Abstract
Objective
To determine whether variants in the estrogen receptor 1 (alpha) and 2 (beta) (ESR1 and ESR2) genes are associated with cognitive impairment in non-demented elderly men and women.
Background
Several single nucleotide polymorphisms (SNPs) on ESR1 and ESR 2 genes have been associated with a range of hormone sensitive diseases such as breast cancer and osteoporosis. Genetic variations in ESR may also influence cognitive aging but are less studied, especially among men.
Methods
We studied 2527 participants enrolled in an ongoing prospective study of community-dwelling elders. Four SNPs from ESR1 and four from ESR2 were analyzed. We measured cognitive function with the Modified Mini-Mental Status Examination (3MS) at baseline and biannually; cognitive impairment was defined as a decline of 5 or more points over 4 years. We calculated odds of developing cognitive impairment across SNPs using gender-stratified logistic regression and adjusted analyses for age, education, baseline 3MS score and in addition for race.
Results
1343 women (mean age 73.4) and 1184 men (mean age 73.7) comprised our cohort. Among women, after multivariate adjustment, 2 of the ESR1 SNPs (rs8179176, rs9340799) and 2 of the ESR2 SNPs (rs1256065, rs1256030) were associated with likelihood of developing cognitive impairment, although the association for rs8179176 was of trend level significance. In men, 1 of the ESR1 SNPs (rs728524) and 2 of the ESR2 (rs1255998, rs1256030) were associated with cognitive impairment. Further adjustment for race attenuated the results somewhat. There was no association between any ESR SNP and level of bioavailable estradiol but testosterone level did vary among 2 of the SNPs (p<0.05).
Conclusion
We found that among non-demented community elders, several SNPS in the ESR1 and ESR2 genes were associated with risk of developing cognitive impairment. These findings suggest that estrogen receptor genetic variants may play a role in cognitive aging.
1. Introduction
The genes for the estrogen receptor 1 and 2 (ESR1 or alpha and ESR2 or beta) have several single nucleotide polymorphisms (SNPs). Differences in ESR1 polymorphism frequencies have been demonstrated among patients with breast cancer, osteoporosis and endometriosis [2,7,9,11,21,24]. The polymorphisms may affect ER gene enhancer activity [17] or gene regulation [1], but how they influence these outcomes is not exactly known. Several polymorphisms have been linked to the modification of the effects of exogenous estrogen on lipid levels in older women [10].
Although not as well studied, genetic variations in ESR may influence cognitive aging. This is important as approximately 50% of genetic factors remain unidentified for sporadic Alzheimer disease (AD). Several case-controls studies [3,12,18], but not all [17], have found an association between several ESR1 polymorphisms (most notably PvuII and XbaI ) and AD. As part of a prospective study of non-demented women, we found that both of the SNPs we examined on ESR1 were associated with likelihood of developing cognitive impairment over 8 years [31]. There are few data on ESR2 polymorphisms and cognitive function or dementia.
Most studies to date have been cross-sectional and only determined the association with advanced cognitive decline such as AD. In addition, few studies have investigated the association of the ESR variations on cognitive aging in men. The goal of this study is to determine whether ESR1 and ESR 2 gene polymorphisms are associated with cognitive decline in both elderly men and women who were non-demented at study onset. We hypothesized that the previously studied ESR1 polymorphisms such as PvuII and XbaI would be associated with cognitive impairment and that in addition, some of the ESR2 polymorphisms would be associated with cognitive outcomes.
2. Methods
2.1. Study population
Participants were part of the Health, Aging and Body Composition (Health ABC) study, a prospective cohort study beginning in 1997 of 3075 community-dwelling elders then aged 70-79 years old and living in Memphis, TN or Pittsburgh, PA. To identify potential participants, a random sample of white and all black Medicare-eligible elders within designated zip code areas were contacted. To be eligible for the study, participants had to report no difficulties with activities of daily living, walking a quarter of a mile, or climbing 10 steps without resting. They also had to be free of life-threatening cancers and could not intend to move out of the study area for at least 3 years. Seventy-seven percent of all eligible participants contacted agreed to participate in the study. All elders participating in the study signed an informed written consent, approved by the institutional review boards at the clinical sites. This study was approved by the institutional review boards of the coordinating center, the University of California, San Francisco.
Of the 3075 participants, 103 were missing ESR1 and ESR2 genotype data. Of the remaining 2972, 18 were missing baseline cognitive data and an additional 427 were missing follow-up cognitive data (220 died, 14 were lost to follow-up and 193 did not have repeat cognitive testing), leaving 2527 participants in our analytic cohort. There were no differences in genotype frequencies among those with and without cognitive follow-up for all 8 of the polymorphisms among women and for 7 of the 8 ER polymorphisms among men. For ESR2 rs1255998, men with the GG genotype were more likely to be missing cognitive follow-up (26%) compared to men with the CC (15%) or CG genotype (18%) (p=0.007).
