Abstract
Muscle VEGF expression is upregulated by exercise. Whether this VEGF response is regulated by transcription and/or post-transcriptional mechanisms is unknown. Hypoxia may be responsible: myocyte PO2 falls greatly during exercise and VEGF is a hypoxia-responsive gene. Whether exercise induces VEGF expression in other organs important to acute physical activity is also unknown. To address these questions, we created a VEGF/Luciferase reporter mouse and measured VEGF transcription, mRNA and protein responses to a) acute exercise and b) short-term hypoxia (FIO2=0.06) in brain (brainstem, cerebellum, cortex, hippocampus and striatum), muscle, lung, heart and liver. Exercise increased VEGF transcription, mRNA and protein in brain (hippocampus only), lungs and skeletal muscles, but not liver or heart. Hypoxia increased VEGF expression only in brain (cortex, hippocampus and striatum). New transcription appears to be a major exercise-induced regulatory step for increasing VEGF expression in muscle, lung and brain. Hippocampal VEGF expression was the only component of the exercise response recapitulated by hypoxia equivalent to the Everest summit.
Keywords: Exercise, hypoxia, skeletal muscle, lung, brain, transcription
1. Introduction
Exercise-induces multiple cellular signals including mechanical stimuli stemming from increased blood flow and muscle contraction and a fall in intracellular oxygen levels. Vascular endothelial growth factor expression is increased in skeletal muscle following just a single period of moderate intensity exercise (Breen et al. 1996). Furthermore, a hypoxic response element (HRE) is present within the VEGF promoter and could potentially bind HIF-1α during periods of low oxygen tension and transctivate the VEGF gene (Forsythe et al. 1996; Kimura et al. 2000). Therefore, intracellular hypoxia has been proposed to be a key signaling mechanism activated during exercise. However, whether VEGF expression is regulated at the transcriptional or post-transcriptional level, by a fall in intracellular oxygen during exercise or additional exercise-related stimuli is unkown. In addition, exercise has been shown to increase both angiogenesis and neurogenesis in regions of the brain. The hippocampal neurogenic response to exercise is thought to be mediated by VEGF expressed within peripheral organs (Fabel et al. 2003; Villar-Cheda et al. 2009). Thus, which organs increase VEGF expression in response to an acute exercise bout and the gene regulatory mechanisms that control VEGF expression in each organ remains to be elucidated.
In this study the gene regulatory mechanisms, used to selectively regulate VEGF expression in individual organs in mice subjected to whole body exercise or acute severe hypoxic exposure were evaluated. Key to this experimental design was the use of VEGF-luciferase reporter mice, developed in our laboratory, that allowed the contributions of in vivo transcriptional and post-transcriptional gene regulatory steps that control organ-specific exercise-induced VEGF gene expression to be measured. Analyses of these key gene regulatory steps in individual organs allowed the identification of organs which selectively respond in vivo to exercise and the predominant regulatory control mechanism that mediates this response. The effects of short-term exposure at rest to 6% oxygen (severe hypoxia equivalent to breathing air on the summit of Mt. Everest) were also evaluated to reveal the organ-specific VEGF gene regulatory responses to a low oxygen condition that may occur in tissue cells during exercise.
We detected coordinate expression of VEGF in brain, lung and skeletal muscles in response to exercise, and found it predominantly but not completely regulated at the transcriptional level. In response to a 2-hour period of breathing 6% O2, the only organ to express increased VEGF was the brain, and this occurred through a predominantly post-transcriptional mechanism.
2. Materials and Methods
2.1 Generation of Transgenic Mice
The mouse VEGF promoter/luciferase (firefly) clone was constructed and kindly provided by Dr. D’Amore (Shima et al. 1996). The VEGF-Luciferase reporter transgenic mouse strain was engineered at the University of California, San Diego (UCSD) Transgenic Mouse and Gene Targeting Core facility. The Kpn I and Sal I restriction DNA fragment of plasmid pVEGF-Luciferase-1217 that included −1217 bp to +0.4 kb of the mouse VEGF gene, the firefly luciferase gene and SV40 polyA was used for pronuclear injection into fertilized oocytes harvested from CB6F1 mice (Harlan Sera-Lab, IN) (Shima et al. 1996). Microinjected oocytes were then implanted into pseudo-pregnant foster mothers. The offspring (F0) were tested for chromosomal integration of the transgene by PCR analysis of mouse, tail DNA using primers specific to the VEGF promoter and luciferase gene (forward primer 5’- GCAGCTGGCCTACCTACCTT; reverse primer 5’-TCGCGGTTGTTACTTGACTG). A homozygous transgenic mouse line was subsequently generated through the crossing of positive siblings. Homozygous VEGF-Luciferase reporter mice identified by the PCR assay were confirmed by crossing with wild-type CB6F1 (−/−) mice and this resulted in 100% offspring being heterozygous (+/−) for the VEGF gene as expected.
2.2 Treadmill exercise protocol
This exercise protocol was modified from a protocol previously used in our laboratory for exercising rats (Breen et al. 1996). Briefly, littermate mice (6–8 weeks of age) were divided into experimental and control groups (N= 6 mice/group). At this stage, preliminary studies showed that VEGF transcriptional levels had reached constant organ-specific levels. Exercise groups were subjected to one treadmill running session at 24 M/min, 10-degree incline for 1 hour. One hour after the exercise session, which allowed time for luciferase and VEGF to be expressed, the mice were anesthetized and skeletal muscles (gastrocnemius, soleus, plantaris and tibialis anterior), heart, lungs, liver and whole brain were dissected within 15 minutes of the final end point from each mouse, frozen in liquid nitrogen and stored at −80°C until analysis.
2.3 Hypoxic Exposure
Mice were exposed to 2 hours of 6% oxygen in a 4-liter chamber ventilated at approximately 1–2 liters/min. Oxygen concentration was monitored with a mass Spectrometer (Perkin Elmer MGA1100). Tissue samples were collected immediately after the hypoxic exposure.
