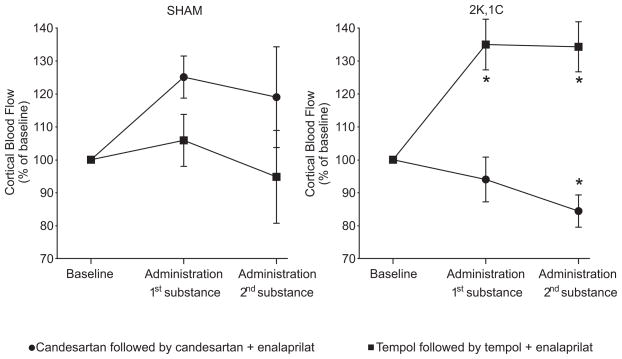
Figure 2.
Mean changes in renal cortical blood flow in Sham and 2K,1C rats during the different acute interventions. *denotes p<0.05 when compared to baseline with the same group, and † denotes p<0.05 when compared to the candesartan, or the combined candesartan + enalaprilat treatment within the same category of animals. All values are mean ± SEM.
Statistics
ANOVA was used to compare multiple data sets. When appropriate, this was followed by Dunnett’s post hoc test. Two data sets within the same group were compared using Student’s t-test for paired comparisons. Relative changes displayed in the figures are for visualization purpose only; statistics were calculated using the original parametric data sets (GraphPad Prism, GraphPad Software, San Diego CA). For all comparisons, p<0.05 was considered statistically significant. All values are expressed as mean ± SEM.
Results
A total of 54 rats were clipped and 24 rats survived until the acute experiments twelve months later, resulting in a survival ratio of 44%. Of the 24 remaining rats, one was excluded due to surgical errors and one due to having an infarcted and atrophied left kidney. All animals that underwent Sham surgery survived.
The body weights did not differ between Sham and 2K,1C rats (Table 1). Both the non-clipped and the clipped kidneys of 2K,1C rats were significantly heavier when corrected for body weight than kidneys of Sham rats.
Table 1.
Body weights (BW), kidney weights (KW), and baseline kidney tissue pO2 in sham or rats with a silver clip placed on the renal artery of the left kidney (2K,1C) prior to the different acute interventions.
| KW/BW (x1000) |
Ratio Kidney Size (Clipped/Non-clipped) | Baseline Cortical pO2 (mmHg) | Baseline Medullary pO2 (mmHg) | |||||
|---|---|---|---|---|---|---|---|---|
| Type | Intervention | N | BW (g) | Non-clipped | Clipped | |||
| Sham | Candesartan and Candesartan + enalaprilat | 7 | 511±12 | - | 2.69±0.08 | - | 43±3 | 28±1 |
| Sham | Tempol and Tempol + enalaprilat | 7 | 492±16 | - | 2.65±0.09 | - | 47±2 | 28±2 |
| 2K,1C | Candesartan and Candesartan + enalaprilat | 8 | 466±23 | 5.43±0.39† | 3.55±0.63* | 0.64±0.08 | 42±3 | 16±2* |
| 2K,1C | Tempol and Tempol + enalaprilat | 7 | 477±7 | 6.45±0.41† | 4.53±0.30* | 0.70±0.02 | 41±1 | 14±1* |
All values are mean ± SEM.
denotes p<0.05 when compared to corresponding Sham group, and
denotes p<0.05 when compared to the clipped kidney within the same group.