2.2. Measurements
The Modified Mini-Mental State Examination (3MS) was administered to all participants during the baseline visit and repeated at the Year 3 and 5 follow-up visits. It is a brief, general cognitive battery with components for orientation, concentration, language, praxis, and immediate and delayed memory [29]. The maximum (best) score is 100. Cognitive impairment was defined as a 3MS decline of 5 or more points at either follow-up visit as has been previously recommended [14].
ESR1 and ESR2 polymorphisms were genotyped by the 5′-nuclease assay [16] four SNPs were analyzed on each gene. Assays were designed using the ABI Assays-on-Demand service and genotyped using the ABI 7900HT (Applied Biosystems). Each assay included as positive controls, individuals whose genotype was confirmed by direct sequencing, and no template controls as negative controls. The test sample included 5% duplicates as controls to which the experimenter was blinded.
Potential covariates included participant gender, age, race, and whether or not they achieved a high-school level of education. At Year 3, health literacy was assessed using the Rapid Estimate of Adult Literacy in Medicine (REALM), scored 0-66 with scores >60 representing a 9th grade reading level or above. In addition, presence of diabetes mellitus and hypertension, history of myocardial infarction (MI) and history of stroke or transient ischemic attack (TIA) were determined using prevalent disease algorithms based on self-report of physician diagnoses and recorded medications, with measurements from the clinic examination used for selected conditions (e.g. diabetes). Depressive symptoms were assessed with the Center for Epidemiologic Studies-Depression Scale (CES-D), with higher scores indicating greater number of symptoms and a score ≥ 16 consistent with possible depression [19]. Apolipoprotein E (APOE) genotypes were analyzed using standard techniques and coded as APOE e4 or no e4. Bioavailable plasma estradiol and free serum testosterone concentrations were measured on a subset of participants (n=831). Estradiol assays were performed at the Royal Marsden Labs (London, England) by an initial extraction by column chromatography and radioimmunoassay using a highly specific rabbit antiserum raised against an estradiol-6-carboxymethyloxime-bovine serum albumin conjugate (EIR, Wurenlingen, Switzerland) and Third Generation Estradiol [I125] reagent (DSL 39120 Diagnostic Systems Laboratories Inc., Texas). The intra-assay and inter-assay coefficients of variation were 7.6% and 17.0%, respectively. Serum concentrations of free testosterone were measured at Wake Forest University using an enzyme immunoassay (EIA) kit (sensitivity = 0.19 pg/ml, detection range = 0.25-100 pg/ml) from Diagnostic Systems Laboratories, Inc. (DSL, Webster, TX). The intra-assay and inter-assay coefficients of variation were 4.9% and 9.0%, respectively, for a low level control (1.9pg/ml) and were 3.6% and 13.0%, respectively, for a high level control (26.6pg/ml).
2.3. Statistical analyses
Analyses were conducted with Stata Release 9 (StataCorp. 2005, College Station, TX). We first tested for Hardy-Weinberg equilibrium and estimated a measure of linkage disequilibrium within each race/ethnicity group. To determine whether each ER SNP was associated with cognitive impairment, the proportion of participants that developed cognitive impairment was calculated by genotype. Since the rates of cognitive impairment were similar in most cases for the heterozygotes and the homozygotes of the most common allele, we combined them to simplify analyses and presentation of results. We used the Mantel-Haenszel method to calculate unadjusted and adjusted odds ratios and 95% confidence intervals of risk of cognitive impairment across genotype, using the most frequent genotype group as the reference. Interactions between race and gender and each of the ER polymorphisms on cognitive impairment were tested using the Breslow-Day test of homogeneity. Since there were statistically significant interactions between gender and 2 of the 8 ER polymorphisms on cognitive impairment, we stratified all analyses by gender. Thus, the total number of comparisons was 16 (8 SNPs for both genders). We controlled for but did not stratify by race since there were no significant interactions between race and the SNPs on cognitive impairment.
Because age, education, baseline 3MS score and race were strongly associated with the outcome and some of the polymorphisms, we adjusted our analyses for these covariates.. Multivariate adjustments were conducted by assigning each participant to a stratum that was defined by the combination of characteristics they exhibited on all of these covariates. The Mantel-Haenszel method was then used to calculate a common odds ratio across the strata, thereby controlling for the characteristics defining them. This provides a way to adjust for the possible confounding effects of the covariates without estimating a model-based parameter for each of them [5]. For a sensitivity analysis, we ran traditional multivariate logistic regression models adjusting for the same covariates.
We also constructed haplotypes for both ESR1 and ESR2 and tested for associations with cognitive impairment using the R software [23].
3. Results
Hardy-Weinberg equilibrium was present in all ESR polymorphisms except for in one of the 8 polymorphisms (ESR1 rs9340799) among blacks (p=0.042). Linkage disequilibrium was very strong between the first 2 ESR1 polymorphisms (D’ ranged from 0.369-0.998 in whites and 0.294-0.999 in blacks) and between all 4 ESR2 polymorphisms (D’ ranged from 0.977-0.996 in whites and 0.819-0.960 in blacks).
