Abstract
Studies in amphibian embryos have suggested that retinoic acid (RA) may function as a signal that stimulates posterior differentiation of the nervous system as postulated by the activation-transformation model for anteroposterior patterning of the nervous system. We have tested this hypothesis in retinaldehyde dehydrogenase-2 (Raldh2) null mutant mice lacking RA synthesis in the somitic mesoderm. Raldh2–/– embryos exhibited neural induction (activation) as evidenced by expression of Sox1 and Sox2 along the neural plate, but differentiation of spinal cord neuroectodermal progenitor cells (posterior transformation) did not occur as demonstrated by a loss of Pax6 and Olig2 expression along the posterior neural plate. Spinal cord differentiation in Raldh2–/– embryos was rescued by maternal RA administration, and during the rescue RA was found to act directly in the neuroectoderm but not the somitic mesoderm. RA generated by Raldh2 in the somitic mesoderm was found to normally travel as a signal throughout the mesoderm and neuroectoderm of the trunk and into tailbud neuroectoderm, but not into tailbud mesoderm. Raldh2–/– embryos also exhibited increased Fgf8 expression in the tailbud, and decreased cell proliferation in tailbud neuroectoderm. Our findings demonstrate that RA synthesized in the somitic mesoderm is necessary for posterior neural transformation in the mouse and that Raldh2 provides the only source of RA for posterior development. An important concept to emerge from our studies is that the somitic mesodermal RA signal acts in the neuroectoderm but not mesoderm to generate a spinal cord fate.
Keywords: Neuroectoderm, Spinal cord, Tailbud, Retinoic acid, Raldh2, Fgf8, Cyp26a1, Olig2, Mouse
1. Introduction
A key objective of embryology is to understand the cell–cell signaling pathways regulating generation of differentiated tissues from undifferentiated stem cells. In amniote vertebrate embryos, the primitive ectoderm (epiblast) consists of a pluripotent embryonic stem cell population which differentiates during primitive streak formation (gastrulation) to produce the three primary germ layers (embryonic ectoderm, mesoderm, and endoderm). As development proceeds, the primitive streak stem cell zone regresses posteriorly and cells emerging from the primitive streak become further differentiated and acquire progressively more posterior characteristics. The activation–transformation model for differentiation of neuroectoderm from primitive ectoderm suggests that an activation event occurs (neural induction) followed by a transforming event which posteriorizes later-forming neuroectoderm (Nieuwkoop, 1952). Neural induction of anterior ectoderm to form prospective brain has been found in amphibian embryos to occur in response to bone morphogenetic protein (BMP) antagonists produced in underlying mesoderm generated in the primitive streak (Sasai and de Robertis, 1997). The involvement of BMP antagonists in amniote neural induction is less clear, but fibroblast growth factor (FGF) produced in the primitive streak and tailbud has been implicated (Stern, 2002). FGF8 signaling is particularly important in the mouse as it is necessary for posterior neural induction as well as generation of mesoderm during gastrulation (Sun et al., 1999). In addition to these signals, paraxial mesoderm emerging from the regressing primitive streak provides another signal needed for differentiation of the posterior nervous system (Muhr et al., 1999). One function of this paraxial mesodermal factor is to attenuate posterior FGF signaling mediated by Fgf8 expressed in the tailbud (Bertrand et al., 2000; Del Corral et al., 2002). Recent studies in avian embryos indicate that retinoic acid (RA) is the paraxial mesodermal factor, and evidence was provided that opposing actions of RA and FGF signaling pathways control posterior neuronal differentiation (Del Corral et al., 2003; Novitch et al., 2003). Treatment of amphibian embryos with exogenous RA suggests that this nuclear receptor ligand may be a factor that causes a posterior transformation of the nervous system, thus supporting the activation–transformation model for anteroposterior subdivision of the nervous system (Durston et al., 1989; Sive et al., 1990). However, it remains unclear if endogenous RA functions as the postulated posterior transformation signal or whether it also acts upstream at the neural induction step (formation of neuroectoderm from primitive ectoderm). Also, it is unknown whether the RA that opposes tailbud FGF signaling is synthesized exclusively in the paraxial mesoderm, and to what extent RA can travel from paraxial mesoderm (or other potential sources) to target tissues.