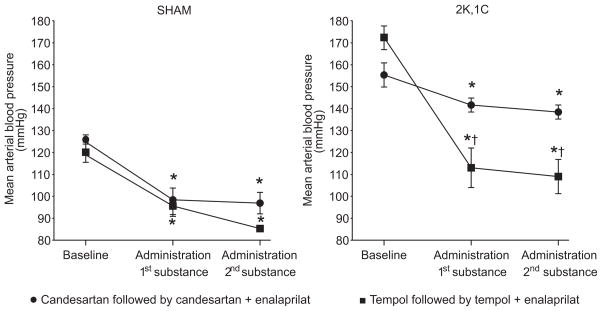
2K,1C rats had elevated MAP, averaging 163±3 (n=22) compared to Sham 123±3 (n=14, P<0.05; Fig. 1 and Table 2). All acute interventions reduced the MAP modestly, but similarly, in elderly Sham rats. The elevated MAP of 2K,1C rats was reduced by candesartan. However, tempol was significantly more effective (Fig. 1). Enalaprilat did not produce a further fall in MAP in any rats administered tempol or candesartan.
Figure 1.
Mean arterial blood pressure in anesthetized Sham and 2K,1C rats during baseline conditions and during the different acute interventions. *denotes p<0.05 when compared to baseline with the same group, and † denotes p<0.05 when compared to the candesartan, or the combined candesartan + enalaprilat treatment within the same category of animals. All values are mean ± SEM.
Table 2.
Response in mean arterial blood pressure (MAP), relative cortical blood flow (CBF) and kidney pO2 to administration of the selective AT2-R blocker PD-123,319 and the combination of PD-123,319 + enalaprilat in a separate group of 2K,1C rats.
| Parameter | Baseline | After PD-123,319 | After PD-123,319 + enalaprilat |
|---|---|---|---|
| MAP (mmHg) | 162±5 | 155±5 | 133±5* |
| CBF (% of baseline) | 100 | 101±11 | 88±9 |
| Cortical pO2 (% of baseline) | 100 | 105±3.3 | 104±5 |
| Medullary pO2 (% of baseline) | 100 | 96±11 | 91±17 |
All values are mean ± SEM of n=7.
denotes p<0.05 when compared to baseline.
The CBF was unchanged after all applied acute interventions in Sham rats. Candesartan did not change CBF significantly in 2K,1C rats (Fig. 2). However, CBF was increased in 2K,1C rats by tempol. CBF did not change further in 2K,1C rats given enalaprilat after tempol but was reduced significantly by enalaprilat following candesartan administration (Fig. 2).
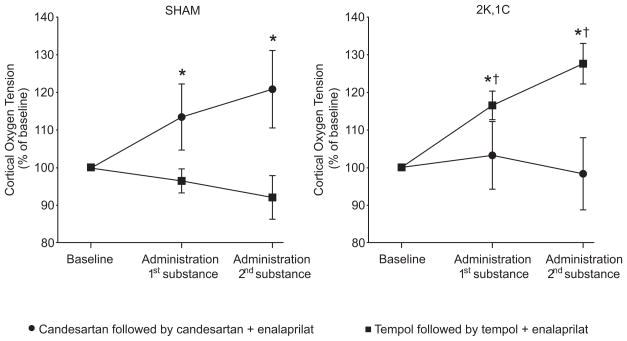
The baseline renal cortical pO2 was similar in 2K,1C and Sham rats (Table 1). Candesartan, or the combination of candesartan and enalaprilat, increased cortical pO2 in Sham rats, whereas tempol had no effect in this group (Fig. 3). Only tempol increased the cortical pO2 in 2K,1C rats after which there was no further change with enalaprilat (Fig. 3).
Figure 3.
Mean changes in renal cortical oxygen tension in Sham and 2K,1C rats during the different acute interventions. *denotes p<0.05 when compared to baseline with the same group, and † denotes p<0.05 when compared to the candesartan, or the combined candesartan + enalaprilat treatment within the same category of animals. All values are mean ± SEM.