There were 1343 women and 1184 men in our analytic cohort (Table 1). The mean (SD) age of the women at baseline was 73.4 (2.9) and the mean age of the men was 73.7 (2.8); 44% of the women and 34% of the men and were black; 22% of the women and 25% of the men had less than a high-school level of education; 30% of women and 28% of men were carriers of the APOE e4 allele. For women, the most common allele frequencies were: ESR1 rs8179176 A=58%, rs9340799 A=50%, rs728524 A=88%, rs3798577 T=53%; ESR2 rs1255998 C=70%, rs1256065 A=72%, rs1256049 G=95%, rs1256030 C=64%. For men, the most common allele frequencies were: ESR1 rs8179176 A=67%, rs9340799 A=52%, rs728524 A=91%, rs3798577 T=54%; ESR2 rs1255998 C=74%, rs1256065 A=68%, rs1256049 G=95%, rs1256030 C=62%. Other than age, education and race, there were no other baseline characteristics listed in Table 1, including cardiovascular risk factors, that were associated consistently with both ESR SNP and cognitive impairment.
Table 1.
Baseline Characteristics of the 2527 Health ABC Participants by Gender
| Characteristic Mean (sd) or % |
Women (N=1343) |
Men (N=1184) |
|---|---|---|
| Age | 73.4 (2.9) | 73.7 (2.8) |
| Black (%) | 44 | 34 |
| Education < high school (%) | 22 | 25 |
| Hypertension (%) | 54 | 47 |
| Diabetes (%) | 13 | 16 |
| Myocardial infarction history (%) | 7 | 14 |
| Stroke/TIA history (%) | 7 | 6 |
| Depression score ≥ 16 (%) | 5 | 4 |
| Current hormone therapy (%) | 23 | - |
| Pittsburgh site (%) | 50 | 51 |
| APOE e4 carrier (%) | 30 | 28 |
| REALM score >60 (%) | 81 | 73 |
| Baseline 3MS score | 91.2 (7.3) | 90.0 (8.1) |
| Testosterone serum level (pg/ml) † | 2.6 (2.0) | 6.2 (3.0) |
| Estradiol plasma level (pg/ml) † | 9.7 (3.0) | 31.2 (13.5) |
Testosterone and Estradiol available for 422 women and 409 men
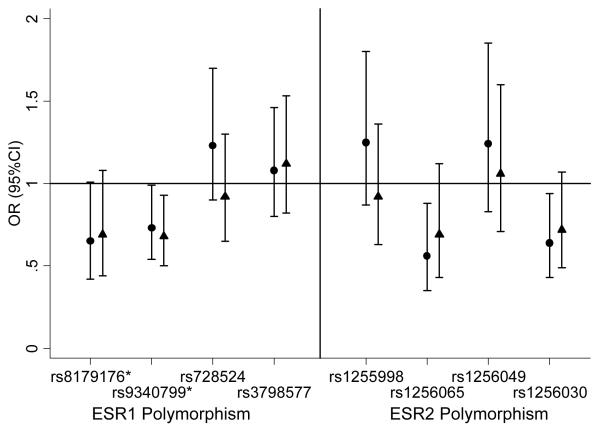
Overall, cognitive impairment (3MS decline ≥5 points over 4 years) occurred in 352 (26%) of the women and 266 (22%) of the men. In unadjusted analyses, cognitive impairment was associated (either statistically or trend-level significance) with 3 of the 4 ESR1 polymorphisms among women: rs8179176 (GG vs. AA or AG, OR=0.61; 95%CI 0.40-0.94), rs9340799 (GG vs. AA or AG, OR=0.76; 95%CI 0.57-1.02) and rs728524 (GG or GA vs. AA, OR=1.33; 95%CI 0.98-1.80) (Table 2a). Heterozygotes and homozygotes that were grouped together did not differ from each other in terms of cognitive impairment except for with rs728524 (GG vs GA, OR=2.36; 95%CI 1.15-4.80). After adjustment for baseline age, education and 3MS score, the association with ESR1 rs8179176 was slightly lessened (GG vs. AA or AG, OR=0.65; 95%CI 0.42-1.01), the association with ESR1 rs9340799 was strengthened (GG vs. AA or AG, OR=0.73; 95%CI 0.54-0.99), and the association with ESR1 rs728524 became non-significant (GG or GA vs. AA, OR=1.23; 95%CI 0.90-1.70). Further adjustment for race led to similar results.(Figure 1a).
Table 2a.
Odds of developing cognitive impairment (unadjusted and multivariate adjusted for age, education and baseline cognition) over 4 years by ESR1 and ESR2 single nucleotide polymorphism genotype in women.