The concept of embryonic tissue differentiation occurring in regions of opposing RA and FGF signals was originally demonstrated in studies of proximodistal outgrowth of chick limb buds (Mercader et al., 2000). In mouse embryos, Fgf8 is required to generate a distal FGF signal needed for limb outgrowth (Lewandoski et al., 2000; Moon and Capecchi, 2000). A gene critical for mouse limb RA synthesis has also been identified. Mouse gene knockout studies revealed that several overlapping alcohol dehydrogenases catalyze the first step of RA synthesis, oxidation of retinol (vitamin A) to retinaldehyde (Molotkov et al., 2002), whereas a single gene encoding retinaldehyde dehydrogenase-2 (Raldh2) is essential for the second step of RA synthesis in most embryonic tissues, i.e. oxidation of retinaldehyde to RA (Niederreither et al., 1999; Mic et al., 2002). Further studies revealed that all-trans-RA is the endogenous ligand needed to rescue Raldh2–/– embryonic development, and that its isomer 9-cis-RA is undetectable and unnecessary (Mic et al., 2003). We have further demonstrated that Raldh2 is required to generate a proximal RA signal in the lateral plate mesoderm that travels distally into the limb bud during outgrowth (Mic et al., 2004b). Thus, Raldh2 and Fgf8 function as the primary genetic factors involved in generating opposing RA and FGF signals needed for limb development. As Raldh2 is also essential for RA synthesis in the paraxial mesoderm, we have now examined Raldh2–/– embryos to test the hypothesis that Raldh2 is required for posterior neural development and to further examine the mechanism of RA action using a genetic loss-of-function model. Our studies indicate that Raldh2 is not required for neural induction of ectoderm emerging from the tailbud, but that it is required for posteriorization of tailbud neuroectoderm to generate cells characteristic of the spinal cord. We demonstrate that RA generated in the somitic mesoderm by RALDH2 is the only source of RA for posterior development and that RA travels throughout the posterior neuroectoderm (trunk and tailbud) and trunk mesoderm, but not into the tailbud mesoderm. Our studies also revealed that the RA which reaches the tailbud neuroectoderm is required to define the anterior border of Fgf8 expression and to stimulate proliferation of neuroectodermal progenitor cells. In summary, our findings indicate that somitic RA generated by Raldh2 is required to drive neuroectoderm to a spinal cord fate and that RA acts directly in posterior neuroectoderm but not mesoderm during this process.
2. Results
2.1. Raldh2 is responsible for all RA activity detected in mouse embryos at E8.5
Genetic studies have revealed that RA signaling activity in mouse embryos is dependent upon Raldh2 encoding an aldehyde dehydrogenase that synthesizes RA (Niederreither et al., 1999; Mic et al., 2002), and Cyp26a1 encoding a P450 that degrades RA (Sakai et al., 2001; Abu-Abed et al., 2001). Raldh2 is initially expressed during mouse development at E7.5 in the paraxial mesoderm, consistent with a role in posterior but not anterior axis development in late primitive streak stage embryos. The sites of RA synthesis and degradation in the posterior region of an E8.5 wild-type mouse embryo are shown by double hybridization with Raldh2 and Cyp26a1 probes (Fig. 1A). Raldh2 mRNA is localized in the somitic paraxial mesoderm anterior to the tailbud while Cyp26a1 mRNA exists in the tailbud, with the gap between the two domains encompassing the presomitic mesoderm.
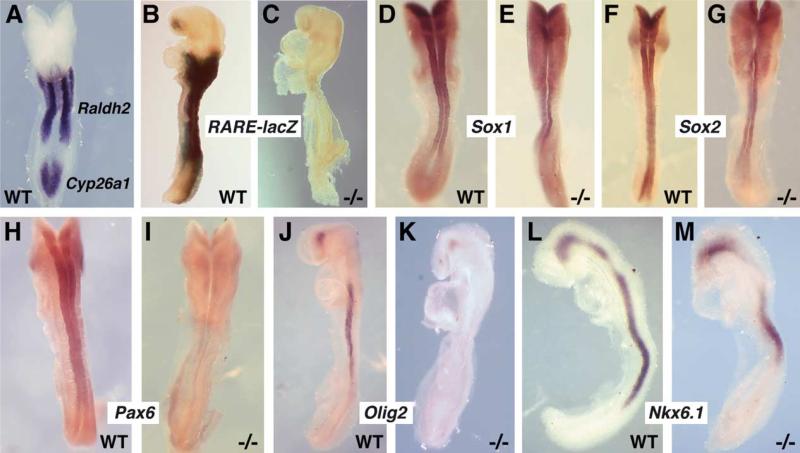
Fig. 1.
