Abstract
Objective
We present results of a feasibility test of a sequential treatment strategy using continuation phase cognitive-behavioral therapy (CBT) to prevent relapse in youths with major depressive disorder (MDD) who have responded to acute phase pharmacotherapy.
Method
Forty-six youths (ages 11–18 years) who had responded to 12 weeks of treatment with fluoxetine were randomized to receive either 6 months of continued antidepressant medication management (MM) or antidepressant MM plus relapse prevention CBT (MM+CBT). Primary outcome was time to relapse, defined as a Childhood Depression Rating Scale-Revised score of 40 or higher and 2 weeks of symptom worsening or clinical deterioration warranting alteration of treatment to prevent full relapse.
Results
Cox proportional hazards regression, adjusting for depression severity at randomization and for the hazard of relapsing by age across the trial, revealed that participants in the MM treatment group had a significantly greater risk for relapse than those in the MM+CBT treatment group (hazard ratio = 8.80; 95% confidence interval 1.01–76.89; χ2 = 3.86, p = .049) during 6 months of continuation treatment. In addition, patient satisfaction was significantly higher in the MM+CBT group. No differences were found between the two treatment groups on attrition rate, serious adverse events, and overall global functioning.
Conclusions
These preliminary results suggest that continuation phase CBT reduces the risk for relapse by eightfold compared with pharmacotherapy responders who received antidepressant medication alone during the 6-month continuation phase.
Keywords: depression, CBT, relapse prevention, sequential treatment
Acute phase psychopharmacological and psychosocial treatment for pediatric major depressive disorder (MDD) has been shown to be effective in reducing symptoms,1–3 but relapse rates in this population range from 34% to 75%.4 In addition, even with the most effective treatments available (e.g., combination psychopharmacology, psychosocial treatments), residual symptoms are common, and remission rates are low.5 Therefore, it is essential to develop effective therapies in the continuation phase of treatment that will lengthen remission and prevent relapse.
The literature on relapse and recurrence prevention for children and adolescents is limited. In a small preliminary study (n = 40), fewer fluoxetine-treated participants (34%) than pill placebo–treated participants (60%) met criteria for relapse. Furthermore, time to relapse was shorter for the placebo group than the fluoxetine group (71.2 ± 9.5 days versus 180.7 ± 17.0 days, respectively; p = .046).6 More recently, in a randomized controlled trial, comparing continuation phase fluoxetine versus pill placebo over 6 months, 42.0% of fluoxetine responders who were randomized to continuation phase fluoxetine relapsed compared with 69.2% who were treated with continuation phase pill placebo.7 The patients treated with placebo had a significantly shorter time to relapse than those who remained taking fluoxetine. However, as noted, more than 40% of patients relapsed over 6 months despite continuing antidepressant treatment.7 This high rate of relapse warrants the development of additional strategies to prevent relapse.
Adult studies have demonstrated that cognitive-behavioral therapy (CBT) therapy delivered in both the acute phase and the continuation phase of treatment can successfully reduce depressive relapse.8,9 Continuation phase cognitive therapy10 significantly reduced the risk of relapse in adults who responded to acute phase cognitive therapy.11,12 Furthermore, the combination of CBT and antidepressant medication delivered as continuation phase treatment after response to acute phase pharmacotherapy has been shown to improve outcomes, including reducing rates of relapse and recurrence in adults.13–18 The rationale for a sequential treatment strategy is to improve symptoms (e.g., mood, concentration, energy) through the use of antidepressant medication and then introduce a psychosocial component in the continuation phase to optimize treatment gains. Patients may be more likely to be receptive to the benefits of the psychosocial treatment because of reduced depressive symptoms and improved clinical status. In addition, psychosocial treatment aids in the reduction of residual symptoms and in the prevention of future relapse.14,15 Of note, augmenting depression treatment as a “second-step strategy” with cognitive therapy also seems to be as effective as augmenting with pharmacotherapy in increasing levels of remission in nonresponders to acute treatment with antidepressant medication.19
To date, there are two studies evaluating continuation phase CBT to prevent relapse in youth. Kroll et al.,20 in a pilot study of continuation phase CBT, added 6 months of continuation phase CBT after acute CBT treatment. During 6 months, continuation phase CBT significantly lowered relapse (6%) compared with historical controls (50%). Clarke and coworkers21 reported inconclusive findings regarding the efficacy of continuation phase treatment in depressed adolescents treated for 8 weeks in the acute phase with adolescent group CBT, adolescent group CBT with a separate parent group, or waitlist control. All of the patients who completed treatment, regardless of response, were randomized to assessments quarterly, assessments quarterly plus booster sessions, or annual assessments for 24 months. Booster sessions did not reduce relapse; however, booster treatment did accelerate remission in patients with depression at the end of the acute phase.21 One limitation of the design is that only one to two continuation phase sessions were provided during 24 months; this “dose” may be insufficient in preventing relapse in vulnerable youths. In addition, this study randomized acute phase completers, regardless of response status. Both of these studies focused on relapse rates during and after continuation phase CBT that followed acute CBT treatment. We are not aware of any studies of sequential treatment of pediatric responders to acute phase pharmacotherapy for MDD followed by continuation phase CBT.
Noting the high burden of illness associated with depression and the high rates of recurrence of depression in youths, Costello and coworkers,22 as part of a National Institute of Mental Health (NIMH) workgroup in mood disorders research, recommended a focus on continuation and maintenance studies of medication and psychotherapy. Can the effective adult continuation treatment strategies of augmenting antidepressant treatment with CBT be applied to youths to prevent relapse of depression? Although there are no published reports on the augmentation of initial CBT treatment with medication treatment in depressed youths, results from the Treatment of Resistant Depression in Adolescents study indicate that adding CBT to antidepressant medication after a failed trial of medication is superior to continued medication alone in adolescents with treatment-resistant depression.23 An efficacious sequential treatment, such as a continuation phase CBT, that can be successfully delivered after response to acute anti-depressant treatment would represent an important contribution to the field. To that end, we developed relapse prevention CBT (RP-CBT), which focuses on reducing residual depressive symptoms and promoting wellness as ameans of reducing risk for depressive relapse.24
In the present study, we present the primary outcomes of the feasibility study of RP-CBT.24 In this pilot study, adolescents who had responded to acute antidepressant treatment were randomized to continuation treatment (24 weeks) with medication management (MM) or MM and RP-CBT (MM+CBT).