| ESR1 SNP Genotype |
N With Genotype |
% Impaired |
Unadjusted OR (95% CI) |
Multivariate OR (95% CI) |
ESR2 SNP Genotype |
N With Genotype |
% Impaired |
Unadjusted OR (95% CI) |
Multivariate OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| rs8179176 | rs1255998 | ||||||||
| GG | 151 | 19 | 0.61 (0.40-0.94) | 0.65 (0.42-1.01) | GG | 171 | 33 | 1.51 (1.06-2.13) | 1.25 (0.87-1.80) |
| AA or AG | 1163 | 27 | 1.0 (ref) | 1.0 (ref) | CC or CG | 1131 | 25 | 1.0 (ref) | 1.0 (ref) |
| rs9340799 | rs1256065 | ||||||||
| GG | 335 | 22 | 0.76 (0.57-1.02) | 0.73 (0.54-0.99) | CC | 144 | 16 | 0.51 (0.32-0.81) | 0.56 (0.35-0.88) |
| AA or AG | 965 | 27 | 1.0 (ref) | 1.0 (ref) | AA or AC | 1155 | 27 | 1.0 (ref) | 1.0 (ref) |
| rs3728524 | rs1256049 | ||||||||
| GG or GA | 255 | 31 | 1.33 (0.98-1.80) | 1.23 (0.90-1.70) | AA or AG | 134 | 32 | 1.39 (0.94-2.04) | 1.24 (0.83-1.85) |
| AA | 1052 | 25 | 1.0 (ref) | 1.0 (ref) | GG | 1181 | 25 | 1.0 (ref) | 1.0 (ref) |
| rs3798577 | rs1256030 | ||||||||
| CC | 1007 | 26 | 1.04 (0.77-1.39) | 1.08 (0.80-1.46) | TT | 197 | 18 | 0.58 (0.39-0.85) | 0.64 (0.43-0.94) |
| TT or TC | 299 | 26 | 1.0 (ref) | 1.0 (ref) | CC or CT | 1108 | 27 | 1.0 (ref) | 1.0 (ref) |
Most common allele frequencies: ESR1 rs8179176 A=58%, rs9340799 A=50%, rs728524 A=88%, rs3798577 T=53%; ESR2 rs1255998 C=70%, rs1256065 A=72%, rs1256049 G=95%, rs1256030 C=64%.
Figure 1a.
The association between ESR1 and ESR2 single nucleotide polymorphisms (SNPs) and risk of developing cognitive impairment in women.
● Adjusted for age, education and baseline 3MS score.
▲ Adjusted for age, education, baseline 3MS score and race.
*rs8179176 is PvuII and rs9340799 is XbaI.
Cognitive impairment was associated with 3 of the 4 ESR2 polymorphisms among women in unadjusted analyses: rs1255998 (GG vs. CC or CG, OR=1.51; 95%CI 1.06-2.13); rs1256065 (CC vs. AA or AC, OR=0.51; 95%CI 0.32-0.81); rs1256030 (TT vs. CC or CT, OR=0.58; 95%CI 0.39-0.85) (Table 2a). Heterozygotes and homozygotes that were grouped together did not differ from each other in terms of cognitive impairment except for with rs1255998 (CC vs CG, OR=0.66; 95%CI 0.50-0.87). Adjustment for baseline age, education and 3MS score led to similar results except for rs1255998, which became non-significant (GG vs. CC or CG, OR=1.25; 95%CI 0.87-1.80). Further adjustment for race resulted in a lessening of the association between rs1256065 (CC vs. AA or AC, OR=0.69; 95%CI 0.43-1.12) and rs1256030 (TT vs. CC or CT, OR=0.72; 95%CI 0.49-1.07) (Figure 1a).
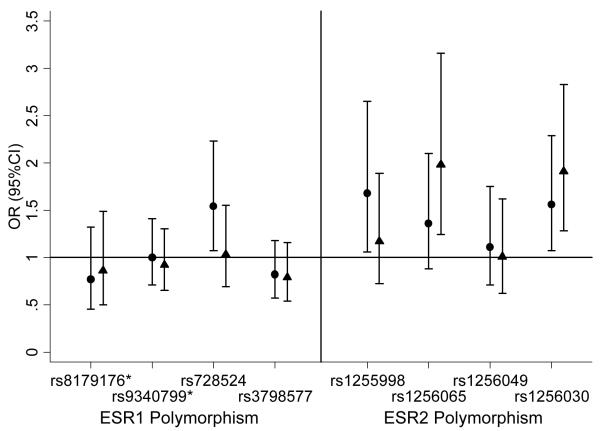
Among men, cognitive impairment was associated with 1 of the 4 ESR1 polymorphisms in unadjusted analyses: rs728524 (GG or GA vs. AA, OR=1.79; 95%CI 1.25-2.57) (Table 2b). Heterozygotes and homozygotes that were grouped together did not differ from each other in terms of cognitive impairment. After adjustment for baseline age, education and 3MS score, results were similar. However, additional adjustment for race resulted in no association (Figure 1b). Cognitive impairment was associated with 2 of the 4 ESR2 polymorphisms among men in unadjusted analyses: ESR2 rs1255998 (GG vs. CC or CG, OR=1.96; 95%CI 1.29-3.00) and ESR2 rs1256030 (TT vs. CC or CT, OR=1.48; 95%CI 1.02-2.14) (Table 2b). Heterozygotes and homozygotes that were grouped together did not differ from each other in terms of cognitive impairment. After adjustment for baseline age, education and 3MS score, cognitive impairment was still significantly associated with rs1255998 (GG vs. CC or CG, OR=1.68; 95%CI 1.06-2.65) and rs1256030 (TT vs. CC or CT, OR=1.56; 95%CI 1.07-2.29). Further adjustment for race made the association with rs1255998 non-significant, but resulted in a significant association with rs126065 (CC vs. AA or AC OR=1.98; 95%CI 1.24-3.16) and strengthened the association with rs1256030 (TT vs. CC or CT, OR=1.91; 95%CI 1.28-2.83) (Figure 1b).