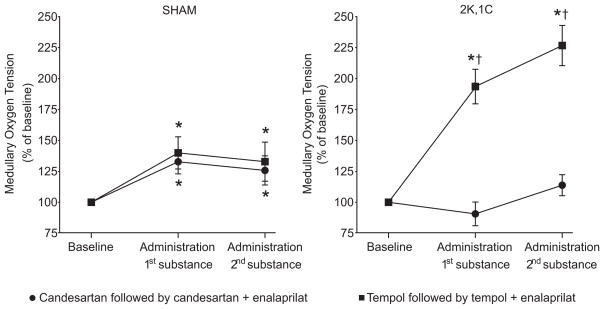
The baseline renal medullary pO2 was reduced in 2K,1C rats compared to Sham (Table 1). Renal medullary pO2 was increased after all the applied acute interventions in Sham, whereas again only tempol increased the medullary pO2 in 2K,1C rats (Fig. 4). Subsequent administration of enalaprilat after tempol did not alter the medullary pO2 further.
Figure 4.
Mean changes in renal medullary oxygen tension in Sham and 2K,1C rats during the different acute interventions. *denotes p<0.05 when compared to baseline with the same group, and † denotes p<0.05 when compared to the candesartan, or the combined candesartan + enalaprilat treatment within the same category of animals. All values are mean ± SEM.
The 2K,1C rats administered PD-123,319 had similar body weight (495±21 g), kidney to body weight ratios (right kidney 5.01±0.62; left kidney 3.71±0.82; P<0.05), right to left kidney weight ratio (0.76), baseline cortical and medullary pO2 (44±1 and 16±1 mmHg, respectively) as the other two 2K,1C groups. Administered PD-123,319 to 2K,1C rats had no effect on any of the investigated parameter. Subsequent addition of enalaprilat caused a modest reduction of MAP (Table 2).
Discussion
The main new findings from this study are that the acute administration of tempol is more effective in lowering MAP in chronic 2K,1C rats than blockade of the renin-angiotensin system with ARB or ACE inhibitor. Furthermore, only tempol increased the blood flow and the tissue pO2 in the clipped kidney, whereas the ARB had no effect. The addition of an ACE inhibitor to rats that had received tempol or candesartan did not improve these responses.
Both the hypertension, and the intrarenal alterations in blood flow and tissue pO2 in early 2K,1C hypertension, are highly dependent on increased Ang II action.17 Whereas we found that the acute inhibition of AT1-Rs or ACE reduced the blood pressure in chronic 2K,1C, the fall in BP was significantly greater following a reduction in ROS with tempol. Furthermore, only tempol effectively increased both the CBF and the tissue pO2 in the renal cortex and medulla in the clipped kidney of chronic 2K,1C, whereas interventions directed against the renin-angiotensin system had no effect on these parameters. The altered function of the clipped kidney likely is a response to prolonged exposure to severely increased Ang II levels, and to a reduced perfusion pressure and medullary pO2. Chronically elevated Ang II can induce self-sustaining mechanisms which maintain ROS production. Thus, prolonged Ang II can reduce the antioxidant defense systems, such as superoxide dismutase, upregulate NADPH oxidase subunits, oxidize tetrahydrobiopterin (BH4) with subsequent uncoupling of NO synthases, stabilize the thromboxane-prostanoid (TP)-receptors and induce vascular and renal inflammation to name but a few.10 The results from the present study show that the effects of tempol differ substantially from those that follow interruption of the renin-angiotensin system, which is consistent with the concept of a self-sustaining ROS producing system.
Tempol, whether given by acute intravenous infusion22 or by a two week subcutaneous infusion7 into rats with early 2K,1C renovascular hypertension reduced both the MAP and the RVR substantially. Oral administration of tempol for two23 or five24 weeks to rats with early 1K,1C renovascular hypertension also reduced the MAP. However, whereas we had found a reduced renal cortical pO2 in the clipped kidney of early 2K,1C hypertensive rats,7,17 the cortical pO2 was maintained in the clipped kidney in the chronic model. The tissue oxygen availability is determined by the interplay between oxygen delivery and consumption. The latter is highly influenced by the tubular Na+ transport25 and therefore by the GFR. Although we did not measure the GFR, previous studies have established that there is a reduced GFR in the clipped kidney of chronic 2K,1C hypertensive rats that has been ascribed to a reduced renal perfusion pressure.26 However, the absence of an atrophic stenotic kidney in this study implies that the increase in systemic pressure in these rats likely overcame the obstruction caused by the clip and that the blood perfusion distally to the mild or moderate obstruction must have been fairly well maintained. If so, it is also likely that the GFR of the clipped kidney would have been substantially higher compared to previous reports.26 A well maintained blood supply may also explain the near-normal pO2 values in the kidney cortex of the clipped kidney in the present model.