METHOD
The present study was an NIMH-funded feasibility trial of a novel continuation phase CBT designed to prevent relapse in adolescents whose depression had improved after acute treatment with antidepressant medication. The experimental treatment was continuation phase CBT plus the antidepressant medication fluoxetine (MM+CBT) compared with the control group of continuation phase antidepressant treatment with fluoxetine (MM). The study was approved by the University of Texas Southwestern Medical Center at Dallas Institutional Review Board. Before conducting any study procedures, participants and parents were informed of the purpose, participant rights, study procedures, and risks and benefits of the study, and all questions were answered. All of the participants and their parents provided written informed consent and assent.
Procedures
Treatment Development
Relapse prevention CBT was supported in part by an NIMH R34 treatment development grant. The RPCBT approach is twofold—targeting residual symptoms that remain after an adequate treatment response, and identifying and enhancing current strengths to promote wellness. The stages of treatment development and a specific description of the treatment are presented elsewhere.24
Study Participants
Recruitment was conducted through clinical referrals to a general child and adolescent psychiatry outpatient clinic, as well as through radio and print advertisements. Participants were young outpatients, ages 11 to 18 years, with a primary diagnosis of MDD for at least 4 weeks, a Children’s Depression Rating Scale-Revised (CDRS-R)25 total score of 40 or higher and a Clinical Global Impression Severity (CGI-S)26 score of 4 or higher. Concurrent psychiatric diagnoses were not exclusionary if secondary to MDD, with the exception of lifetime history of any psychotic disorder (including psychotic depression), bipolar disorder, anorexia nervosa, or bulimia, or history of alcohol or substance abuse within 6 months. Participants were required to be in good general medical health and of normal intelligence as determined by clinician judgment. Patients were excluded if they had a concurrent medical condition that would interfere with the study or endanger themselves; were pregnant or lactating females not using adequate contraception; had first-degree relatives with bipolar I disorder; had severe suicidal ideation (active ideation with plan and intent) requiring inpatient treatment; had previous failure of or intolerance to fluoxetine; or were currently taking other psychotropic medications, other than stable attention-deficit/hyperactivity disorder (ADHD) treatment. Because of the length of the study, ADHD treatment was allowed. No changes in ADHD treatment were permitted after randomization.
Evaluation
An initial telephone screen reviewing inclusion and exclusion criteria was conducted with a potential participant’s parent; eligible participants were scheduled for a diagnostic interview. After consent and assent, an evaluator interviewed parents and patients separately using the Kiddie Schedule for Affective Disorders and Schizophrenia (K-SADS-PL)27 to determine whether the child met criteria for the study. The evaluator obtained family psychiatric history using the Family History Research Diagnostic Criteria.28 One week later, a psychiatrist or licensed psychologist interviewed the participant and parent to determine course and severity of depressive illness using the K-SADS-PL, CDRS-R, and CGI-S. The participants who continued to have a CDRS-R score of 40 or higher and who met all inclusion criteria and no exclusion criteria were scheduled to start acute treatment within 5 to 10 days (baseline visit). The research coordinator obtained socioeconomic status through parent-report of occupation level and education level; this information was used to calculate the Hollingshead four/factor index of social status.29
Acute Phase Treatment
The acute phase treatment consisted of 12 weeks of pharmacotherapy. The participants began open treatment with fluoxetine at the baseline visit (week 0), with an initial dose of 10 mg/day for 1 week, after which dosage was increased to 20 mg/day. After 6 weeks of treatment, dosing could be increased to 30 to 40 mg if there was minimal or no response (i.e., CGI ≥3). Fluoxetine could be reduced to 10 mg if intolerable side effects were present. The child and adolescent psychiatrist conducted pharmacotherapy visits weekly for weeks 1 to 4 and every other week until week 12 (8 total visits of acute phase pharmacotherapy). These visits included supportive clinical management (e.g., contact with schools, referrals for treatment for family members). No concomitant psychotherapy or psychotropic medications (other than ADHD treatment) were allowed during the study, including the continuation phase. Participants were discontinued if they exhibited poor adherence (e.g., did not regularly attend visits or were not compliant with medication) or if they had a poor response to fluoxetine.
Although the above methodology was used for 91.6% (66/72) of acute phase participants, 6 additional participants were recruited directly at the point of randomization (i.e., after adequate acute medication treatment). Adolescents who were currently being treated with a selective serotonin reuptake inhibitor (SSRI) were screened by telephone and evaluated by an independent evaluator (IE) after written informed consent and assent. Study inclusion and exclusion criteria were applied to these participants. Diagnosis was made using the K-SADS-PL, and baseline CDRS-R and CGI-S were rated based on worst of episode. Randomization CDRS-R and CGI-S were rated based on the past 2 weeks (consistent with participants who received acute treatment through the study). Participants already being treated with an SSRI were required to be on treatment for a minimum of 12 weeks and no more than 18 weeks. Although dose was not controlled during acute treatment, participants were required to be taking an adequate dose before randomization. The evaluator determined response to treatment based on the clinical interview with the adolescent and the parent, as well as through consultation with the treating community physician.
Randomization
After 12 weeks of acute phase fluoxetine treatment, participants were assessed for treatment response by an IE. Nonresponders at week 12 were discontinued from the study and given treatment referrals. Participants who had an adequate treatment response, defined as a CGI-Improvement score of 1 (very much improved) or 2 (much improved), and at least 50% reduction in depressive symptoms on the CDRS-R were randomized to continued MM or continued MM plus RP-CBT (MM+CBT). Thus, randomization was based on symptom improvement (treatment response) and not remission of depressive illness (absence of symptoms).
Randomization was stratified by two levels of response: adequate responders, as previously defined (participants with a CGI score of 1 or 2 and a 50% reduction in symptoms), and remitters, defined as having a CDRS-R score of 28 or lower in addition to the above response criteria. Randomization was also stratified by age: younger (11–14 years) and older (15 years and older).