RA is unnecessary for neural induction but needed for neuronal differentiation. (A) Double hybridization to detect Raldh2 and Cyp26a1 mRNAs in an E8.5 wild-type (WT) embryo. (B–C) RARE-lacZ expression (RA activity) in E8.5 wild-type and Raldh2–/– (–/–) embryos; Raldh2–/– embryos totally lack RA activity. (D–E) Sox1 and (F–G) Sox2 mRNAs were examined at E8.5 by whole-mount in situ hybridization; these Sox genes are markers of neural induction and were still expressed all along the neural tube of Raldh2–/– embryos (Raldh2–/– embryos have reduced neural plate folding in the hindbrain region, but neural tube closure occurs in the spinal cord). (H–I) Pax6 mRNA in wild-type and Raldh2–/– embryos at E8.5; the Raldh2–/– embryo lacks Pax6 mRNA in the posterior neural tube, whereas the wild-type embryo exhibits expression throughout the hindbrain and spinal cord. (J–K) Olig2 mRNA is not detected in the posterior neural tube of an E8.5 Raldh2–/– embryo, but expression is still observed in a small domain of the forebrain. (L–M) Nkx6.1 mRNA is still detected in the brain and posterior neural tube of an E8.5 Raldh2–/– embryo with a posterior border of expression at the anterior end of the tailbud (in both wild-type and mutant there is a gap of expression in the hindbrain, with the mutant having a larger gap).
RA activity was examined in embryos carrying the RARE-lacZ RA-reporter transgene (Rossant et al., 1991). RARE-lacZ expression indicated that RA signaling normally occurs within the Raldh2 expression domain (plus to a certain extent anterior and posterior), but that all RA signaling activity is lost in Raldh2–/– embryos (N=3; Fig. 1B–C). It can also be observed that RA activity in wild-type embryos is reduced in the tailbud where Cyp26a1 is expressed, consistent with its role in RA degradation.
2.2. RA is not required for neural induction
As ectoderm emerges from the tailbud, Sox1 and Sox2, encoding high-mobility group (HMG) transcription factors, are expressed in ectoderm that has undergone neural induction (Pevny et al., 1998). Thus, Sox1 and Sox2 play a role in differentiating neuroectoderm from non-neural ectoderm which will form epidermis. In order to examine whether RA is needed for neural induction, we examined E8.5 wild-type and Raldh2–/– embryos for expression of Sox1 and Sox2 mRNAs. Raldh2–/– embryos expressed Sox1 (N=2) and Sox2 (N=2) at relatively normal levels in neuroectoderm, indicating that RA is unnecessary for neural induction (Fig. 1D–G). The expression domains of Sox1 and Sox2 appear wider in the hindbrain region of Raldh2–/– embryos compared to wild-type due to incomplete closure of the neural tube in the mutant.
2.3. Raldh2 is required for posterior neuronal differentiation
Posteriorization of neuroectoderm during the late stages of gastrulation allows hindbrain and spinal cord to be differentiated from forebrain and midbrain (Muhr et al., 1999). During the process of hindbrain specification, several Hox genes including Hoxb1 are normally induced, and this is lost in Raldh2–/– embryos (Niederreither et al., 2000). This demonstrates that RA is required for the early stages of neural posteriorization when hindbrain is forming. Here we have examined the role of RA at later stages of development when the spinal cord is developing. During spinal cord development, overlapping expression of the homeobox transcription factors Pax6 and Nkx6.1 in the posterior neural tube are required for the basic helix-loop-helix transcription factor Olig2 to be expressed all along the anteroposterior axis of the spinal cord in a ventral location (Marquardt and Pfaff, 2001). Genetic studies in the mouse have demonstrated that Olig2 is required for spinal cord motor neuron differentiation (Lu et al., 2002; Takebayashi et al., 2002). We found that a lack of RA synthesis in Raldh2–/– mouse embryos resulted in a loss of Pax6 (N=3) and Olig2 (N=3) expression in the posterior neural tube at E8.5 (Fig. 1H–K). A small expression domain of Olig2 that normally exists in the forebrain was not eliminated in Raldh2–/– embryos (Fig. 1J–K). We also previously reported that the forebrain expression domain of Pax6 (not shown here) is still retained in Raldh2–/– embryos (Mic et al., 2004a). We found that Nkx6.1 expression remained in both anterior and posterior neural tissues of Raldh2–/– embryos (N=3), indicating that RA is not required for its expression (Fig. 1L–M). Our findings indicate that RA signaling is not needed during neural induction, but that RA is needed for undifferentiated posterior neuroectoderm to differentiate to a spinal cord fate. This requirement for RA during posterior neural transformation is conserved between mammalian and avian embryos as investigations with quail and chick embryos have also shown that RA signaling is necessary to induce Pax6 and Olig2 but not Nkx6.1 in the spinal cord (Del Corral et al., 2003; Novitch et al., 2003).