Table 2b.
Odds of developing cognitive impairment (unadjusted and multivariate adjusted for age, education and baseline cognition) over 4 years by ESR1 and ESR2 single nucleotide polymorphism genotype in men.
| ESR1 SNP Genotype |
N With Genotype |
% Impaired |
Unadjusted OR (95% CI) |
Multivariate adjusted OR (95% CI) |
ESR2 SNP Genotype |
N With Genotype |
% Impaired |
Unadjusted OR (95% CI) |
Multivariate adjusted OR (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| rs8179176 | rs1255998 | ||||||||
| GG | 115 | 18 | 0.74 (0.45-1.21) | 0.77 (0.45-1.32) | GG | 110 | 35 | 1.96 (1.29-3.00) | 1.68 (1.06- 2.65) |
| AA or AG | 1034 | 23 | 1.0 (ref) | 1.0 (ref) | CC or CG | 1039 | 21 | 1.0 (ref) | 1.0 (ref) |
| rs9340799 | rs1256065 | ||||||||
| GG | 245 | 23 | 1.03 (0.73-1.44) | 1.00 (0.71-1.41) | CC | 139 | 26 | 1.23 (0.82-1.85) | 1.36 (0.88- 2.10) |
| AA or AG | 899 | 22 | 1.0 (ref) | 1.0 (ref) | AA or AC | 997 | 22 | 1.0 (ref) | 1.0 (ref) |
| rs3728524 | rs1256049 | ||||||||
| GG or GA | 171 | 32 | 1.79 (1.25-2.57) | 1.54 (1.07-2.23) | AA or AG | 122 | 25 | 1.21 (0.78-1.86) | 1.11 (0.71- 1.75) |
| AA | 977 | 20 | 1.0 (ref) | 1.0 (ref) | GG | 1044 | 22 | 1.0 (ref) | 1.0 (ref) |
| rs3798577 | rs1256030 | ||||||||
| CC | 249 | 20 | 0.87 (0.61-1.23) | 0.82 (0.57-1.18) | TT | 169 | 28 | 1.48 (1.02-2.14) | 1.56 (1.07- 2.29) |
| TT or TC | 889 | 23 | 1.0 (ref) | 1.0 (ref) | CC or CT | 971 | 21 | 1.0 (ref) | 1.0 (ref) |
Most common allele frequencies: ESR1 rs8179176 A=67%, rs9340799 A=52%, rs728524 A=91%, rs3798577 T=54%; ESR2 rs1255998 C=74%, rs1256065 A=68%, rs1256049 G=95%, rs1256030 C=62%.
Figure 1b.
The association between ESR1 and ESR2 single nucleotide polymorphisms (SNPs) and risk of developing cognitive impairment in men.
● Adjusted for age, education and baseline 3MS score.
▲ Adjusted for age, education, baseline 3MS score and race.
*rs8179176 is PvuII and rs9340799 is XbaI.
We found statistically significant interactions between gender and 2 of the 8 ER polymorphisms on cognitive impairment. ESR2 rs1256065 genotypes AA and AC vs. CC were associated with lower rates of cognitive impairment in women and higher rates in men (p for interaction =0.005). The ESR2 rs1256030 genotype TT vs. CC and CT was associated with lower rates of cognitive impairment in women and higher rates in men (p for interaction =0.001). There were no significant associations between plasma estradiol levels and any of the 8 polymorphisms in men or women. However, testosterone level did vary among 2 of the SNPs associated with cognitive impairment (ESR2 rs1256065 for women and ESR rs1255998 in men, p<0.05 for both). When we added testosterone concentration to the adjusted model, results did not change appreciably, nor did the gender interactions. There was no interaction with APOE e4 and ESR SNPs on cognitive impairment.
Traditional multivariate logistic regression models yielded very similar estimates of odds ratios and confidence intervals. In addition, haplotype analyses were in broad agreement with our polymorphism analyses [4]. For example, in both men and women, those with ESR1 haplotypes containing an A in rs728524 were less likely to have cognitive impairment than those with a G in that position. Women with ESR2 haplotypes containing a C in rs1256065 were less likely to have cognitive impariment than those with an A in that position. Men with ESR2 haplotypes containing a C in rs1255998 and in rs1256030 (especially GAGT) had less cognitive impairment than others.
4. Discussion
Among approximately 2500 community-dwelling elders, risk of developing cognitive impairment was associated with 2 of the 4 ESR1 and 2 of the 4 ESR2 SNPs among women and among men, risk of cognitive impairment was associated with 2 of the 4 ESR2 SNPs. Additional adjustment for race, however, tended to diminish some of the associations although in other cases, it strengthened them.