2.4 Luciferase Activity Assay
Luciferase activity was measured in tissue homogenates with the Luciferase Assay System (Promega). Total cellular protein in homogenates was measured with the DC-Protein Assay (Bio-Rad). Luciferase activity is reported as luciferase units per ug total protein.
2.5 Real Time RT-PCR Analysis of VEGF mRNA Levels
Tissues were homogenized with TRIzol Reagent (Invitrogen) for isolation of total cellular RNA. 200 ul of Trizol reagent was used to homogenize each soleus and 500 ul of reagent for 30–40 mg additional skeletal muscles and organs. A further purification step was performed using RNeasy Mini columns (Qiagen) with the additional DNase I step to remove contaminating genomic DNA. 1 ng of DNase-free RNA was then reverse transcribed to cDNA with the Thermoscript RT-PCR system (Invitrogen). RT products were amplified with SYBR-Green PCR kit reagents (SuperArray) and primers specific for VEGF165 or ribosomal RNA. The annealing temperature was set to 58°C. The VEGF primers used were: Forward primer: CGTTTAACTCAAGCTGCCTCGC, and Reverse primer: CTTCCAGGAGTACCCCGACGAGATA. The data presented was normalized to ribosomal protein L13a (L13A). Several control genes were also measured to ensure that they were not influenced by hypoxia or exercise (RealTimePrimers). VEGF mRNA levels were calculated with the comparative method (2ΔΔCt method).
2.6 VEGF Measurement
Samples were homogenized in Passive Lysis buffer (#E194A, Promega). Extraction buffer volumes used were 200 ul for each soleus and 500 ul for 30–40 mg of gastrocnemius, plantaris, T.A., heart, lung, liver and brain. VEGF protein levels in the samples were measured with a mouse specific VEGF ELISA kit (R&D, Minneapolis, MN). VEGF levels are expressed as ng VEGF per ug total protein.
2.7 Statistical Analysis
Using Statview software, statistical significance between organs collected from the experimental and control groups was evaluated using a T-test. In the case where several skeletal muscle types were analyzed, a 2 way ANOVA was used to identify an overall skeletal muscle difference between rest and exercise and a T-test was used to identify significant muscle type exercise responses. A one-way ANOVA comparing muscle type exercise responses (fold change in luciferase activity, mRNA and protein) revealed that these exercise responses were not different between muscle types (i.e. soleus, plantaris lateral gastrocnemius, medial gastrocnemius, and T.A.). P<0.05 was considered statistically significant. Data are expressed as Mean ± standard deviation (S.E.M.).
3. Results
3.1 Exercise-Induced Coordinate VEGF Transcription, mRNA and Protein Responses in Brain and Lungs
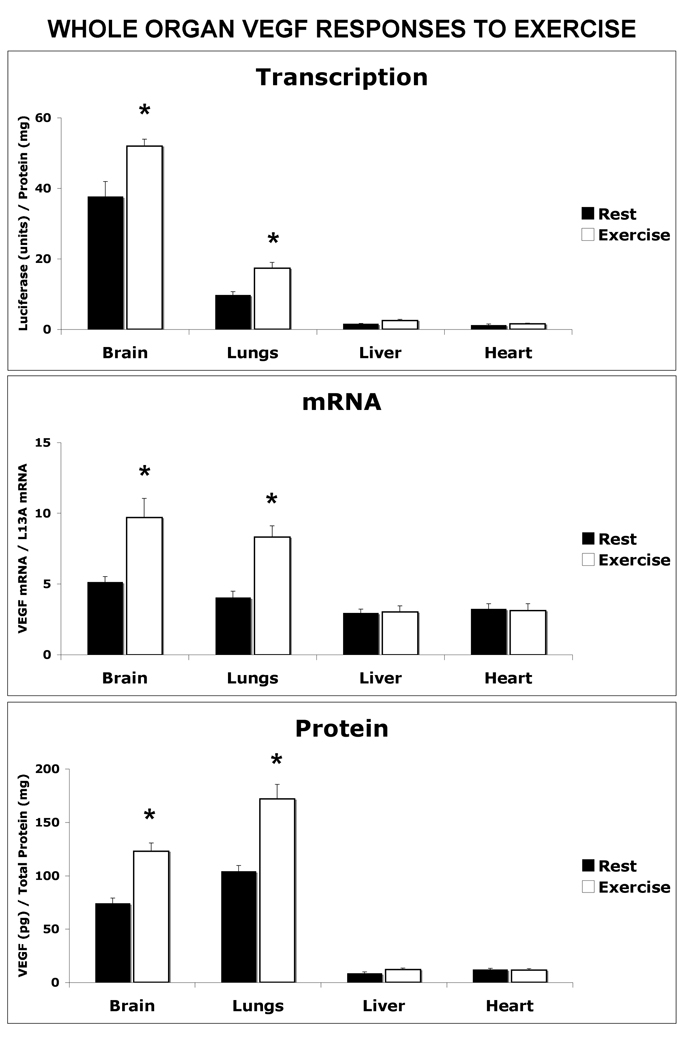
Figure 1 presents VEGF transcription, mRNA and protein levels in tissues other than skeletal muscle following an acute exercise bout. After one hour of exercise, VEGF transcriptional activity had increased 38% in brain (p<0.05), 78% in the lungs (p<0.01), and 62% in liver (p<0.05), compared to the resting group. Luciferase activity in the heart did not differ between the rested and exercised groups. Post-exercise VEGF mRNA levels were also increased, in the brain by 88% (p<0.05) and the lungs by 105% (p<0.01) above the levels in non-exercised mice. Liver VEGF mRNA levels were not increased despite exercise-induced transcriptional activation. In the same mice, a coordinate increase in VEGF protein levels was measured in the brain (66%) and lungs (66%). VEGF protein levels were not significantly different between the resting and control groups in the liver or heart.
Figure 1. One Hour of Exercise Signals an Increase in Transcriptional-Regulated VEGF Gene Expression in Brain and Lung.