2.4. Distribution of RA from its site of synthesis in the somitic mesoderm
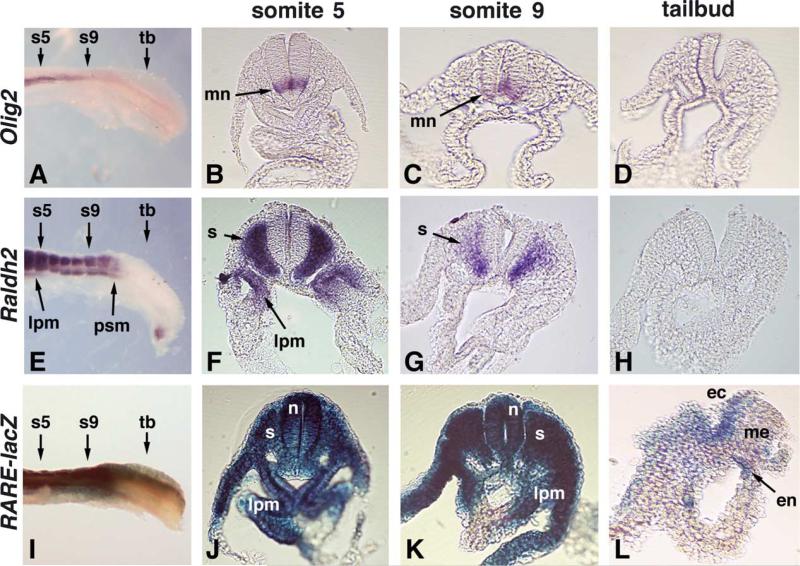
Previous studies suggested that Raldh2 may be responsible for synthesizing the RA needed for spinal cord motor neuron differentiation as Raldh2 is expressed in somitic mesoderm adjacent to the neural tube (Del Corral et al., 2003; Novitch et al., 2003). Our genetic studies have now shown that Raldh2 is responsible for all RA detected posteriorly in mouse embryos at E8.5 and that Raldh2 is necessary for Olig2 expression in the spinal cord. Thus, it can be concluded that RA is needed for posterior differentiation in mouse embryos and that Raldh2 expressed in the somitic mesoderm is the only source of RA synthesis for this process in mouse embryos. However, the extent of RA distribution from sites of Raldh2 expression during posterior development is unclear. This was examined by directly comparing the expression patterns of Olig2, Raldh2, and RARE-lacZ along the anteroposterior and dorsoventral axes of 10-somite stage mouse embryos (Fig. 2). Raldh2 expression was observed in the somites with a posterior border in the presomitic mesoderm where the next somite was just beginning to condense (Fig. 2E–H). Olig2 expression was observed ventrally in the developing motor neuron field adjacent to the Raldh2 expression domain (Fig. 2A–D). RARE-lacZ expression was observed at high levels in the trunk mesoderm and neural tube and at lower levels further posterior in the tailbud (Fig. 2I–L). Transverse sections showed that RARE-lacZ is expressed throughout the dorsoventral axis of the neural tube adjacent to somite 5 and somite 9, thus demonstrating that RA synthesized by Raldh2 in the somites can travel to all portions of the posterior neural tube (Fig. 2F,G,J,K). Transverse sections also revealed that RARE-lacZ expression occurs at lower levels in the tailbud neuroectoderm and endoderm but not mesoderm (Fig. 2L). These findings provide evidence that RA may function cell non-autonomously during posterior neuronal differentiation, with Raldh2 generating RA in the somitic mesoderm that travels to the neural tube where it may induce Pax6 and Olig2. As RA was not localized to any particular region of the neural tube, this may explain its ability to induce not only Olig2, which is limited to the developing motor neuron domain, but also Pax6 which is expressed more widely across the dorsoventral axis of the neural tube (Marquardt and Pfaff, 2001). Also, we conclude that the RA generated by Raldh2 in the somitic mesoderm travels posteriorly to nascent tailbud neuroectoderm, suggesting that RA may also function in this location.
Fig. 2.
Raldh2 functions cell non-autonomously for generation of RA detected in the spinal cord and tailbud. Wild-type embryos at E8.5 (10 somite stage) were compared for expression of the target gene Olig2 (A–D), for the source of RA synthesis by Raldh2 (E–H), and for the distribution of RA activity detected with RARE-lacZ (I–L). For each, transverse sections are shown at somite 5 (s5), somite 9 (s9), and the tailbud (tb). Raldh2 expression is limited to the somites, anterior presomitic mesoderm, and anterior lateral plate mesoderm. A high level of RA activity (RARE-lacZ) is observed throughout the dorsoventral axis of the neural tube (J,K); the developing motor neuron field (marked by Olig2 expression) is included within this region of high RA activity (B,C). Weaker RA activity is also detected in the tailbud, but is absent from the mesoderm (I, L). ec, ectoderm; en, endoderm; lpm, lateral plate mesoderm; me, mesoderm; mn, motor neuron field; n, neural tube; psm, presomitic mesoderm; s, somite; tb, tailbud.