Continuation Phase Treatment
All randomized participants (MM and MM+CBT) were evaluated by the psychiatrist every other week for weeks 12 to 16 and monthly from weeks 16 to 36, with additional visits allowed as needed. Participants were maintained on the medication dose to which they responded because this approach is typical of continuation phase pharmacotherapy treatment.30 No changes in antidepressant medication dosage were allowed after randomization. The protocol included a planned discontinuation of medication for both groups at week 24, if not clinically contraindicated (i.e., participant, family, and/or psychiatrist believed it was clinically necessary to continue medication treatment). It has been recommended that youths receive 6 to 9 months of overall treatment for MDD.7 This planned discontinuation is consistent with treatment depression guidelines in both adult and pediatric depression.
Participants randomized to MM+CBT began CBT sessions at week 13. These participants received a total of 8 to 11 sessions of continuation phase CBT over a 6-month period. Generally, the sessions occurred weekly for 4 weeks, biweekly for 2 months, and optional monthly booster sessions for 3 months. Sessions were typically 1 hour in length, the exception being sessions one and two, which were 1.5 hours in length and included a family component. Family sessions were included as part of the CBT protocol, with a minimum of three family sessions.
The CBT therapists included one doctoral/level psychologist (B.D.K.), one masters/level psychologist (J.M.J.), and one post-doctoral psychology fellow (Avery Hoenig). Training of the therapists included weekly case conferences and manual review for 6 months before the first patient began therapy. After that time, weekly supervision of therapists was conducted by the senior author. For quality control purposes, all sessions were audiorecorded, and 20.8% (41 tapes) of these tapes were randomly selected to be rated by masters- or doctoral-level therapists on the Cognitive Therapy Rating Scale.32 One hundred percent of selected tapes were rated as acceptable (Cognitive Therapy Rating Scale ≥40).
Outcome Measures
Primary outcome measures were completed at weeks 12 (randomization baseline), 24, and 36 by IEs who were blind to treatment assignment. The IEs in this trial were experienced clinicians with at least 5 years of experience conducting diagnostic and severity ratings on youths in clinical depression trials. All had received training in at least one outside investigator meeting, and all had been certified through multisite depression trials.
Relapse
Time to relapse was the primary outcome variable. We also included significant clinical deterioration (as recommended by Rush et al.32) as an outcome, given that it is not ethical to withhold treatment in controlled trials until the patient has had a complete relapse. Thus, we defined relapse in two ways: a one-time CDRS-R score of 40 or higher with a history of 2 weeks of symptom worsening based on patient and parent report or clinical history, and clinical deterioration in which the CDRS-R score was less than 40, but the clinician noted significant deterioration that would suggest full relapse if the patient’s treatment was not altered. Thus, for the present study, relapse status was a binomial outcome variable operationally defined as “relapse” or “nonrelapse” based on a score of depression severity.
Functioning, Feasibility, Satisfaction, and Safety
Additional outcomes included depression severity and functioning, feasibility (attrition, medication discontinuation during continuation phase treatment, and client satisfaction), and safety. Depression severity was measured throughout the study using the CDRS-R and CGI. The CGI was used to measure acute treatment response (which determined randomization). The CDRS-R score was used to measure remission and relapse. This measure was completed by the initial diagnostic interviewer at screening, by the treating psychiatrist at each visit (during the acute and continuation phases), and by the IE at each assessment point (weeks 12, 24, and 36). On the CDRSR, a score of 40 or higher is generally considered to indicate the presence of MDD.25 Global functioning was based on the Children’s Global Assessment Scale (CGAS),33 which requires the IE to assess the participant’s overall functioning on a scale of 0 to 100, with higher scores indicating greater functioning. Patient severity and global functioning were obtained by the IE by interviewing both the patient and the parent independently and making a clinical decision for the rating based on these two interviews (i.e., the summary score for the CDRS-R).
Attrition was calculated based on the frequency of dropout, unrelated to relapse outcomes. Medication discontinuation was planned at week 24. Participants, the family, and the child and adolescent psychiatrist decided whether to continue medication based on clinical need. This decision was documented by the study coordinator. Client satisfaction was measured by the Client Satisfaction Questionnaire-8,34,35 an eight-item scale on which patients and parents rate their satisfaction with treatment on a scale of 1 to 4, with higher ratings being indicative of higher satisfaction. Safety was assessed through serious adverse event (SAE) recording. Serious adverse events were tracked throughout acute and continuation treatment. The SAEs were defined according to the Food and Drug Administration criteria, which included any adverse event that led to death; was life threatening; required hospitalization; led to disability, congenital anomaly, or birth defect; or required intervention to prevent permanent impairment or damage, or other important medical events that required medical or surgical intervention (e.g., failed suicide attempt).
Data Analysis
Acute phase baseline demographic and clinical characteristics were compared between the MM and MM+CBT treatment groups using an independent sample t test (for continuous outcomes) and a likelihood ratio χ2 (for categorical outcomes). Similarly, baseline characteristics were compared between participants who entered the continuation phase and those who did not enter the continuation phase.
A Cox proportional hazards regression, with adjustment for CDRS-R total score at randomization and for the hazard of relapsing by age across the trial (i.e., absorbing age in the model), was used to compare time to relapse between the participants in the MM group and those in the MM+CBT group. Hazard ratios were also estimated. As part of the survival analysis, right censoring was used in the present study. Censoring occurred when incomplete information was available about the survival time (or time to relapse) of a given participant (the information was incomplete because the participant did not have an event during the time that the participant was part of the study). In our study, censoring (or a censored observation) meant a participant who dropped out of the study without relapsing or who completed the trial without relapsing. Overall, 73.9% of the participants in the present trial were censored. Approximately 86.3% of the participants in the MM+CBT group were censored, whereas approximately 62.5% of those in the MM group were censored.
Additional outcomes of global functioning (CGAS), CDRS-R at exit, satisfaction with treatment (Client Satisfaction Questionnaire-8, patient, parent) were compared between the two treatment groups using an independent sample t test. Categorical measures of rate of attrition and rate of discontinuation of medication after 24 weeks were also compared between the two treatment groups using the χ2 statistic.