In contrast to the kidney cortex, we detected a significantly reduced pO2 in the outer medulla of the clipped kidneys of chronic 2K,1C hypertensive rats. This might contribute to progressive kidney dysfunction, as proposed in other models of hypoxia-induced kidney damage.15,18 Therefore, it is important to elucidate the mechanisms involved and to identify potential interventions to restore the pO2 in the medulla of the clipped kidney. The results from the present study show that tempol not only reduced the MAP, but effectively increased the blood flow and cortical and medullary pO2 in the clipped kidney. These effects were not influenced by subsequent administration of an ACE inhibitor, which implies that tempol had corrected any effects due to ongoing Ang II generation, but the mechanism was not studied further in these experiments. It could entail a reduction by tempol of renal medullary O2.− with enhanced NO bioavailability which would increase blood flow and thereby oxygen delivery, and also increase the efficiency of mitochondria to produce ATP. NO reversibly inhibits mitochondrial respiration by competing for the binding site of oxygen.27,28 This effect of NO is potentiated at the low pO2 levels recorded in the medulla.
Chade et al reported that an acute intrarenal infusion of tempol into pigs fed a high fat diet and studied 12 weeks after a renal artery stenosis failed to improve the reduced levels of renal blood flow, cortical perfusion or GFR.29 In contrast, we detected large increases in renal blood flow and cortical perfusion in rats studied at an even more prolonged stage of 2K,1C renovascular disease. It is possible that the ten-fold increase in low density lipoprotein cholesterol in the pigs fed a high fat diet, which were accompanied by extensive perivascular and tubulointestinal fibrosis and neovascularization which were not pronounced in the kidneys in our study, had limited the hemodynamic response to tempol in the hypercholesterolemic pig model.30 Alternatively, the rather modest degree of renal artery stenosis which did not induce renal atrophy in our rat model may have provided an opportunity for a reduction in ROS to be apparent as an increase in blood flow and oxygenation.
Ang II induces NO release via AT2-Rs within the clipped kidney in early 2K,1C and thereby sustains blood flow and pO2 in that kidney.17 Indeed, enalaprilat did reduce CBF modestly in the clipped kidney after candesartan, consistent with a residual role for AT2-Rs in maintaining cortical flow, if not oxygenation in the chronic model. However, the selective AT2-R blocker PD-123,319 had no effect on either MAP, CBF or kidney oxygenations, whereas the effects of enalaprilat persisted after blockade of the AT2-Rs blockade. Therefore, we conclude that the involvement of AT2-Rs is not very evident in the kidneys of rats with chronic 2K,1C. The reason for this change in the importance of the AT2-Rs is presently unknown. In contrast to the AT2-Rs, tempol retains its efficiency from the early to the chronic 2K,1C phase of modest hypertension in the rat.