2.5. Maternal administration of RA rescues Olig2 expression via a direct effect on the neuroectoderm
We have previously shown that development of Raldh2–/– embryos can be substantially rescued by a low dose maternal dietary RA supplement that returns embryonic RA levels to approximately the same level observed in wild-type embryos (Mic et al., 2003). We found here that Olig2 expression was rescued in E9.5 Raldh2–/– embryos following such maternal RA administration starting at E6.75. Progressively more Olig2 expression was observed with longer RA treatments such that treatment ending at E7.75 resulted in very little Olig2 expression at E9.5 (N=2), treatment ending at E8.5 provided substantial expression of Olig2 at E9.5 (N=2), and continuous treatment up to the point of analysis at E9.5 gave results indistinguishable from untreated wild-type controls (N=2) (Fig. 3A–D). It can be concluded that continuous RA signaling is needed to induce Olig2 expression as new neural tube tissue is generated from the tailbud.
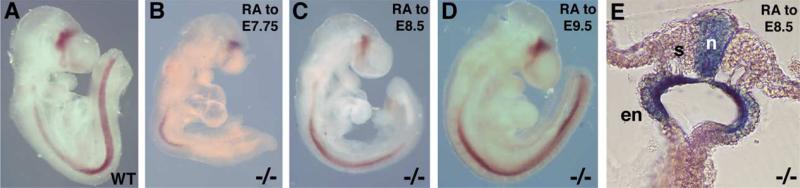
Fig. 3.
Maternal dietary RA treatment rescues spinal cord Olig2 expression in Raldh2–/– embryos. (A) Olig2 expression in E9.5 wild-type embryo (untreated control). Olig2 expression is also shown in E9.5 Raldh2–/– embryos following maternal dietary RA treatment from E6.5–E7.75 (B), E6.75–E8.25 (C), or E6.75–E9.5 (D); progressively more Olig2 expression along the anteroposterior axis of the spinal cord is observed in Raldh2–/– embryos with longer RA treatments. (E) RARE-lacZ expression was examined in an E8.5 Raldh2–/– embryo treated with RA from E6.75–E8.5; shown is a transverse section through the spinal cord at the level of somite 9 demonstrating that maternally administered RA has reached the neural tube and endoderm, but is absent in the somitic mesoderm. en, endoderm; n, neural tube; s, somite.
In order to examine the mechanism whereby maternal RA rescues embryonic Olig2 expression, we examined RARE-lacZ expression in E8.5 Raldh2–/– embryos following low dose maternal dietary RA administration from E6.75 to the point of analysis at E8.5. A transverse section of such an embryo shows that RA signaling activity is occurring throughout the neural tube and endoderm, but not in the mesoderm (Fig. 3E). Interestingly, RARE-lacZ expression was not observed in the somitic mesoderm of Raldh2–/– embryos subjected to this dietary RA rescue (N=4), indicating that the low dose of exogenous RA entering the embryo was not distributed evenly (Fig. 3E); compare to pattern of endogenous RA in wild-type embryos (Fig. 2K). This phenomenon is clearly dose-dependent as large doses of exogenous RA have previously been shown to induce RARE-lacZ in all cells of the embryo at E8.5 (Rossant et al., 1991; Mic et al., 2002). As the normal site of RA synthesis in the somitic mesoderm did not exhibit RA signaling activity in Raldh2–/– embryos under these rescue conditions, this suggests that RA does not need to act in the mesoderm during posterior neuronal differentiation.
2.6. RA generated by Raldh2 limits the anterior border of Fgf8 expression in the tailbud
Fgf8 is normally expressed in the tailbud, but its expression is extinguished anteriorly as cells exit the tailbud (Bertrand et al., 2000; Del Corral et al., 2002). There have been conflicting results concerning the ability of RA to effect posterior Fgf8 expression, with results in avian embryos suggesting that RA reduces Fgf8 expression in the tailbud (Del Corral et al., 2003), and results in Xenopus embryos suggesting that RA induces Fgf8 expression in the tailbud (Moreno and Kintner, 2004). Our observation that RA synthesized in the somitic mesoderm of mouse embryos enters the tailbud stimulated us to examine this tissue more closely in Raldh2–/– embryos. We performed double hybridizations to examine expression of Fgf8 and Uncx4.1 in the same embryo; Uncx4.1 is a marker for the posterior portion of each somite (Mansouri et al., 1997). Examination of E8.5 Raldh2–/– and wild-type embryos matched for somite number demonstrated that the somites were smaller and more densely packed in the absence of RA, that the distance from the posterior end of the embryo to the most recently formed somite was increased, and that Fgf8 expression extended further anterior in the tailbud (N=3; Fig. 4A). When matched for somite number, Raldh2–/– embryos appeared overall slightly larger than wild-type embryos, with size increases noticed in the tailbud and head, but a size decrease apparent in the trunk where somites are located. This suggests that in the absence of RA the length of the tailbud increases along its anteroposterior axis at the expense of the trunk, with more Fgf8 expression occurring anteriorly. Our findings thus indicate that RA normally limits the anteroposterior extent of Fgf8 expression in the mouse tailbud, consistent with previous results in avian embryos (Del Corral et al., 2003). Whether this reflects a direct effect of RA on the Fgf8 gene remains to be determined, but this possibility is supported by our results with RARE-lacZ indicating that RA generated by Raldh2 in the somitic mesoderm normally travels to the tailbud neuroectoderm which expresses Fgf8 at relatively high levels.