All analyses were intent-to-treat. The level of significance for all tests was set at p ≤ .05.
RESULTS
Participant Characteristics
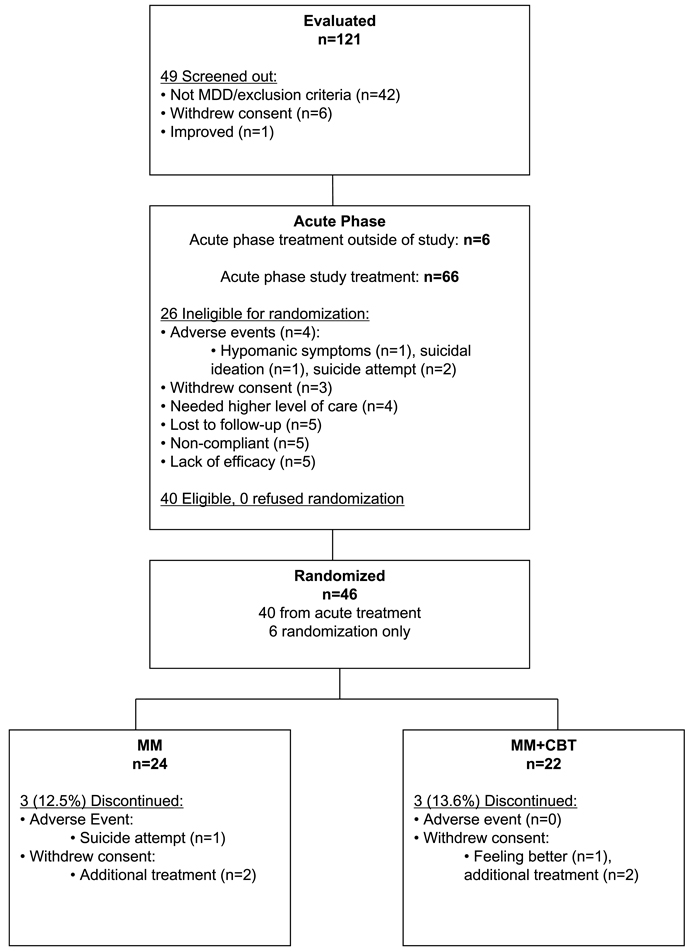
One hundred twenty-one patients were screened for the study, and 66 patients were entered into acute treatment (Fig. 1). Of these, 40 were eligible for randomization; all 40 agreed to randomization. In addition, 6 adolescents already taking SSRI and responding to treatment were screened and entered through the randomization-only option. Thus, 46 total participants were randomized. Demographic and clinical characteristics were similar between those who were randomized (n = 46) and those who were not (n = 26; results not shown). Furthermore, within the 46 randomized participants, there were no significant differences between those who received acute treatment through the study protocol (n = 40) and those who entered at the point of randomization (n = 6) on acute baseline demographics, depression severity (CDRS-R, illness duration), or CDRS-R at randomization baseline (results not shown).
Fig. 1.
Consort diagram.
Forty-six participants were randomized to MM (n = 24) or MM+CBT (n = 22). The continuation phase sample consisted of 24 male (52.2%) and 22 female participants (47.8%). Participants’ ages ranged from 11 to 18 years, with an average age of 14.3 years (SD 1.9 years). The majority of participants (n = 34, 73.9%) were white. The average age of onset of the current major depressive episode was 13.4 years (SD 2.1 years). Two thirds of the adolescents had a comorbid psychiatric disorder, and there were no significant differences between the MM and MM+CBT treatment groups on number of comorbid diagnoses at baseline (t = 0.69, p = .49). Approximately 48% of the total continuation phase sample had a positive maternal history of depression, whereas approximately 18% had a positive paternal history of depression.
The average CDRS-R total score at acute baseline (week 0) was 58.0 (SD 9.1). The average CDRS-R total score at randomization baseline (week 12) was 26.6 (SD 5.2). There were no significant differences between the MM and MM+CBT groups on CDRS-R total score at acute baseline (t = 1.49, p = .14) or at randomization baseline (t = 0.11, p = .91). Medication dosage (milligrams of fluoxetine) at the time of randomization was similar (MM = 26.67, SD 10.07; MM+CBT = 30.0, SD 9.26, t = −1.17, p = .25). Dosages for the two subjects who were taking a different SSRI antidepressant medication were converted to equivalent doses of fluoxetine.
Demographic and clinical characteristics for participants who were randomized (N = 46) are shown in Table 1; participant characteristics were similar between the MM and MM+CBT treatment groups. During continuation phase treatment, the MM participants attended an average of 5.6 visits (range 1–9), whereas MM+CBT participants attended an average of 5.8 MM visits (range 1–10) and an average of 8.98 CBT visits (range 0–14).
TABLE 1.