The present study has some limitations, mainly relating to methodological difficulties. Due to the massive fibrosis resulting from the clip placed on the renal artery, it is very difficult to measure total kidney blood flow and split-kidney function in these rats. We therefore choose to use laser-Doppler methodology since the main focus of this study was to measure hemodynamic alterations occurring in response to acute drug administrations. The laser-Doppler technique is suitable for acute experiments, as described previously,31 if care is taken to confirm each measurement location. However, the disadvantage is that the hemodynamic significance of the clip cannot be verified, as discussed further above. A second limitation was the apparently rather modest degree of renal artery stenosis engendered which in fact was accompanied by renal hypertrophy, rather than renal atrophy. Furthermore, all animals were chronically treated with HHR throughout the course of the study to maximize survival of the clipped animals. It is also possible that only animals with only a modest renal artery restriction survived until the actuate experiments twelve months later and that this influenced the results of the present study. Although previous studies have shown that HHR indeed lowers blood pressure, this treatment has been shown to have only a marginal influence on the development of renal alterations such albuminuria, glomerolusclerosis, cytokine levels, renal blood flow response to blockade of the nitric oxide system, kidney oxygen tension and intrarenal levels of Ang II and angiotensinogen in several different models of hypertension-induced kidney damage including rats with spontaneous hypertension (SHR), 5/6 nephrectomized, deoxycorticosterone-acetate (DOCA) hypertension rats and 2K,1C.9,32–37 These previous reports, together with the fact that the HHR therapy was discontinued 14 days before the acute experiments, indicate that this procedure should have no major influence on the results reported in the present study.
In conclusion, acute administration of tempol had superior antihypertensive efficiency and improvement of renal blood perfusion and oxygenation compared to an ARB in this model. Whether this will translate into differences in long-term renoprotection during ischemic nephropathy warrants further study.
Perspectives
Unilateral renal artery stenosis in man can lead to hypertension, renal atrophy and reduced renal function. Presently, no treatments have been found to prevent progressive kidney dysfunction in this setting. A controlled trial of renal artery angioplasty in patients with renal artery stenosis found no beneficial effects on glomerular filtration rate at one year.38 Current pharmacologic therapy is equally unsatisfactory.39 ACE inhibitors and ARBs are effective in reducing blood pressure, but can worsen renal insufficiency.8 Chronic renal hypoxia has been considered an underlying cause of progressive CKD.40 Thus, our finding that the acute administration of tempol is effective both in reducing the blood pressure and in improving the renal oxygenation in the clipped kidney at both the early17 and the chronic phase suggests a possible role for drugs of this class in prevention or management of hypertension and renal insufficiency, at least in patients with modest degrees of renal artery stenosis. This warrants further study.
Acknowledgments
We thank Emily Wing Kam Chan for preparing and editing the manuscript.
Sources of Funding