To our knowledge, no previous studies have investigated the role of ESR2 SNPs and cognition in women or the role of either the ESR1 or ESR2 SNPs and cognition in men. However, our results are generally in line with prior investigations of ESR1 SNPs and risk of development of AD in women. Case-control studies have reported that the ESR1 PvuII and XbaI polymorphisms are associated with AD and cognitive impairment [3,6,15]. We previously found, as part of a prospective study, that the PvuII and XbaI polymorphisms were associated with rates of 4-year cognitive decline in older women [31]. These SNPs correspond to ESR1 rs8179176 (PvuII) and rs9340799 (XbaI) and thus, our and other groups’ prior results for XbaI and PvuII were confirmed in the present study suggesting that these associations appear to be robust. Recently, Kravitz and colleagues reported that 2 ESR1 SNPs (including rs9340799) but no ESR2 SNPs were associated with cross-sectional cognitive performance in middle-aged women [13]. Interestingly, in that multi-ethnic cohort, the associations between the SNPs and cognitive function varied by race/ethnicity.
The strong association between the ESR1 and ESR2 SNPs and likelihood of developing cognitive decline is also consistent with a large body of animal and molecular data suggesting that estrogen exerts a beneficial effect on the central nervous system [20]. Estrogen receptors are located throughout the brain, especially in areas involved in learning and memory such as the hippocampus and amygdale [27] and the enzymes necessary for sex steroid biosynthesis have been identified in these same regions [28]. Ovarectomized ESR1 knock-out mice have impaired performance on a hippocampal-dependent cognitive task that is reversed with estradiol administration [8]. The abundant localization of the ESR2 in human hippocampus supports a role for this receptor in cognition as well [22,25]. Indeed, number of ESR2 has been found to be increased in the brains of patients with AD compared to elderly controls [25].
The polymorphisms may affect ESR gene enhancer activity [17] or gene regulation [1], but how they influence cognitive outcomes is not exactly known. It is possible that the SNPs influence sex hormone levels and this in turn, affects cognitive function. Some previous studies have reported that certain ESR1 variants are associated with estradiol levels in older women but not in men [26]. Additionally, an ESR2 polymorphism (a polymorphic dincucleotide CA repeat in the noncoding 3′-portion of the gene) has been associated with total and free testosterone levels in young women [30]. However, we did not find any significant associations between plasma estradiol levels and any of the 8 polymorphisms in men or women. While 2 of the SNPs were associated with serum concentration of free testosterone, statistical adjustment for this measure did not account for the association between the SNP and cognitive impairment. Finally, there is animal data and emerging human studies to suggest that there may be an interaction of ESR1 SNPs with the APOE e4 allele on risk of developing AD [6]. However, we did not find an interaction with APOE e4 and any of the ESR SNPs on cognitive impairment.
Compared with previous association studies, our study has several strengths. First, we had a relatively large sample with 1343 women and 1184 men who had genetic analysis and cognitive testing over time. Second, we had a wealth of data on comorbidities and demographics that we could adjust for in order to determine if the association between ESR SNPs and cognitive decline were independent of these possible confounders. Thirdly, we were able to determine if there were gender and race interactions for the association between genetic variations in ESR1 and ESR2 and cognitive impairment since our cohort was comprised of nearly half men and women and Blacks and Whites. While we did not find an interaction with the ESR SNPs and race on cognitive decline, we did find differences in SNP frequencies by race and the association with cognitive decline was attenuated with adjustment for race. Interestingly, there was a gender interaction with the 2 ESR2 SNPs and risk of cognitive impairment whereby both SNPs conferred a decreased risk of cognitive impairment in women and an increased risk in men. This was not accounted for by adjustment for level of sex hormones.
While this is the first study to examine the prospective association between ESR1 and ESR2 NPs and cognitive function in a large prospective study of elderly men and women, we are limited in the interpretation of our findings due to the lack of etiology of cognitive impairment. Participants in our cohort also did not undergo a clinical evaluation to determine whether the cognitive impairment was due to vascular disease or AD. We also had some participants without follow-up cognitive testing which may have limited our ability to evaluate prospective associations with change in cognitive function. Furthermore, we did not conduct correction for multiple comparisons, although we limited our analyses to studying the 8 SNPS listed only.
We found that among non-demented community elders, several SNPS in the ESR1 and ESR2 genes were associated with risk of cognitive impairment. These findings suggest that estrogen receptor genetic variants may play a role in cognitive aging.
Acknowledgments
Funded by N01-AG-6-2101, N01-AG-6-2103, N01-AG-6-2106 and R01-AG021918. This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging.
Footnotes
There are no actual or potential conflicts of interest for any of the other authors.