Demographic and Baseline Clinical Characteristics
| Participant Characteristics | Randomized Sample (n = 46) | Treatment Group | Test Statistic |
pa | |
|---|---|---|---|---|---|
| MM (n = 24) | MM+CBT (n = 22) | ||||
| Demographics | |||||
| Age, mean ± SD, y | 14.3 ± 1.9 | 14.4 ± 2.2 | 14.3 ± 1.7 | t = 0.17 | .86 |
| Sex, % (n) | χ2 = 0.09 | .75 | |||
| Male | 52.2 (24) | 50.0 (12) | 54.5 (12) | ||
| Female | 47.8 (22) | 50.0 (12) | 45.5 (10) | ||
| Race, % (n) | χ2= 1.38 | .24 | |||
| White | 73.9 (34) | 66.7 (16) | 81.8 (18) | ||
| Nonwhite | 26.1 (12) | 33.3 (8) | 18.2 (4) | ||
| Family incomeb | χ2 = 0.07 | .79 | |||
| $12,500–$24,999 | 8.3 (3) | 13.3 (2) | 4.8 (1) | ||
| $25,000–$49,999 | 27.8 (10) | 20.0 (3) | 33.3 (7) | ||
| $50,000–$69,999 | 13.9 (5) | 20.0 (3) | 9.5 (2) | ||
| $70,000 and up | 50.0 (18) | 46.7 (7) | 52.4 (11) | ||
| SES scale, (range 8–66), % (n)c | χ2 = 1.19 | .27 | |||
| Score 8–39 (categories 3 to 5) | 30.4 (14) | 37.5 (9) | 22.7 (5) | ||
| Score 40–66 (categories 1 and 2) | 69.6 (32) | 62.5 (15) | 77.3 (17) | ||
| SES raw score, mean ± SDc | 46.5 ± 13.7 | 44.9 ± 14.2 | 48.3 ± 13.2 | t = 0.83 | .41 |
| Clinical characteristics | |||||
| CDRS-R total at screening, mean ± SDd | 58.0 ± 9.1 | 59.9 ± 8.3 | 55.9 ± 9.6 | t = 1.49 | .14 |
| Randomization baseline (week 12) CDRS-R | 26.6 ± 5.2 | 26.7 ± 5.1 | 26.5 ± 5.4 | t = 0.11 | .91 |
| Total, mean ± SDe | |||||
| Current episode length (weeks), mean ± SD | 31.6 ± 26.3 | 35.0 ± 27.4 | 28.0 ± 25.1 | t = 0.90 | .37 |
| No. episodes, mean ± SD | 1.2 ± 0.49 | 1.2 ± 0.41 | 1.3 ± 0.56 | t = 0.75 | .45 |
| No. comorbidities, mean ± SD | 0.95 ± 0.86 | 1.04 ± 0.90 | 0.86 ± 0.83 | t = 0.69 | .49 |
| Age of onset, current episode, mean ± SD | 13.4 ± 2.1 | 13.3 ± 2.2 | 13.5 ± 1.9 | t = 0.20 | .84 |
| Positive maternal history of depression, %(n)f | 47.7 (21) | 45.5 (10) | 50.0 (11) | χ2 = 0.09 | .76 |
| Positive paternal history of depression, %(n)f | 18.2 (8) | 27.3 (6) | 9.1 (2) | χ2 = 2.53 | .11 |
Note: MM= antidepressant medication management; MM+CBT = antidepressant medication management plus relapse prevention cognitive-behavioral therapy; SES = socioeconomic status; CDRS-R: Children’s Depression Rating Scale-Revised. The means presented in this table are the arithmetic means; n = participants per group (total sample size 46 participants); nonwhite for the MM group = African American, Hispanic, Other (self-identified race/ethnicity), and nonwhite for the MM+CBT group = Hispanic.
Tested for differences between the MM and MM+CBT treatment groups on each demographic/clinical characteristic in a separate model.
Ten missing observations.
SES scale based on Hollingshead30 four-factor index.
Measured (at screening) before week 0.
Measured (at week 12) before randomizing to MM or MM+CBT.
Two missing observations associated with the MM group.
Hazard of Relapse
The primary outcome (time to relapse) was evaluated using a Cox proportional hazards regression, with adjustment for CDRS-R total score at randomization and for the hazard of relapsing by age across the trial (i.e., absorbing age in the model). The Cox proportional hazards regression revealed that participants in the MM treatment group had a significantly greater risk for relapse than those in the MM+CBT treatment group (hazard ratio 8.80; 95% confidence interval 1.01–76.89; χ2 = 3.86, p = .049). After adjustment for these (continuously measured) covariates, the hazard of relapse for those who received MM treatment was approximately eight times greater than the hazard of relapse for those who received MM+CBT treatment.
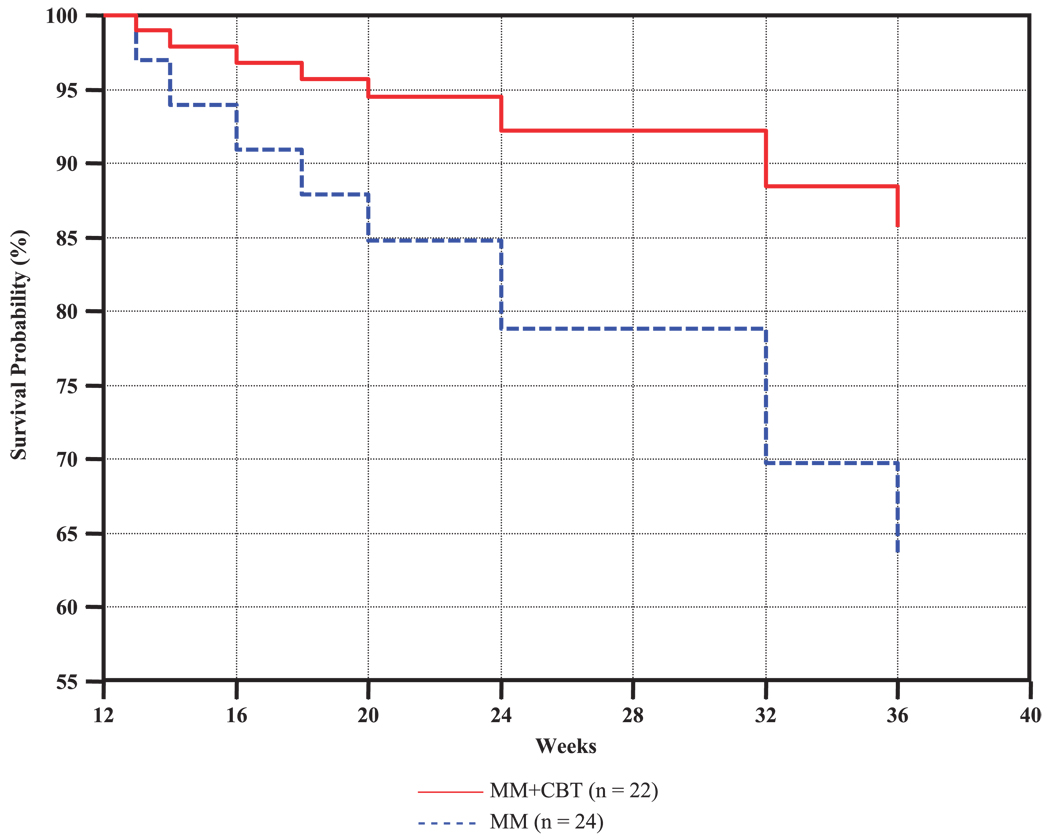
Figure 2 displays the survival curves, plotted at the mean of all covariates in the model, and indicates that the survival probability (of not relapsing) is greater at all periods across the trial for the MM+CBT group than for the MM group. As shown in Figure 2, at weeks 16, 20, 24, and 36 (or within 4, 8, 12, and 24 weeks of radomization, respectively), the estimated probability of relapse for the MM group was approximately 8%, 15%, 21%, and 37%, respectively, compared with approximately 4%, 5%, 8%, and 15%, respectively, for the MM+CBT group.