VEGF-Luciferase reporter mice were subjected to a one-hour exercise bout (24 M/min, 100 incline) before the collection of non-skeletal muscle organs and measurement of VEGF transcriptional activity, mRNA and protein levels compared to non-exercised (resting) mice. A) VEGF Transcription: The brain, lungs, liver and heart were dissected from control (Rest) and 1 hour post-exercise (Exercise) and assayed for luciferase activity by luminometer. The data are expressed as luciferase (RLU)/ total protein (µg). B) VEGF/ mRNA Levels: The relative VEGF mRNA levels were measured in each organ by real time RT-PCR and normalized to the housekeeping gene, ribosomal L13A. C) VEGF Protein levels: Tissue levels of VEGF were measured in homogenates using a mouse specific VEGF ELISA. The data in each graph is represented as the mean ± the SEM, n=6, *indicates p<0.05.
3.2 Regional Analysis of Cerebral VEGF Expression in Response to Exercise
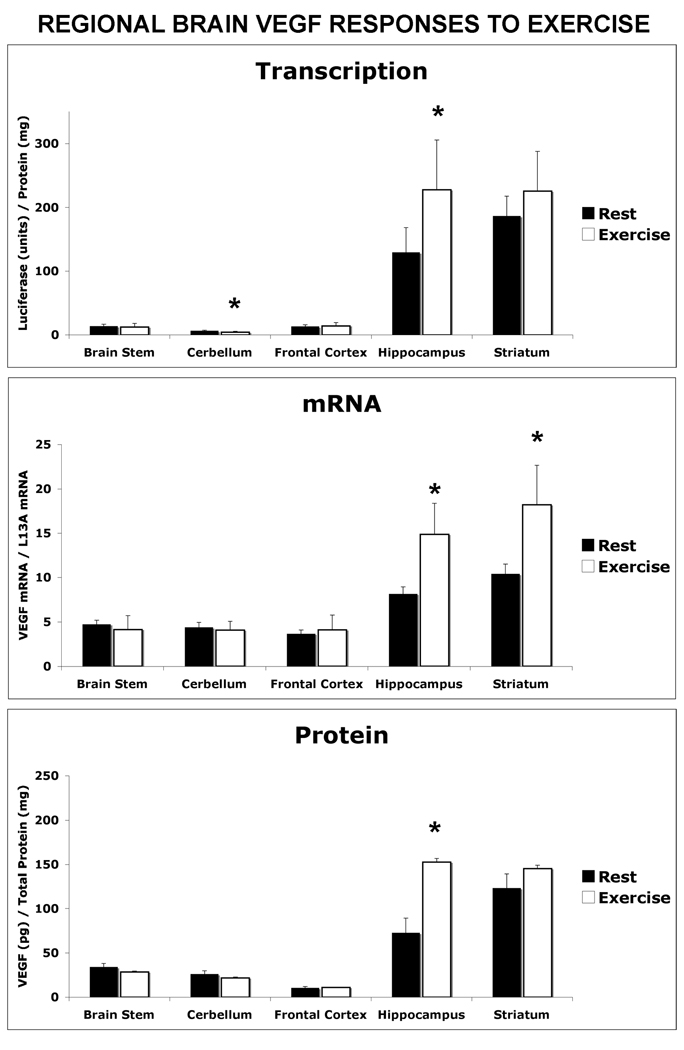
Upon further dissection of the total brain into functional distinct anatomical regions, it was revealed that the major increase in VEGF expression occurs in the hippocampus (Figure 2). VEGF luciferase activity was elevated by 76% (p<0.05), in coordination with an increase in mRNA level by 110% (p<0.01) and final protein product by 82% (p<0.05). In addition a decrease of 35% in VEGF luciferase reporter activity was detected in the cerebellum from exercised mice compared to the control group. However, this transcriptional response did not result in a change in cerebellum VEGF mRNA or proteins levels.
Figure 2. Exercise-Induced VEGF Expression is Predominant in the Hippocampus.
From additional groups of resting and post-exercise VEGF-Luciferase mice the brain was dissected into functional subregions and assayed for VEGF transcription, mNRa and protein levels. Regions assayed included the brain stem, cerebellum, frontal cortex, hippocampus and striatum. The data in each graph is represented as the mean ± the SEM, n=6. Significant difference between exercised and resting mice at confidence levels of * P<0.05
3.3 Hind Limb Locomotor Muscle VEGF Transcriptional Activation in Responses to Exercise
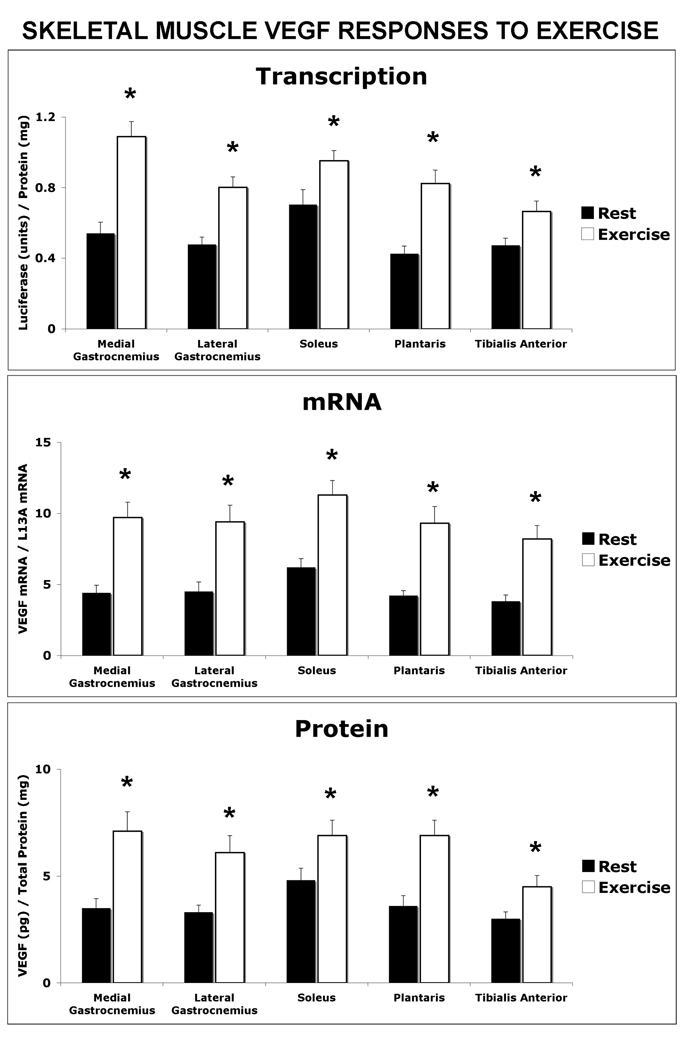
Post-exercise, hind limb skeletal muscles also revealed co-ordinate increases in VEGF transcription, mRNA and protein levels (Figure 3). The exercise response was not different among the skeletal muscle types analyzed. In the medial gastrocnemius from exercise mice VEGF luciferase activity was increased by 102% above resting levels (p<0.02), mRNA increased by 121% (p< 0.01) and protein by 103% (p<0.01). The lateral gastrocnemius muscle showed a similar response that consisted of VEGF luciferase activity increasing by 68% above resting levels (p<0.02), mRNA increasing by 85% (p< 0.01) and protein by 109% (p<0.01). Likewise, VEGF luciferase activity was increased by 35% (p<0.05), mRNA levels were increased by 82% (p<0.01) and VEGF protein was increased by 44% (p<0.05) in the soleus from exercise mice compared to the soleus from resting mice. The plantaris revealed a 41% increased in exercise-induced VEGF luciferase activity compared to the resting group and 121% (p<0.01) and 91% (P<0.01) increases in VEGF mRNA and protein levels, respectively. The tibialis anterior from exercised mice revealed 41% (p<0.01), 216% (p<0.01), and 50% (p<0.01) increases in luciferase activity, mRNA and VEGF levels, respectively, compared to non-exercised mice.