REFERENCES
- [1].Albagha OM, McGuigan FE, Reid DM, Ralston SH. Estrogen receptor alpha gene polymorphisms and bone mineral density: haplotype analysis in women from the United Kingdom. J Bone Miner Res. 2001;16(1):128–34. doi: 10.1359/jbmr.2001.16.1.128. [DOI] [PubMed] [Google Scholar]
- [2].Anderson TI, Wooster R, Laake K, Collins N, Warren W, Skrede M, Elles R, Tveit KM, Johnston SR, Dowsett M, Olsen AO, Moller P, Stratton MR, Borresen-Dale AL. Screening for ESR mutations in breast and ovarian cancer patients. Hum Mutat. 1997;9(6):531–6. doi: 10.1002/(SICI)1098-1004(1997)9:6<531::AID-HUMU6>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- [3].Brandi ML, Becherini L, Gennari L, Racchi M, Bianchetti A, Nacmias B, Sorbi S, Mecocci P, Senin U, Govoni S. Association of the estrogen receptor alpha gene polymorphisms with sporadic Alzheimer’s disease. Biochem Biophys Res Commun. 1999;265(2):335–8. doi: 10.1006/bbrc.1999.1665. [DOI] [PubMed] [Google Scholar]
- [4].Clayton D, Chapman J, Cooper J. Use of unphased multilocus genotype data in indirect association studies. Genet Epidemiol. 2004;27(4):415–28. doi: 10.1002/gepi.20032. [DOI] [PubMed] [Google Scholar]
- [5].Clayton D, Hills M. Statistical Methods in Epidemiology. Oxford University Press; 1993. [Google Scholar]
- [6].Corbo RM, Gambina G, Ruggeri M, Scacchi R. Association of estrogen receptor alpha (ESR1) PvuII and XbaI polymorphisms with sporadic Alzheimer’s disease and their effect on apolipoprotein E concentrations. Dement Geriatr Cogn Disord. 2006;22(1):67–72. doi: 10.1159/000093315. [DOI] [PubMed] [Google Scholar]
- [7].Deng HW, Li J, Li JL, Johnson M, Gong G, Recker RR. Association of VDR and estrogen receptor genotypes with bone mass in postmenopausal Caucasian women: different conclusions with different analyses and the implications. Osteoporos Int. 1999;9(6):499–507. doi: 10.1007/s001980050177. [DOI] [PubMed] [Google Scholar]
- [8].Fugger HN, Foster TC, Gustafsson J, Rissman EF. Novel effects of estradiol and estrogen receptor alpha and beta on cognitive function. Brain Res. 2000;883(2):258–64. doi: 10.1016/s0006-8993(00)02993-0. [DOI] [PubMed] [Google Scholar]
- [9].Georgiou I, Syrrou M, Bouba I, Dalkalitsis N, Paschopoulos M, Navrozoglou I, Lolis D. Association of estrogen receptor gene polymorphisms with endometriosis. Fertil Steril. 1999;72(1):164–6. doi: 10.1016/s0015-0282(99)00198-3. [DOI] [PubMed] [Google Scholar]
- [10].Herrington DM, Howard TD, Hawkins GA, Reboussin DM, Xu J, Zheng SL, Brosnihan KB, Meyers DA, Bleecker ER. Estrogen-receptor polymorphisms and effects of estrogen replacement on high-density lipoprotein cholesterol in women with coronary disease. N Engl J Med. 2002;346(13):967–74. doi: 10.1056/NEJMoa012952. [DOI] [PubMed] [Google Scholar]
- [11].Ioannidis JP, Ralston SH, Bennett ST, Brandi ML, Grinberg D, Karassa FB, Langdahl B, van Meurs JB, Mosekilde L, Scollen S, Albagha OM, Bustamante M, Carey AH, Dunning AM, Enjuanes A, van Leeuwen JP, Mavilia C, Masi L, McGuigan FE, Nogues X, Pols HA, Reid DM, Schuit SC, Sherlock RE, Uitterlinden AG. Differential genetic effects of ESR1 gene polymorphisms on osteoporosis outcomes. Jama. 2004;292(17):2105–14. doi: 10.1001/jama.292.17.2105. [DOI] [PubMed] [Google Scholar]
- [12].Isoe-Wada K, Maeda M, Yong J, Adachi Y, Harada H, Urakami K, Nakashima K. Positive association between an estrogen receptor gene polymorphism and Parkinson’s disease with dementia. Eur J Neurol. 1999;6(4):431–5. doi: 10.1046/j.1468-1331.1999.640431.x. [DOI] [PubMed] [Google Scholar]
- [13].Kravitz HM, Meyer PM, Seeman TE, Greendale GA, Sowers MR. Cognitive functioning and sex steroid hormone gene polymorphisms in women at midlife. Am J Med. 2006;119(9 Suppl 1):S94–S102. doi: 10.1016/j.amjmed.2006.07.030. [DOI] [PubMed] [Google Scholar]
- [14].Kuller LH, Shemanski L, Manolio T, Haan M, Fried L, Bryan N, Burke GL, Tracy R, Bhadelia R. Relationship between ApoE, MRI findings, and cognitive function in the Cardiovascular Health Study. Stroke. 1998;29(2):388–98. doi: 10.1161/01.str.29.2.388. [DOI] [PubMed] [Google Scholar]