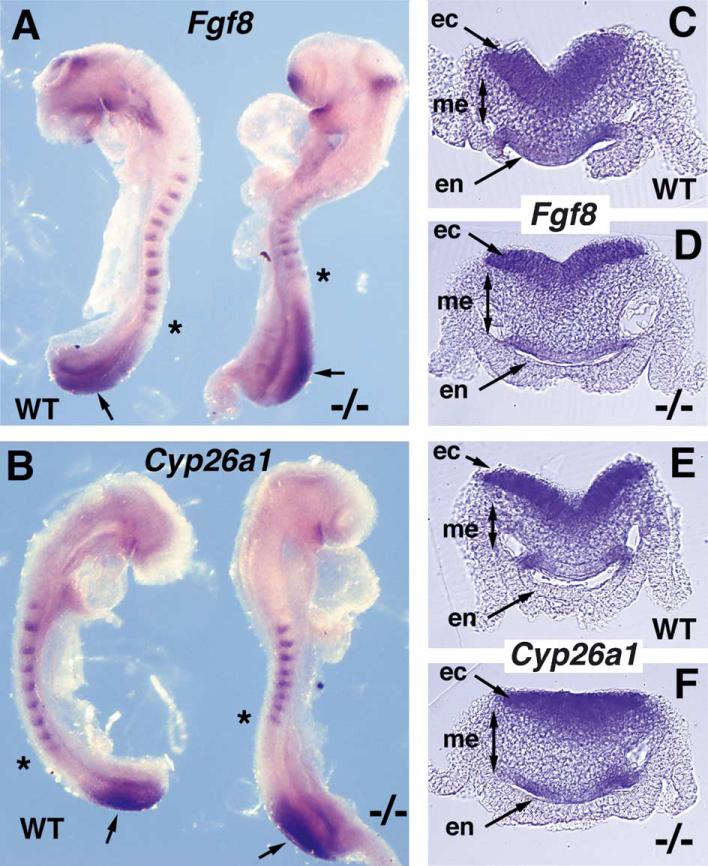
Fig. 4.
Fgf8 and Cyp26a1 expression in the tailbud of Raldh2–/– embryos. (A) Double hybridization showing Fgf8 mRNA in the tailbud and Uncx4.1 mRNA in somites; Raldh2–/– embryos exhibit an expansion of the tailbud along its anteroposterior axis characterized by an anterior shift in the border of Fgf8 expression (wild-type and mutant each have 11 somites with the most posterior somite marked by an asterisk). (B) Double hybridization showing Cyp26a1 mRNA in the tailbud and Uncx4.1 mRNA in somites; the Cyp26a1 tailbud expression domain is not significantly altered in Raldh2–/– embryos (wild-type has 9 somites and mutant has 8 somites with the most posterior somite marked by an asterisk). (C–F) Transverse sections through the tailbud of embryos stained for Fgf8 or Cyp26a1 mRNA (plane of sections depicted by arrows in panels A and B); expression of these genes in the three germ layers is not significantly altered in the mutant; however, Raldh2–/– tailbuds have an expanded mesodermal zone. ec, ectoderm; en, endoderm; me, mesoderm.
We examined transverse sections through the tailbud of embryos stained for Fgf8 expression to determine if a lack of RA effected expression in the ectodermal, mesodermal, or endodermal cell populations. Raldh2–/– embryos were found to maintain relatively normal expression of Fgf8 in all three germ layers (Fig. 4C–D). However, these experiments revealed that the mesodermal compartment was expanded in Raldh2–/– embryos (Fig. 4D). These findings suggest that a lack of RA results not only in an expansion of the tailbud along its anteroposterior axis, but also an expansion of its mesodermal component along the dorsoventral axis.