Figure 3. Hind Limb (Gastrocnemius, Soleus and Tibialis Anterior) Displayed a Coordinate Increase in Exercise-Induced VEGF Transcription mRNA and Protein.
Hind limb muscles were collected from resting and post-exercise VEGF-Luciferase mice and assayed for VEGF transcription, mRNA and proteins levels. The data in each graph is represented as the mean ± the SEM, n=6. Significant difference between exercised and resting mice at confidence levels of * P<0.05.
3.3 Selective Cerebral Response to 6% O2 Exposure
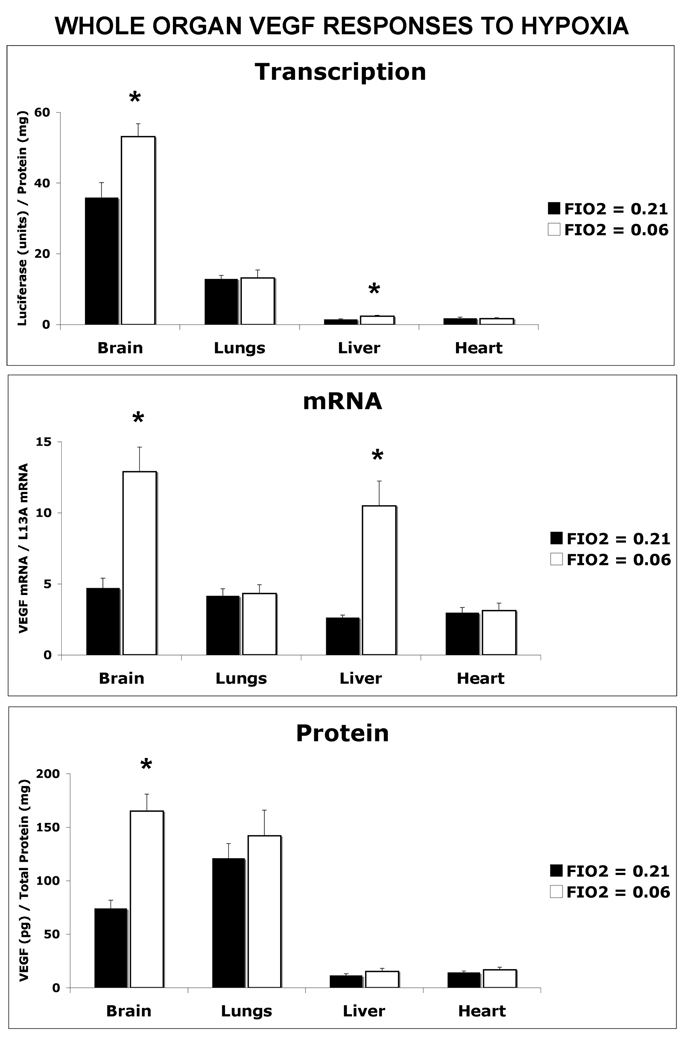
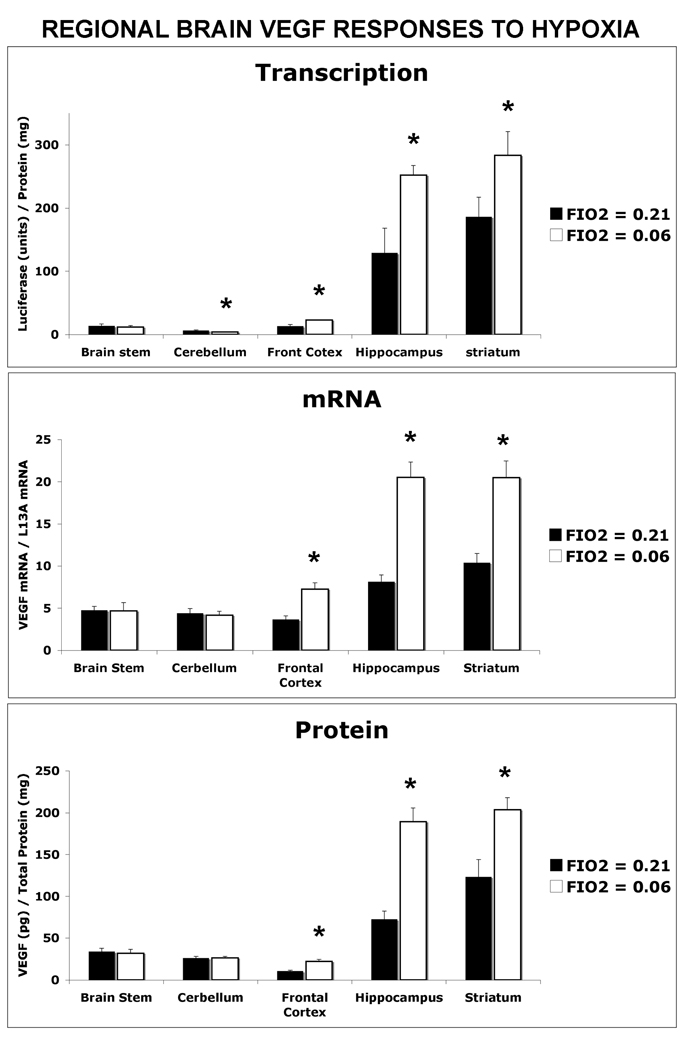
The VEGF transcriptional response to hypoxia differed among the organs analyzed (Figure 4–Figure 6). Following a period of breathing 6% oxygen for two hours, luciferase activity was found to increase by 48% (p<0.05) in the brain and 56% (p<0.05) in the liver compared to their respective normoxic controls (Figure 4). VEGF mRNA and protein levels also increased by 174% (p<0.01) and 123% (p<0.01), respectively, in hypoxic brain compared to normoxic controls (Figure 4). However, in liver despite an increase in both VEGF transcriptional activation (56%, p<0.05) and mRNA levels (297%, p<0.01), there was no increase in the amount of VEGF protein. In lung, heart (Figure 4), and locomotor muscles (Figure 6), VEGF luciferase activity, mRNA and proteins levels in the hypoxic group were not significantly different from those in the normoxic control group. Upon further analysis of the anatomical regions of the brain (Figure 5), VEGF luciferase activity was found to be increased in the frontal cortex by 76% (p<0.05), the hippocampus by 95% (P<0.01) and striatum by 52% (p<0.05) under