Fig. 2.
Survival curves, plotted at the mean of all covariates in the model, showing the survival probability (of not relapsing) at a given week across the trial for the MM+CBT and MM treatment groups. The survival model, comparing MM+CBT with MM, included the following continuously measured covariates: CDRS-R total score at randomization and age across the trial. CDRS-R = Children’s Depression Rating Scale-Revised; MM= antidepressant medication management; MM+CBT = antidepressant medication management plus relapse prevention cognitive-behavioral therapy.
Functioning, Feasibility, Satisfaction, and Safety
Functioning
Table 2 presents depression severity (CDRS-R) and global functioning (CGAS) results at the end of treatment. There were no differences in depression severity ratings or overall global functioning as assessed by the IE at week 36 or exit (CDRS-R: t = 1.7, p = .09, CGAS: t = .42, p = .68).
TABLE 2.
Functioning, Feasibility, and Satisfaction Outcomes
| MM (n = 24) |
MM+CBT (n = 22) |
Test Statistic |
p | |
|---|---|---|---|---|
| Mean ± SD | Mean ± SD | |||
| CGAS | 63.5 ± 10.1 | 64.8 ± 9.7 | t = −0.42 | .68 |
| CDRS-R | 33.6 ± 14.1 | 27.4 ± 8.9 | t = 1.72 | .09 |
| CSQ-8, patient | 3.58 ± 0.43 | 3.98 ± 0.05 | t = −3.82 | .001 |
| CSQ-8, parent | 3.67 ± 0.42 | 3.88 ± 0.42 | t = −1.80 | .09 |
| Percent (n) | Percent (n) | |||
| Attrition | 8.3 (2) | 13.6 (3) | χ2 = 0.28 | .60 |
| Able to | 29.2 (7) | 36.4 (8) | χ2 = 0.27 | .60 |
| discontinue | ||||
| medication |
Note: CGAS = Children’s Global Assessment Scale; CDRS-R = Children’s Depression Rating Scale-Revised; CSQ-8 = Client Satisfaction Questionnaire-8; MM = antidepressant medication management; MM+CBT = antidepressant medication management plus relapse prevention cognitive-behavior therapy.
Feasibility
Overall, there was a low dropout rate in this continuation phase study (5/46; 11%). Figure 1 includes the number of participants that discontinued continuation phase treatment, unrelated to relapse status. In the MM group, 12.5% (3/24) of the patients discontinued study treatment, compared with 13.6% (3/22) in the MM+CBT group (p = .60). In the MM group, one participant dropped out because of a suicide attempt, and two withdrew consent to obtain psychosocial treatment. In the MM+CBT group, one withdrew consent because of improvement, and two withdrew consent to obtain additional types of treatment.
There were no differences between the MM and MM+CBT groups on discontinuation of medication (p = .60). At week 24, all participants were scheduled to have medication discontinued, although medication was allowed to continue if clinically indicated. Only approximately one third were able to discontinue their medications, with 29% (7/24) of the MM group and 36% (8/22) of the MM+CBT group discontinuing medication (χ21 = .27, p = .60). Relapse rates were assessed between those who discontinued medication and those who did not, regardless of treatment assignment, and relapse rates were not associated with medication discontinuation status.
Satisfaction
Patient satisfaction levels indicate higher levels of satisfaction in participants who received the MM+CBT treatment compared with the MM-only group (p = .001; Table 2). Parent-reported satisfaction levels between the two treatment groups was not statistically significant (p = .09) but did show similar results. Those parents whose children received the CBT reported higher levels of satisfaction than those parents whose children received medication only.
Safety
Four participants experienced an SAE during continuation phase treatment. Two events occurred in the MM-only group (both suicide attempts, one of which resulted in hospitalization); and two, in the MM+CBT group (hospitalization for diabetic ketoacidosis and a suicide attempt that did not result in hospitalization). These events were reported to the University of Texas Southwestern Medical Center at Dallas IRB and the study data safety monitoring board (DSMB).
DISCUSSION
This article presents the first randomized controlled pilot study of a sequential treatment strategy in youths with depression. The addition of continuation phase CBT after response to acute phase pharmacotherapy significantly lengthened time to relapse. Adolescents who did not receive continuation phase CBT had an eightfold greater risk for relapse than those who received CBT. Estimated probabilities of relapse at 36 weeks were 37% in the MM group and 15% in the MM+CBT group. Of importance, the probability of relapse in participants remaining only taking medication was similar to the results of Emslie and coworkers,7 which showed that 42% of participants who remained taking fluoxetine after acute treatment experienced a relapse of depression. Certainly, medication alone may not be sufficient in reducing relapse in adolescent depression because more than one third of those youths will have a return of depressive symptoms. This study demonstrated that continuation phase CBT may reduce this risk of depressive relapse.
Because this was a 9-month trial, the investigators had several concerns regarding the feasibility and acceptability of continuation phase treatment in adolescents. One concern was that adolescents would not want to begin a trial of psychotherapy once they had improved with antidepressant treatment or would not continue through the full course of CBT treatment. Although participants had shown improvement and despite an increase in patient burden (i.e., increased number of sessions), patient satisfaction was actually higher in the MM+CBT group than in the MM group. In addition, continuation phase attrition was similar for both treatment groups. There were no differences in medication discontinuation between the two groups. Of note, only one third of the adolescents were able or willing to discontinue medication at week 24 (planned discontinuation).