2.7. Expression of Cyp26a1 in the tailbud is not regulated by endogenous RA
We also examined the effect of a loss of RA on Cyp26a1 expression in the tailbud. Previous studies have indicated that Cyp26a1 expression in the mouse tailbud can be repressed by excess RA (Fujii et al., 1997), but Cyp26a1 expression in the Xenopus tailbud was found to be induced by excess RA (Moreno and Kintner, 2004). We found that Cyp26a1 was expressed at a similar level in the tailbuds of E8.5 Raldh2–/– and wild-type embryos matched for somite number, with no significant expansion anteriorly into the larger tailbud that forms in the absence of RA (N=3; Fig. 4B). Also, Raldh2–/– embryos were found to maintain relatively normal expression of Cyp26a1 in all three germ layers of the tailbud (Fig. 4E–F). Our loss-of-function findings thus indicate that endogenous RA is unnecessary for Cyp26a1 expression in the tailbud, and unnecessary to limit the anterior border of Cyp26a1 expression. We suggest that the previous observations of RA-mediated alterations in posterior Cyp26a1 expression are the results of the use of excess exogenous RA and do not reflect endogenous RA action. In support of this suggestion, recent studies show that zebrafish Cyp26a1 expression is responsive to exogenous RA but not endogenous RA generated by Raldh2 (Dobbs-McAuliffe et al., 2004).
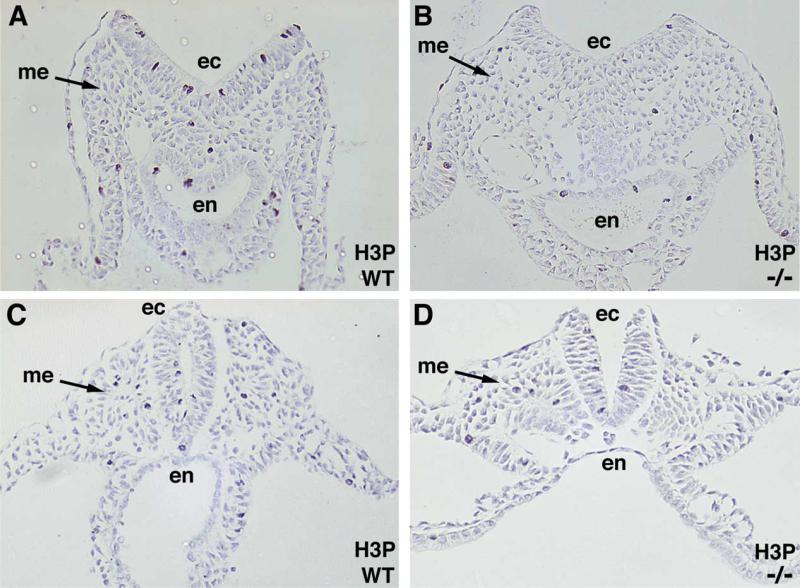
2.8. RA is required to maintain a high level of cell proliferation in the tailbud neuroectoderm
In order to determine if a lack of RA altered cell proliferation during posterior development, we examined tailbuds and spinal cords of E8.5 (10-somite) wild-type and Raldh2–/– embryos with antibodies against phosphohis-tone 3 which is detectable only in cells undergoing mitosis. We found that Raldh2–/– tailbuds had a significantly lower mitotic index in the neuroectoderm compared to wild-type embryos (2.8±0.6% vs. 8.1±0.5%; mutant vs.wild-type; p<0.001), but that the mitotic indices were not significantly different for the underlying mesoderm (2.5±0.2% vs. 3.5±0.3%; mutant vs.wild-type) or endoderm (3.5±1.2% vs. 4.5±0.4%; mutant vs.wild-type) (Fig. 5A–B). In contrast, in these same embryos there was no significant difference in neuroectodermal cell proliferation further anterior in the neural tube at the level of somite 9 (6.2±1.1% vs. 7.2±0.7%; mutant vs.wild-type) (Fig. 5C–D); this provides evidence that the reduction in cell proliferation observed in the tailbud neuroectoderm of E8.5 Raldh2–/– embryos is not due to poor health of the embryos. These findings suggest that endogenous RA, which we have shown normally travels from the somitic mesoderm to the tailbud neuroectoderm, is needed to generate a high level of neuroectodermal cell proliferation specifically in the tailbud.
Fig. 5.
RA stimulates cell proliferation in tailbud neuroectoderm. Shown are transverse sections through the tailbud (A–B) and posterior neural tube at the level of somite 9 (C–D) stained with antibodies against phosphohistone 3 (H3P) to detect cells undergoing mitosis; note the decrease in H3P staining in Raldh2–/– tailbud neuroectoderm, but not neural tube neuroectoderm. These sections are representative of those used to determine mitotic indices. ec, ectoderm; en, endoderm; me, mesoderm.