this acute period of severe hypoxia compared to the normoxic group. VEGF mRNA levels were also increased by 97% (p<0.05), 151% (p<0.01) and 97% (p<0.01) in the frontal cortex, hippocampus and striatum, respectively, in response to hypoxia. VEGF protein levels in the hypoxic group were also increased by 112% (p<0.05), 161% (p<0.01) and 65% (P<0.05) in the frontal cortex, hippocampus and striatum, respectively, to similar brain regions in the normoxic mouse group. Similar to the exercise response, acute hypoxia also led to a decrease in VEGF luciferase activity (41%, p<0.05) in the cerebellum that did not result in a change in mRNA or protein product levels.
Figure 4. Short-term Exposure to 6% Oxygen Selectively Signals Transcriptional Regulated VEGF Expression in the Brain.
VEGF-Luciferase reporter mice were exposed to an hypoxic environment (6% oxygen, FIO2 = 0.06) for 2 hours prior to measurement of VEGF transcription, mRNA and protein levels in non-skeletal muscle organs. Values were compared to mice maintained in a normoxic environment (FIO2 = 0.21). Values are the mean ± SEM, n= 6 mice per FIO2 group. Significant difference between FIO2 = 0.21 and FIO2 = 0.06 are reported with confidence levels of *p<0.05.
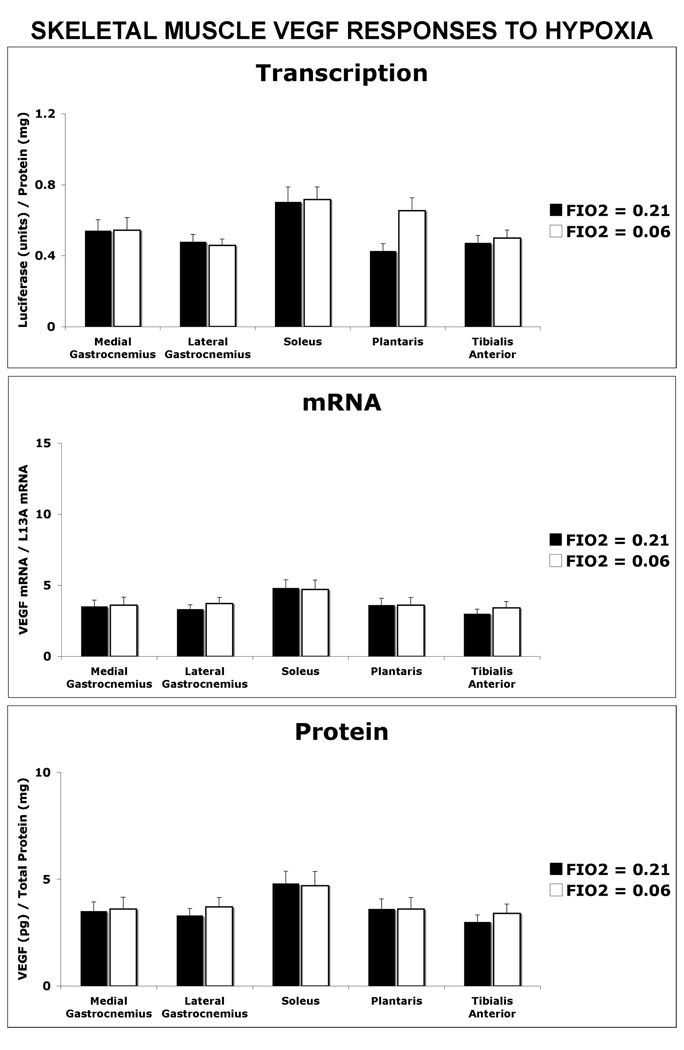
Figure 6. Hypoxic Exposure Did Not Alter Skeletal Muscle VEGF Expression.
Mice were exposed to FIO2 = 0.21 or FIO2 = 0.06 for 2 hours and the medial gastrocnemius, lateral gastrocnemius, soleus, plantaris, tibialis anterior were isolated post-hypoxic exposure and assayed for changes in VEGF transcription, mRNA and protein. No significant differences between FIO2 groups was detected, n=6 mice per group.