- [15].Lambert JC, Harris JM, Mann D, Lemmon H, Coates J, Cumming A, St-Clair D, Lendon C. Are the estrogen receptors involved in Alzheimer’s disease? Neurosci Lett. 2001;306(3):193–7. doi: 10.1016/s0304-3940(01)01806-7. [DOI] [PubMed] [Google Scholar]
- [16].Livak KJ. Allelic discrimination using fluorogenic probes and the 5′ nuclease assay. Genet Anal. 1999;14(56):143–9. doi: 10.1016/s1050-3862(98)00019-9. [DOI] [PubMed] [Google Scholar]
- [17].Maruyama H, Toji H, Harrington CR, Sasaki K, Izumi Y, Ohnuma T, Arai H, Yasuda M, Tanaka C, Emson PC, Nakamura S, Kawakami H. Lack of an association of estrogen receptor alpha gene polymorphisms and transcriptional activity with Alzheimer disease. Arch Neurol. 2000;57(2):236–40. doi: 10.1001/archneur.57.2.236. [DOI] [PubMed] [Google Scholar]
- [18].Mattila KM, Axelman K, Rinne JO, Blomberg M, Lehtimaki T, Laippala P, Roytta M, Viitanen M, Wahlund L, Winblad B, Lannfelt L. Interaction between estrogen receptor 1 and the epsilon4 allele of apolipoprotein E increases the risk of familial Alzheimer’s disease in women. Neurosci Lett. 2000;282(12):45–8. doi: 10.1016/s0304-3940(00)00849-1. [DOI] [PubMed] [Google Scholar]
- [19].McDowell I, Newell C. Measuring Health, a Guide to Rating Scales and Questionnaires. 2nd ed Oxford University Press; New York: 1996. [Google Scholar]
- [20].McEwen BS. Clinical review 108: The molecular and neuroanatomical basis for estrogen effects in the central nervous system. J Clin Endocrinol Metab. 1999;84(6):1790–7. doi: 10.1210/jcem.84.6.5761. [DOI] [PubMed] [Google Scholar]
- [21].Modugno F, Zmuda JM, Potter D, Cai C, Ziv E, Cummings SR, Stone KL, Morin PA, Greene D, Cauley JA. Association of estrogen receptor alpha polymorphisms with breast cancer risk in older Caucasian women. Int J Cancer. 2005;116(6):984–91. doi: 10.1002/ijc.21105. [DOI] [PubMed] [Google Scholar]
- [22].Osterlund MK, Grandien K, Keller E, Hurd YL. The human brain has distinct regional expression patterns of estrogen receptor alpha mRNA isoforms derived from alternative promoters. J Neurochem. 2000;75(4):1390–7. doi: 10.1046/j.1471-4159.2000.0751390.x. [DOI] [PubMed] [Google Scholar]
- [23].R Development Core Team . R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2005. [Google Scholar]
- [24].Rivadeneira F, van Meurs JB, Kant J, Zillikens MC, Stolk L, Beck TJ, Arp P, Schuit SC, Hofman A, Houwing-Duistermaat JJ, van Duijn CM, van Leeuwen JP, Pols HA, Uitterlinden AG. Estrogen receptor beta (ESR2) polymorphisms in interaction with estrogen receptor alpha (ESR1) and insulin-like growth factor I (IGF1) variants influence the risk of fracture in postmenopausal women. J Bone Miner Res. 2006;21(9):1443–56. doi: 10.1359/jbmr.060605. [DOI] [PubMed] [Google Scholar]
- [25].Savaskan E, Olivieri G, Meier F, Ravid R, Muller-Spahn F. Hippocampal estrogen beta-receptor immunoreactivity is increased in Alzheimer’s disease. Brain Res. 2001;908(2):113–9. doi: 10.1016/s0006-8993(01)02610-5. [DOI] [PubMed] [Google Scholar]
- [26].Schuit SC, de Jong FH, Stolk L, Koek WN, van Meurs JB, Schoofs MW, Zillikens MC, Hofman A, van Leeuwen JP, Pols HA, Uitterlinden AG. Estrogen receptor alpha gene polymorphisms are associated with estradiol levels in postmenopausal women. Eur J Endocrinol. 2005;153(2):327–34. doi: 10.1530/eje.1.01973. [DOI] [PubMed] [Google Scholar]
- [27].Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388(4):507–25. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- [28].Stoffel-Wagner B. Neurosteroid metabolism in the human brain. Eur J Endocrinol. 2001;145(6):669–79. doi: 10.1530/eje.0.1450669. [DOI] [PubMed] [Google Scholar]
- [29].Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–8. [PubMed] [Google Scholar]
- [30].Westberg L, Baghaei F, Rosmond R, Hellstrand M, Landen M, Jansson M, Holm G, Bjorntorp P, Eriksson E. Polymorphisms of the androgen receptor gene and the estrogen receptor beta gene are associated with androgen levels in women. J Clin Endocrinol Metab. 2001;86(6):2562–8. doi: 10.1210/jcem.86.6.7614. [DOI] [PubMed] [Google Scholar]
- [31].Yaffe K, Lui L, Grady D, Stone K, Morin P. Estrogen Receptor I Polymorphisms and Risk of Cognitive Impairment in Older Women. Biological Psychiatry. 2002;51(8):677–82. doi: 10.1016/s0006-3223(01)01289-6. [DOI] [PubMed] [Google Scholar]