This work was supported by grants from NHLBI (HL-68686), NIDDIK (DK-07183, DK-36079, DK-49870 and DK-077858) and from the George E. Schreiner Chair of Nephrology.
Footnotes
Conflict of Interest/Disclosure
None.
References
- 1.Sadjadi J, Puttaparthi K, Welborn MB, Rogers TE, Moe O, Clagett GP, Turnage RH, Levi M, Modrall JG. Upregulation of autocrine-paracrine renin-angiotensin systems in chronic renovascular hypertension. J Vasc Surg. 2002;36:386–392. doi: 10.1067/mva.2002.125016. [DOI] [PubMed] [Google Scholar]
- 2.Goldblatt H, Lynch J, Hanzal R, Summerville W. Studies on experimental hypertension I. The production of persistent elevation of systolic blood pressure by means of renal ischemia. J Exp Med. 1934;59:347–379. doi: 10.1084/jem.59.3.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Navar LG, Ichihara A, Chin SY, Imig JD. Nitric oxide-angiotensin II interactions in angiotensin II-dependent hypertension. Acta Physiol Scand. 2000;168:139–147. doi: 10.1046/j.1365-201x.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilcox CS, Cardozo J, Welch WJ. AT1 and TxA2/PGH2 receptors maintain hypertension throughout 2K,1C Goldblatt hypertension in the rat. Am J Physiol. 1996;271:R891–896. doi: 10.1152/ajpregu.1996.271.4.R891. [DOI] [PubMed] [Google Scholar]
- 5.Welch WJ, Blau J, Xie H, Chabrashvili T, Wilcox CS. Angiotensin-induced defects in renal oxygenation: role of oxidative stress. Am J Physiol Heart Circ Physiol. 2005;288:H22–28. doi: 10.1152/ajpheart.00626.2004. [DOI] [PubMed] [Google Scholar]
- 6.Gill PS, Wilcox CS. NADPH oxidases in the kidney. Antioxid Redox Signal. 2006;8:1597–1607. doi: 10.1089/ars.2006.8.1597. [DOI] [PubMed] [Google Scholar]
- 7.Welch WJ, Mendonca M, Aslam S, Wilcox CS. Roles of oxidative stress and AT1 receptors in renal hemodynamics and oxygenation in the postclipped 2K,1C kidney. Hypertension. 2003;41:692–696. doi: 10.1161/01.HYP.0000052945.84627.8F. [DOI] [PubMed] [Google Scholar]
- 8.van de Ven PJ, Beutler JJ, Kaatee R, Beek FJ, Mali WP, Koomans HA. Angiotensin converting enzyme inhibitor-induced renal dysfunction in atherosclerotic renovascular disease. Kidney Int. 1998;53:986–993. doi: 10.1111/j.1523-1755.1998.00840.x. [DOI] [PubMed] [Google Scholar]
- 9.Welch WJ, Baumgartl H, Lubbers D, Wilcox CS. Renal oxygenation defects in the spontaneously hypertensive rat: role of AT1 receptors. Kidney Int. 2003;63:202–208. doi: 10.1046/j.1523-1755.2003.00729.x. [DOI] [PubMed] [Google Scholar]
- 10.Wilcox CS. Oxidative stress and nitric oxide deficiency in the kidney: a critical link to hypertension? Am J Physiol Regul Integr Comp Physiol. 2005;289:R913–935. doi: 10.1152/ajpregu.00250.2005. [DOI] [PubMed] [Google Scholar]
- 11.Palm F, Buerk DG, Carlsson PO, Hansell P, Liss P. Reduced nitric oxide concentration in the renal cortex of streptozotocin-induced diabetic rats: effects on renal oxygenation and microcirculation. Diabetes. 2005;54:3282–3287. doi: 10.2337/diabetes.54.11.3282. [DOI] [PubMed] [Google Scholar]
- 12.Palm F, Cederberg J, Hansell P, Liss P, Carlsson PO. Reactive oxygen species cause diabetes-induced decrease in renal oxygen tension. Diabetologia. 2003;46:1153–1160. doi: 10.1007/s00125-003-1155-z. [DOI] [PubMed] [Google Scholar]
- 13.Palm F, Friederich M, Carlsson PO, Hansell P, Teerlink T, Liss P. Reduced nitric oxide in diabetic kidneys due to increased hepatic arginine metabolism: implications for renomedullary oxygen availability. Am J Physiol Renal Physiol. 2008;294:F30–37. doi: 10.1152/ajprenal.00166.2007. [DOI] [PubMed] [Google Scholar]