Figure 5. Brain Regional Responses to Hypoxia.
Brain dissections were also performed on additional groups of hypoxia-exposed (6% oxygen, FIO2 = 0.06, 2 hours) and normoxic (FIO2 = 0.21) and each functional cerebral region analyzed for changes in VEGF transcription, mRNA and protein levels. Values are the mean ± SEM, n= 6 mice per FIO2 group. Significant difference between FIO2 = 0.21 and FIO2 = 0.06 are reported with confidence levels of *p<0.05.
4. Discussion
In this study organ-specific VEGF gene expression was measured in response to 1 hour of exercise or a 2-hour exposure to acute severe hypoxia (FIO2 = 0.06). We found that in response to exercise, there is a coordinate increase in VEGF gene expression in some but not all organs studied. In contrast, mice exposed to 6% O2 responded with a selective increase in cerebral VEGF expression.
4.1 Exercise-Regulated VEGF Expression
The VEGF-luciferase reporter mouse used in this study allowed the contribution of VEGF transcription and post-transcriptional control mechanisms to overall VEGF expression to be evaluated. In exercise-responsive tissues, a positive correlation between increased VEGF transcriptional activation and mRNA levels was observed. Likewise, in most tissues a direct relationship exists between VEGF mRNA levels and the amount of new VEGF protein produced. The exception to this observation was the liver in which hypoxic exposure resulted in a 3-fold increase in VEGF mRNA level that was not accompanied by VEGF accumulation. The model of Stefanini et al. predicts that newly expressed VEGF transiently and simultaneously increases in plasma and tissue and returns to baseline values at a rate dictated by the permeability between tissue and blood fluid compartments. Thus, the liver may rapidly clear newly synthesized bioactive VEGF while a delay in the skeletal muscle or brain could allow bioactive VEGF to initiate angiogenic and/or neurogenic responses (Stefanini et al. 2008).
4.2 Selective brain responses to acute hypoxia
The present in vivo data also support both hypoxia-induced transcription and posttranscriptional mRNA stabilization as mechanisms for increasing VEGF expression in the brain. A previous study by Marti et al. (Marti et al. 1998) reported a predominant cerebral VEGF response to 6% O2. Hypoxic exposure time in the study of Marti et al. was for a longer period of 6 hours and revealed additional hypoxic VEGF responses in the kidney, testis, lung, heart and liver. However, the brain is thought to be very sensitive to even small changes in systemic oxygen level. HIF-1α accumulation is detectable in the brain with 18% O2 ventilation in contrast to the kidney and liver that require the inspired O2 concentration to drop below a 6% O2 threshold (Sick et al. 1982; Stroka et al. 2001). Cerebral VEGF expression in response to hypoxia is restricted to the frontal cortex, hippocampus and striatum. In support of this finding, the cortex and hippocampus have also been reported to increase HIF-1 levels in response to 3 hours of 8% O2 exposure (Bani Hashemi et al. 2008). Thus, the extent of VEGF expression in response to severe short-term hypoxic exposure differs from hippocampus-restricted VEGF expression in response to exercise
Exercise causes a substantial increase in VEGF transcription in the brain. The data presented in this study suggest that transcription, rather then central nervous system hypoxia-induced post-transcriptional regulation, is the predominant cerebral VEGF gene regulatory mechanism in response to exercise. Exercise has also been reported to increase both neurogenesis and angiogenesis in the hippocampus (Fabel et al. 2003; Villar-Cheda et al. 2009). However, previous reports have suggested that VEGF expression was not directly occurring in cerebral cells but rather VEGF expressed in peripheral organs and circulating throughout the vasculature signaled hippocampal neurogenesis (Fabel et al. 2003). Since VEGF is not expected to readily cross the blood brain barrier this would suggest that new VEGF-dependent blood vessel formation indirectly signaled the accompanying formation of new neurons. However, our data suggest that VEGF transcription is occurring in the brain and thus VEGF could also potentially play a direct role in cerebral neurogenesis and neuroprotection (Ruiz de Almodovar et al. 2009).
4.3 Pulmonary Parenchymal VEGF Response to Acute Exercise
In hypoxia, well-known cardio-respiratory responses include a) increasing tidal volume, respiratory frequency and ventilation causing increased cyclical strain on the parenchyma and airways b) pulmonary arterial constriction and c) modestly increased heart rate and cardiac output. Exercise requires additional increases in ventilation, increasing tissue strain, and cardiac output, leading to even larger elevations in pulmonary vascular pressures (Wagner et al. 1986). In our study, exercise, but not hypoxic exposure, signaled pulmonary VEGF transcriptional activation. Thus, presumably, an increase in ventilation and/or blood flow, and not hypoxia per se, provided the exercise-related signals to transactivate the VEGF promoter. In the lung this increase in available VEGF could regulate vascular permeability and/or play a role in maintaining the integrity of the alveolar capillary barrier (Birukova et al. 2008).
4.4 Differences in Skeletal and Cardiac Muscle VEGF Response to Exercise
All hind limb muscles examined in the present study increased VEGF transcription in response to exercise. Furthermore, the exercise response was found not to be statistically different when the medial and lateral heads of the gastrocnemius, soleus, plantaris and tibialis anterior were compared. Consistent with this finding type IIb and IId mRNA levels, indicative of fiber type composition, were also not different between the mouse medial and lateral gastrocnemius in our study (data not shown). The gastrocnemius response in the mouse is different from that reported in the rat, which reveals a greater exercise-induced VEGF and angiogenic response in the white portion of the gastrocnemius following exercise training of rats with bilateral femoral artery ligation. (Lloyd et al. 2003). This difference between the rat and mouse is likely to reflect a difference in the fiber type composition between these two species.