These findings, as in adult studies, suggest that sequencing treatments (i.e., CBT after antidepressant therapy) seems to be effective in preventing relapse. It is not clear from this study as to when the CBT can be effectively introduced. Waiting 3 months before initiating the CBT treatment is lengthy, particularly for those who have an earlier response to treatment. Certainly, it is important in future studies to determine the best methods for optimizing outcomes.
There are several limitations to the present study. First, this was a small pilot feasibility study. Despite the small sample size, however, the results on the primary outcome (time to relapse) were positive. Another limitation was that assessments were not continued after relapse or withdrawal from treatment. Therefore, it is unknown whether participants who withdrew because of adverse events or withdrawal of consent experienced a relapse of depression. Furthermore, severity and length of depressive relapse episodes are unknown. Finally, there was a difference in clinical contact time between the two groups. In the protocol, we only required two additional visits to the clinic in the MM+ CBT condition to reduce the subject burden of additional clinic appointments on patients and families, and most MM visits were at least 30 to 45 minutes long and consisted of supportive clinical care in addition to symptom review. However, there was more clinical contact time in the MM+CBT condition. We could have added a sham therapy to the MM group as a control for contact time between the two conditions; however, we designed the study to be more consistent with real-world practice.
In conclusion, effective acute phase interventions for youths with depression have been described.1–3 In a recent editorial, Ryan36 emphasized that treatments are needed that maximize remission beyond the achievement of adequate response. Furthermore, the addition of psychotherapy may be a way to achieve greater efficacy of antidepressants.23,36 This pilot study shows that sequencing continuation phase CBT after acute phase medication response may be a promising strategy. At present, the investigators are conducting a larger randomized controlled trial to further evaluate this sequential treatment strategy in youths with depression.
Acknowledgments
Funding for this study was provided by NIMH R34 MH072737 (PI: Betsy Kennard). The authors acknowledge the support of NIMH R01 MH39188 (PI: Graham Emslie), which provided for the start-up phase of this project. The authors also acknowledge the support of NIMH K24 01571 (to Robin Jarrett).
The authors thank Avery Hoenig, Ph.D. (one of the CBT therapists); Puja Patel; Alyssa Parker; Ashley Melson; Jaime Murphy; Jeanne Rintelmann; Jarrette Moore; Kirti Saxena, M.D.; Paul Croarkin, D.O.; and Maryam Rezai, M.D., for valuable contributions. In addition, the authors also thank David Brent, M.D.; Greg Clarke, Ph.D.; John Curry, Ph.D.; Kim Poling, M.S.W.; Kevin Stark, Ph.D.; and his graduate students for careful manual review; and John Rush, M.D., for consultation, and Eric J. Nestler, M.D., Ph.D., for support of the project.
Footnotes
Clinical Trials Registry: Cognitive Behavioral Therapy for Depression Relapse Prevention in Children and Adolescents. URL: http://www.clinicaltrials.gov. Unique identifier: NCT00158301.
Disclosure: Dr. Emslie receives research support from the National Institute of Mental Health, Eli Lilly, Organon, Shire, Somerset, Forest Laboratories, and Biobehavioral Diagnostics; is a consultant for Eli Lilly, Forest Laboratories, GlaxoSmithKline, Wyeth-Ayerst, Shire, and Biobehavioral Diagnostics; and is on the speakers’ bureau for McNeil. The other authors report no conflicts of interests.
REFERENCES
- 1.Cheung AH, Emslie GJ, Mayes TL. Review of the efficacy and safety of antidepressants in youth depression. J Child Psychol Psychiatry. 2005;46:735–754. doi: 10.1111/j.1469-7610.2005.01467.x. [DOI] [PubMed] [Google Scholar]
- 2.TADS Team. Fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents with Depression Study (TADS) randomized controlled trial. JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- 3.Weisz JR, McCarty CA, Valeri SM. Effects of psychotherapy for depression in children and adolescents: a meta-analysis. Psychol Bull. 2006;132:132–149. doi: 10.1037/0033-2909.132.1.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kennard BD, Emslie GJ, Mayes TL, Hughes JL. Relapse and recurrence in pediatric depression. Child Adolesc Psychiatr Clin N Am. 2006;15:1057–1079. doi: 10.1016/j.chc.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Kennard B, Silva S, Vitiello B, et al. The TADS Team. Remission and residual symptoms after short-term treatment in the Treatment of Adolescents with Depression Study (TADS) J Am Acad Child Adolesc Psychiatry. 2006;45:1404–1411. doi: 10.1097/01.chi.0000242228.75516.21. [DOI] [PubMed] [Google Scholar]
- 6.Emslie GJ, Heiligenstein JH, Hoog SL, et al. Fluoxetine treatment for prevention of relapse of depression in children and adolescents: a double-blind, placebo-controlled study. J Am Acad Chil Adolesc Psychiatry. 2004;43:1397–1405. doi: 10.1097/01.chi.0000140453.89323.57. [DOI] [PubMed] [Google Scholar]
- 7.Emslie GJ, Kennard BD, Mayes TL, et al. Fluoxetine versus placebo in preventing relapse of major depression in children and adolescents. Am J Psychiatry. 2008;165:459–467. doi: 10.1176/appi.ajp.2007.07091453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nierenberg AA. Long-term management of chronic depression. J Clin Psychiatry. 2001;62 Suppl 6:17–21. [PubMed] [Google Scholar]
- 9.Vittengl JR, Clark LA, Dunn TW, Jarrett RB. Reducing relapse and recurrence in unipolar depression: a comparative meta-analysis of cognitive therapy’s effects. J Consult Clin Psychol. 2007;75:475–488. doi: 10.1037/0022-006X.75.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jarrett RB, Vittengl JR, Clark LA. Preventing recurrent depression. In: Whisman MA, editor. Adapting Cognitive Therapy for Depression: Managing Complexity and Comorbidity. New York: Guilford Press; 2008. [Google Scholar]