- 14.James PE, Goda F, Grinberg OY, Szybinski KG, Swartz HM. Intrarenal pO2 measured by EPR oximetry and the effects of bacterial endotoxin. Adv Exp Med Biol. 1997;411:557–568. doi: 10.1007/978-1-4615-5865-1_69. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M. Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol. 2003;14:1825–1832. doi: 10.1097/01.asn.0000074239.22357.06. [DOI] [PubMed] [Google Scholar]
- 16.Anderson WP, Denton KM, Woods RL, Alcorn D. Angiotensin II and the maintenance of GFR and renal blood flow during renal artery narrowing. Kidney Int Suppl. 1990;30:S109–113. [PubMed] [Google Scholar]
- 17.Palm F, Connors SG, Mendonca M, Welch WJ, Wilcox CS. Angiotensin II type 2 receptors and nitric oxide sustain oxygenation in the clipped kidney of early Goldblatt hypertensive rats. Hypertension. 2008;51:345–351. doi: 10.1161/HYPERTENSIONAHA.107.097832. [DOI] [PubMed] [Google Scholar]
- 18.Fine LG, Orphanides C, Norman JT. Progressive renal disease: the chronic hypoxia hypothesis. Kidney Int Suppl. 1998;65:S74–78. [PubMed] [Google Scholar]
- 19.Nangaku M, Inagi R, Miyata T, Fujita T. Hypoxia and Hypoxia-Inducible Factor in Renal Disease. Nephron Exp Nephrol. 2008;110:e1–e7. doi: 10.1159/000148256. [DOI] [PubMed] [Google Scholar]
- 20.Wilcox CS. Use of angiotensin-converting-enzyme inhibitors for diagnosing renovascular hypertension. Kidney Int. 1993;44:1379–1390. doi: 10.1038/ki.1993.392. [DOI] [PubMed] [Google Scholar]
- 21.Liss P, Nygren A, Revsbech NP, Ulfendahl H. Intrarenal oxygen tension measured by a modified Clark electrode at normal and low blood pressure and after injection of x-ray contrast media. Pflügers Arch. 1997;434:705–711. doi: 10.1007/s004240050455. [DOI] [PubMed] [Google Scholar]
- 22.Guron GS, Grimberg ES, Basu S, Herlitz H. Acute effects of the superoxide dismutase mimetic tempol on split kidney function in two-kidney one-clip hypertensive rats. J Hypertens. 2006;24:387–394. doi: 10.1097/01.hjh.0000200511.02700.99. [DOI] [PubMed] [Google Scholar]
- 23.Dobrian AD, Schriver SD, Prewitt RL. Role of angiotensin II and free radicals in blood pressure regulation in a rat model of renal hypertension. Hypertension. 2001;38:361–366. doi: 10.1161/01.hyp.38.3.361. [DOI] [PubMed] [Google Scholar]
- 24.Christensen FH, Stankevicius E, Hansen T, Jorgensen MM, Valverde VL, Simonsen U, Buus NH. Flow- and acetylcholine-induced dilatation in small arteries from rats with renovascular hypertension--effect of tempol treatment. Eur J Pharmacol. 2007;566:160–166. doi: 10.1016/j.ejphar.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 25.Lassen NA, Munck O, Thaysen JH. Oxygen consumption and sodium reabsorption in the kidney. Acta Physiol Scand. 1961;51:371–384. doi: 10.1111/j.1748-1716.1961.tb02147.x. [DOI] [PubMed] [Google Scholar]
- 26.Jackson B, Johnston CI. Angiotensin-converting enzyme inhibition in renal disease; contrasting effects on renal function in renal artery stenosis and progressive renal injury. J Hum Hypertens. 1989;3 (Suppl 1):107–115. [PubMed] [Google Scholar]
- 27.Koivisto A, Matthias A, Bronnikov G, Nedergaard J. Kinetics of the inhibition of mitochondrial respiration by NO. FEBS Lett. 1997;417:75–80. doi: 10.1016/s0014-5793(97)01258-1. [DOI] [PubMed] [Google Scholar]
- 28.Koivisto A, Pittner J, Froelich M, Persson AE. Oxygen-dependent inhibition of respiration in isolated renal tubules by nitric oxide. Kidney Int. 1999;55:2368–2375. doi: 10.1046/j.1523-1755.1999.00474.x. [DOI] [PubMed] [Google Scholar]