None of the muscles examined showed a VEGF response to inspired 6% O2 (FIO2 = 0.06). This is an extreme degree of hypoxia, especially imposed acutely, and is the equivalent of the PO2 of inspired air on the summit of Mt. Everest (West 1999). Our aim in this present study was to gain insight into whether a fall in intracellular PO2 during exercise or additional exercise-related stimuli signaled VEGF transcriptional and/or post-transcriptional response. This absence of a VEGF response to short-term hypoxia in the mouse differs from what our laboratory has previously reported in rat gastrocnemius (where FIO2 was 0.12) – that VEGF mRNA levels were increased even at rest under hypoxic conditions (Breen et al. 1996; Tang et al. 2004). Gavin et al. recently reported a doubling of VEGF mRNA when C57BL/6J mice were subjected 6% oxygen for 2 hours in the soleus, plantaris and gastrocnemius (Gavin et al. 2006). The cause for this discrepancy between the data presented in this study and that of Gavin et al. is unclear but may be due to mouse strain differences (Chan et al. 2005; Gavin et al. 2006; Helisch et al. 2006; Zwemer et al. 2007).
A single exercise bout also did not augment cardiac muscle VEGF expression. In rats some but not all studies have shown that exercise training is accompanied by an increase in cardiac VEGF (Belabbas et al. 2008; Marini et al. 2008). In the mouse strain FBV an acute exercise response has been reported to increased VEGF expression (Wu et al. 2009). This is not the case in our study of CB6F1 mice, even though the mice were run at a greater intensity the study reported by Wu et al. (Wu et al. 2009), and suggests that there may be mouse strain differences in the cardiac VEGF response to acute exercise. Some investigators have suggested that a decrease in levels of anti-angiogenic molecules, such as endostatin (Gu et al. 2006), with only a modest increase in cardiac VEGF (or other pro-angiogenic factors) provide the cellular conditions for cardiac angiognesis in response to exercise training.
4.5 Experimental Limitations
It should be noted that throughout this study a reporter gene, luciferase, was used to monitor transcriptional activation of the VEGF promoter in vivo. This does not permit the absolute rate of transcription to be measured. The data collected are limited to revealing relative differences in transcriptional activation. Furthermore we have chosen to compare two conditions, an acute exercise bout and exposure to 6% O2, in an attempt to distinguish how much of the exercise response was due to a fall in intracellular PO2. An FIO2 of 0.06 was chosen as the lowest tolerable level of inspired O2 in unacclimatized mice and coincidentally produces an inspired PO2 equal to that on the summit of Mt. Everest. However, exposure to even this extreme oxygen environment may not have recreated the same intracellular PO2 that occurs during exercise in man - as low as 3 mmHg (Richardson et al. 1999).
4.6 Conclusions
In response to acute exercise, it is clear that some but not all organs increase VEGF expression. The exercise-responsive organs (the brain, lung, and locomotor skeletal muscles) represent a subset of organs that are critical to the oxygen transport system. What is remarkable is that each exercise-responsive organ utilizes a common gene regulatory mechanism reflected by a linear relationship between new VEGF transcription and VEGF mRNA levels. Irrespective of the transcriptional mechanism to increase VEGF mRNA, most tissues (except the liver) translate VEGF mRNA into a proportional number of VEGF molecules. The liver may be highly specialized to clear newly synthesized, secreted VEGF. Interestingly, severe, short-term hypoxia (FIO2 = 0.06) stimulated VEGF expression exclusively in the brain through a combined transcriptional and post-transcriptional mechanism different from that seen during exercise. While our study was limited in that the intracellular PO2 levels during exercise could not be measured, the data suggest that exercise and hypoxia regulate VEGF gene expression through distinct and organ specific-regulatory pathways.
The global ramifications of this study are that exercise may provide a controlled cellular mechanism to increase VEGF expression in regions of the brain that are important for memory and sensory control. VEGF expression, both peripherally in skeletal muscles and lung as well as within cerebral cells, may coordinate the regulation of neurogenesis and angiogenesis in response to exercise and potentially provide neuroprotection against an ischemic or excitotoxic episode. This has many positive implications for the rehabilitation and neuronal repair in several diseases including Alzheimer, Parkinson’s disease and stroke [for review see (Ruiz de Almodovar et al. 2009)].
Acknowledgements
This work was funded by grants NIH PO1 HL 17731-28, TRDRP 12RT-006 and NIH R01 HL084821-03.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bani Hashemi S, Braun J, Bernhardt WM, Rascher W, Dotsch J, Trollmann R. HIF-1alpha subunit and vasoactive HIF-1-dependent genes are involved in carbon monoxide-induced cerebral hypoxic stress response. Eur J Appl Physiol. 2008;104:95–102. doi: 10.1007/s00421-008-0776-9. [DOI] [PubMed] [Google Scholar]
- Belabbas H, Zalvidea S, Casellas D, Moles JP, Galbes O, Mercier J, Jover B. Contrasting Effect of Exercise and Angiotensin Ii Hypertension on in Vivo and in Vitro Cardiac Angiogenesis in Rats. Am J Physiol Regul Integr Comp Physiol. 2008 doi: 10.1152/ajpregu.00014.2008. [DOI] [PubMed] [Google Scholar]