- 11.Jarrett RB, Basco MR, Risser R, et al. Is there a role for continuation phase cognitive therapy for depressed outpatients? J Consult Clin Psychol. 1998;66:1036–1040. doi: 10.1037//0022-006x.66.6.1036. [DOI] [PubMed] [Google Scholar]
- 12.Jarrett RB, Kraft D, Doyle J, Foster BM, Eaves GG, Silver PC. Preventing recurrent depression using cognitive therapy with and without a continuation phase: a randomized controlled trial. Arch Gen Psychiatry. 2001;58:381–388. doi: 10.1001/archpsyc.58.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bockting C, Schene A, Spinhoven P, et al. Preventing relapse/recurrent in recurrent depression with cognitive therapy: a randomized controlled trial. J Consult Clin Psychol. 2005;73:647–657. doi: 10.1037/0022-006X.73.4.647. [DOI] [PubMed] [Google Scholar]
- 14.Fava GA, Grandi S, Zielezny M, Canestrari R, Morphy MA. Cognitive behavioral treatment of residual symptoms in primary major depressive disorder. Am J Psychiatry. 1994;151:1295–1299. doi: 10.1176/ajp.151.9.1295. [DOI] [PubMed] [Google Scholar]
- 15.Fava GA, Grandi S, Zielezny M, Rafanelli C, Canestrari R. Four-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry. 1996;153:945–947. doi: 10.1176/ajp.153.7.945. [DOI] [PubMed] [Google Scholar]
- 16.Fava GA, Rafanelli C, Grandi S, Canestrari R, Morphy MA. Six-year outcome for cognitive behavioral treatment of residual symptoms in major depression. Am J Psychiatry. 1998;155:1443–1445. doi: 10.1176/ajp.155.10.1443. [DOI] [PubMed] [Google Scholar]
- 17.Paykel ES, Scott J, Teasdale JD, et al. Prevention of relapse in residual depression by cognitive therapy: a controlled trial. Arch Gen Psychiatry. 1999;56:829–835. doi: 10.1001/archpsyc.56.9.829. [DOI] [PubMed] [Google Scholar]
- 18.Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68:615–623. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 19.Thase ME, Friedman ES, Biggs MM, et al. Cognitive therapy versus medication in augmentation and switch strategies as second-step treatments: a STAR*D report. Am J Psychiatry. 2007;164:753–760. doi: 10.1176/ajp.2007.164.5.739. [DOI] [PubMed] [Google Scholar]
- 20.Kroll L, Harrington R, Jayson D, Fraser J, Gowers S. Pilot study of continuation cognitive-behavioral therapy for major depression in adolescent psychiatric patients. J Am Acad Child Adolesc Psychiatry. 1996;35:1156–1161. doi: 10.1097/00004583-199609000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Clarke GN, Rohde P, Lewinsohn PM, Hops H, Seeley JR. Cognitive-behavioral treatment of adolescent depression: efficacy of acute group treatment and booster sessions. J Am Acad Child Adolesc Psychiatry. 1999;38:272–279. doi: 10.1097/00004583-199903000-00014. [DOI] [PubMed] [Google Scholar]
- 22.Costello EJ, Pine DS, Hammen C, et al. Development and natural history of mood disorders. Biol Psychiatry. 2002;52:529–542. doi: 10.1016/s0006-3223(02)01372-0. [DOI] [PubMed] [Google Scholar]
- 23.Brent D, Emslie G, Clarke G, et al. Switching to another SSRI or to venlafaxine with or without cognitive behavioral therapy for adolescents with SSRI-resistant depression: the TORDIA randomized controlled trial. JAMA. 2008;299:901–913. doi: 10.1001/jama.299.8.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kennard BD, Stewart SM, Hughes JL, Jarrett RB, Emslie GJ. Developing cognitive behavioral therapy to prevent depressive relapse in youth. Cogn Behav Pract. doi: 10.1016/j.cbpra.2008.02.006. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poznanski E, Mokros H. Children’s Depression Rating Scale-Revised (CDRS-R) Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- 26.Guy W, editor. ECDEU Assessment Manual for Psychopharmacology: Publication ADM 76-338. Washington: US Department of Health, Education, and Welfare; 1976. pp. 113pp. 534–147.–537. [Google Scholar]
- 27.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children–Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 28.Andreasen NC, Rice J, Endicott J, Reich T, Coryell W. The family history approach to diagnosis. Arch Gen Psychiatry. 1986;43:421–429. doi: 10.1001/archpsyc.1986.01800050019002. [DOI] [PubMed] [Google Scholar]
- 29.Hollingshead AB. Four Factor Index of Social Status. New Haven: Yale University Department of Sociology; 1975. [Google Scholar]
- 30.Hughes CW, Emslie GJ, Crismon ML, et al. Texas Children’s Medication Algorithm Project: update from Texas Consensus Conference Panel on medication treatment of childhood major depressive disorder. J Am Acad Chil Adolesc Psychiatry. 2007;46:667–686. doi: 10.1097/chi.0b013e31804a859b. [DOI] [PubMed] [Google Scholar]
- 31.Young JE, Beck AT. Cognitive Therapy Scale Rating Manual. Philadelphia: Center for Cognitive Therapy; 1980. [Google Scholar]
- 32.Rush AJ, Koran LM, Keller MB, et al. The treatment of chronic depression, part 1: study design and rationale for evaluating the comparative efficacy of sertraline and imipramine as acute, crossover, continuation, and maintenance phase therapies. J Clin Psychiatry. 1998;59:589–597. [PubMed] [Google Scholar]
- 33.Shaffer D, Gould MS, Brasic J, et al. A Children’s Global Assessment Scale (CGAS) Arch Gen Psychiatry. 1983;40:1228–1231. doi: 10.1001/archpsyc.1983.01790100074010. [DOI] [PubMed] [Google Scholar]
- 34.Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plann. 1979;2:197–207. doi: 10.1016/0149-7189(79)90094-6. [DOI] [PubMed] [Google Scholar]
- 35.Nguyen TD, Attkisson CC, Stegner BL. Assessment of patient satisfaction: development and refinement of a service evaluation questionnaire. Eval Program Plann. 1983;6:299–314. doi: 10.1016/0149-7189(83)90010-1. [DOI] [PubMed] [Google Scholar]
- 36.Ryan N. Continuation treatment with antidepressants in child and adolescent major depression. Am J Psychiatry. 2008;165:411–412. doi: 10.1176/appi.ajp.2008.08010115. [DOI] [PubMed] [Google Scholar]