- 29.Chade AR, Krier JD, Rodriguez-Porcel M, Breen JF, McKusick MA, Lerman A, Lerman LO. Comparison of acute and chronic antioxidant interventions in experimental renovascular disease. Am J Physiol Renal Physiol. 2004;286:F1079–1086. doi: 10.1152/ajprenal.00385.2003. [DOI] [PubMed] [Google Scholar]
- 30.Chade AR, Bentley MD, Zhu X, Rodriguez-Porcel M, Niemeyer S, Amores-Arriaga B, Napoli C, Ritman EL, Lerman A, Lerman LO. Antioxidant intervention prevents renal neovascularization in hypercholesterolemic pigs. J Am Soc Nephrol. 2004;15:1816–1825. doi: 10.1097/01.asn.0000130428.85603.6b. [DOI] [PubMed] [Google Scholar]
- 31.Hansell P. Evaluation of methods for estimating renal medullary blood flow. Renal Physiol Biochem. 1992;15:217–230. doi: 10.1159/000173457. [DOI] [PubMed] [Google Scholar]
- 32.Welch WJ, Wilcox CS. AT1 receptor antagonist combats oxidative stress and restores nitric oxide signaling in the SHR. Kidney Int. 2001;59:1257–1263. doi: 10.1046/j.1523-1755.2001.0590041257.x. [DOI] [PubMed] [Google Scholar]
- 33.Odum J, Purkiss J, Naish PF. Influence of differing classes of antihypertensive agents on mesangial kinetics following partial renal ablation in rats. Nephrol Dial Transplant. 1994;9:131–137. [PubMed] [Google Scholar]
- 34.Sanz-Rosa D, Cediel E, de las Heras N, Miana M, Balfagon G, Lahera V, Cachofeiro V. Participation of aldosterone in the vascular inflammatory response of spontaneously hypertensive rats: role of the NFkappaB/IkappaB system. J Hypertens. 2005;23:1167–1172. doi: 10.1097/01.hjh.0000170379.08214.5a. [DOI] [PubMed] [Google Scholar]
- 35.Vanourkova Z, Kramer HJ, Huskova Z, Vaneckova I, Opocensky M, Chabova VC, Tesar V, Skaroupkova P, Thumova M, Dohnalova M, Mullins JJ, Cervenka L. AT1 receptor blockade is superior to conventional triple therapy in protecting against end-organ damage in Cyp1a1-Ren-2 transgenic rats with inducible hypertension. J Hypertens. 2006;24:2465–2472. doi: 10.1097/01.hjh.0000251909.00923.22. [DOI] [PubMed] [Google Scholar]
- 36.Kobori H, Ozawa Y, Suzaki Y, Nishiyama A. Enhanced intrarenal angiotensinogen contributes to early renal injury in spontaneously hypertensive rats. J Am Soc Nephrol. 2005;16:2073–2080. doi: 10.1681/ASN.2004080676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klanke B, Cordasic N, Hartner A, Schmieder RE, Veelken R, Hilgers KF. Blood pressure versus direct mineralocorticoid effects on kidney inflammation and fibrosis in DOCA-salt hypertension. Nephrol Dial Transplant. 2008;23:3456–3463. doi: 10.1093/ndt/gfn301. [DOI] [PubMed] [Google Scholar]
- 38.van Jaarsveld BC, Krijnen P, Pieterman H, Derkx FH, Deinum J, Postma CT, Dees A, Woittiez AJ, Bartelink AK, Man in ‘t Veld AJ, Schalekamp MA. The effect of balloon angioplasty on hypertension in atherosclerotic renal-artery stenosis. Dutch Renal Artery Stenosis Intervention Cooperative Study Group. N Engl J Med. 2000;342:1007–1014. doi: 10.1056/NEJM200004063421403. [DOI] [PubMed] [Google Scholar]
- 39.Dworkin LW, Wilcox CS. Medical management of patients with renal artery stenosis. In: Wilcox CS, editor. Therapy in Nephrology and Hypertension. Philadelphia: Saunders and Elsevier; 2008. pp. 647–659. [Google Scholar]
- 40.Fine LG, Bandyopadhay D, Norman JT. Is there a common mechanism for the progression of different types of renal diseases other than proteinuria? Towards the unifying theme of chronic hypoxia. Kidney Int Suppl. 2000;75:S22–26. [PubMed] [Google Scholar]