- Birukova AA, Moldobaeva N, Xing J, Birukov KG. Magnitude-dependent effects of cyclic stretch on HGF- and VEGF-induced pulmonary endothelial remodeling and barrier regulation. Am J Physiol Lung Cell Mol Physiol. 2008 doi: 10.1152/ajplung.90236.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen EC, Johnson EC, Wagner H, Tseng HM, Sung LA, Wagner PD. Angiogenic growth factor mRNA responses in muscle to a single bout of exercise. J Appl Physiol. 1996;81:355–361. doi: 10.1152/jappl.1996.81.1.355. [DOI] [PubMed] [Google Scholar]
- Chan CK, Pham LN, Zhou J, Spee C, Ryan SJ, Hinton DR. Differential expression of pro- and antiangiogenic factors in mouse strain-dependent hypoxia-induced retinal neovascularization. Lab Invest. 2005;85:721–733. doi: 10.1038/labinvest.3700277. [DOI] [PubMed] [Google Scholar]
- Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/mcb.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavin TP, Westerkamp LM, Zwetsloot KA. Soleus, plantaris and gastrocnemius VEGF mRNA responses to hypoxia and exercise are preserved in aged compared with young female C57BL/6 mice. Acta Physiol (Oxf) 2006;188:113–121. doi: 10.1111/j.1748-1716.2006.01609.x. [DOI] [PubMed] [Google Scholar]
- Gu JW, Shparago M, Tan W, Bailey AP. Tissue endostatin correlates inversely with capillary network in rat heart and skeletal muscles. Angiogenesis. 2006;9:93–99. doi: 10.1007/s10456-006-9035-z. [DOI] [PubMed] [Google Scholar]
- Helisch A, Wagner S, Khan N, Drinane M, Wolfram S, Heil M, Ziegelhoeffer T, Brandt U, Pearlman JD, Swartz HM, Schaper W. Impact of mouse strain differences in innate hindlimb collateral vasculature. Arterioscler Thromb Vasc Biol. 2006;26:520–526. doi: 10.1161/01.ATV.0000202677.55012.a0. [DOI] [PubMed] [Google Scholar]
- Kimura H, Weisz A, Kurashima Y, Hashimoto K, Ogura T, D'Acquisto F, Addeo R, Makuuchi M, Esumi H. Hypoxia response element of the human vascular endothelial growth factor gene mediates transcriptional regulation by nitric oxide: control of hypoxia-inducible factor-1 activity by nitric oxide. Blood. 2000;95:189–197. [PubMed] [Google Scholar]
- Lloyd PG, Prior BM, Yang HT, Terjung RL. Angiogenic growth factor expression in rat skeletal muscle in response to exercise training. Am J Physiol Heart Circ Physiol. 2003;284:H1668–H1678. doi: 10.1152/ajpheart.00743.2002. [DOI] [PubMed] [Google Scholar]
- Marini M, Falcieri E, Margonato V, Trere D, Lapalombella R, di Tullio S, Marchionni C, Burattini S, Samaja M, Esposito F. Partial persistence of exercise-induced myocardial angiogenesis following 4-week detraining in the rat. Histochem Cell Biol. 2008;129:479–487. doi: 10.1007/s00418-007-0373-8. [DOI] [PubMed] [Google Scholar]
- Marti HH, Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci U S A. 1998;95:15809–15814. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Leigh JS, Wagner PD, Noyszewski EA. Cellular PO2 as a determinant of maximal mitochondrial O(2) consumption in trained human skeletal muscle. J Appl Physiol. 1999;87:325–331. doi: 10.1152/jappl.1999.87.1.325. [DOI] [PubMed] [Google Scholar]
- Ruiz de Almodovar C, Lambrechts D, Mazzone M, Carmeliet P. Role and therapeutic potential of VEGF in the nervous system. Physiol Rev. 2009;89:607–648. doi: 10.1152/physrev.00031.2008. [DOI] [PubMed] [Google Scholar]
- Shima DT, Kuroki M, Deutsch U, Ng YS, Adamis AP, D'Amore PA. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post-transcriptional regulatory sequences. J Biol Chem. 1996;271:3877–3883. doi: 10.1074/jbc.271.7.3877. [DOI] [PubMed] [Google Scholar]
- Sick TJ, Lutz PL, LaManna JC, Rosenthal M. Comparative brain oxygenation and mitochondrial redox activity in turtles and rats. J Appl Physiol. 1982;53:1354–1359. doi: 10.1152/jappl.1982.53.6.1354. [DOI] [PubMed] [Google Scholar]
- Stefanini MO, Wu FT, Mac Gabhann F, Popel AS. A compartment model of VEGF distribution in blood, healthy and diseased tissues. BMC Syst Biol. 2008;2:77. doi: 10.1186/1752-0509-2-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroka DM, Burkhardt T, Desbaillets I, Wenger RH, Neil DA, Bauer C, Gassmann M, Candinas D. HIF-1 is expressed in normoxic tissue and displays an organ-specific regulation under systemic hypoxia. Faseb J. 2001;15:2445–2453. doi: 10.1096/fj.01-0125com. [DOI] [PubMed] [Google Scholar]
- Tang K, Breen EC, Wagner H, Brutsaert TD, Gassmann M, Wagner PD. HIF and VEGF relationships in response to hypoxia and sciatic nerve stimulation in rat gastrocnemius. Respir Physiol NeurobiolRespir Physiol Neurobiol. 2004;144:71–80. doi: 10.1016/j.resp.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Villar-Cheda B, Sousa-Ribeiro D, Rodriguez-Pallares J, Rodriguez-Perez AI, Guerra MJ, Labandeira-Garcia JL. Aging and sedentarism decrease vascularization and VEGF levels in the rat substantia nigra. Implications for Parkinson's disease. J Cereb Blood Flow Metab. 2009;29:230–234. doi: 10.1038/jcbfm.2008.127. [DOI] [PubMed] [Google Scholar]
- Wagner PD, Gale GE, Moon RE, Torre-Bueno JR, Stolp BW, Saltzman HA. Pulmonary gas exchange in humans exercising at sea level and simulated altitude. J Appl Physiol. 1986;61:260–270. doi: 10.1152/jappl.1986.61.1.260. [DOI] [PubMed] [Google Scholar]
- West JB. Barometric pressures on Mt. Everest: new data and physiological significance. J Appl Physiol. 1999;86:1062–1066. doi: 10.1152/jappl.1999.86.3.1062. [DOI] [PubMed] [Google Scholar]
- Wu G, Rana JS, Wykrzykowska J, Du Z, Ke Q, Kang P, Li J, Laham RJ. Exercise-induced expression of VEGF and salvation of myocardium in the early stage of myocardial infarction. Am J Physiol Heart Circ Physiol. 2009;296:H389–H395. doi: 10.1152/ajpheart.01393.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwemer CF, Song MY, Carello KA, D'Alecy LG. Strain differences in response to acute hypoxia: CD-1 versus C57BL/6J mice. J Appl Physiol. 2007;102:286–293. doi: 10.1152/japplphysiol.00536.2006. [DOI] [PubMed] [Google Scholar]