3. Discussion
3.1. RA is required for posterior neural transformation but not for neural induction
During vertebrate neural development, neuroectoderm becomes differentiated from epidermal ectoderm in the process of neural induction (Sasai and De Robertis, 1997; Stern, 2002). Also, anterior neuroectoderm (forebrain/midbrain) becomes differentiated from posterior neuroectoderm (hindbrain/spinal cord) at an early stage. One mechanism proposed for anteroposterior differentiation is the activation–transformation model in which activation (neural induction) is followed by transformation of the posterior neuroectoderm in response to a posteriorizing signal (Nieuwkoop, 1952). Posterior expansion of the hindbrain upon administration of exogenous RA to amphibian embryos suggested that RA may be a factor normally needed for posterior transformation of the nervous system (Durston et al., 1989; Sive et al., 1990). Exogenous RA also results in an expansion of hindbrain in chick and mouse embryos as previously reviewed (Maden, 2002). Recent studies on avian embryos indicate that spinal cord neuronal differentiation is blocked in the absence of endogenous RA signaling (Del Corral et al., 2003; Novitch et al., 2003). However, these studies did not address whether endogenous RA signaling is needed for neural induction of ectoderm emerging from the tailbud or only for posterior differentiation of neuroectoderm progenitor cells to form neurons of the spinal cord. Also, it is not clear if the role of endogenous RA in neuronal differentiation is conserved in mouse embryos. During mouse development, Raldh2 is initially expressed at E7.5 in the paraxial mesoderm (Niederreither et al., 1997), consistent with a role in synthesizing RA for patterning the posterior axis in late primitive streak embryos. Our analysis here of Raldh2–/– embryos demonstrates that RA is not required for neural induction as expression of Sox1 and Sox2 proceeded normally all along the neural plate of embryos lacking RA activity. Thus, although exogenous RA treatment has been reported to increase Sox1 expression in mouse embryonic stem cells (Guan et al., 2001; Wichterle et al., 2002; Gottlieb, 2002), endogenous RA is not required for normal induction of Sox1 in the mouse embryo neural plate. We did find that endogenous RA was required for further differentiation of posterior neuroectoderm as the spinal cord motor neuron marker Olig2 failed to be expressed all along the posterior neural tube. We found that continuous RA treatment was required to rescue Olig2 expression in the posterior neural tube of Raldh2–/– embryos, thus demonstrating that RA continually acts to posteriorize nascent neuroectoderm as it emerges from the tailbud. Our results provide strong support for RA as a posterior transforming factor needed for spinal cord formation as hypothesized in the activation-transformation model of neural development (Nieuwkoop, 1952), and we suggest that this developmental mechanism may be generally applicable to vertebrate embryos.
3.2. RA synthesized in the somitic mesoderm acts cell non-autonomously in the posterior neuroectoderm
The results presented here demonstrate that RA generated by Raldh2 in the somitic mesoderm performs two cell non-autonomous functions during posterior development: (1) stimulation of cell proliferation in tailbud neuroectoderm and (2) stimulation of neuronal differentiation in neuroectodermal cells exiting the tailbud. The ability of Raldh2 to perform these two different functions is reinforced by our observation that RA synthesized in the somitic mesoderm travels to the adjacent spinal cord neuroectoderm as well as the more distant tailbud neuroectoderm. These observations were made possible through the use of mouse embryos carrying the RARE-lacZ transgene which allowed us to follow where endogenous RA signaling activity occurs in wild-type embryos, and where RA signaling occurs in Raldh2–/– embryos rescued by maternal RA treatment. Such experiments have not been possible in avian, amphibian, or fish embryos due to lack of such a transgene. With the tools available for analysis of RA signaling in mouse embryos we have found that Raldh2 expressed in somitic mesoderm is the only source of endogenous RA for posterior neural development. We also found that maternal dietary administration of a low dose of RA was able to rescue posterior neuronal differentiation in Raldh2–/– embryos, and interestingly we observed that the RA signaling activity restored to these mutant embryos was limited to the neuroectoderm and endoderm and was not detected in the somitic mesoderm. This could be due to a differential ability of maternal RA to reach different embryonic tissues, or could be due to differing levels of RA degradation or RA-binding proteins in epithelial versus mesenchymal cells. These findings demonstrate a direct requirement for RA in the neuroectoderm during neuronal differentiation and indicate that the RA signal does not need to perform an additional function in the somitic mesoderm in order to initiate Olig2 expression in the posterior neural tube. A direct role for RA in the neuroectoderm has also been suggested from studies in chick embryos where Olig2 expression was lost following expression of a dominant-negative RA receptor in the posterior neural tube (Novitch et al., 2003).
3.3. Opposing RA and FGF signals in the tailbud stem cell zone
A mechanism of tissue differentiation involving opposing RA and FGF signals has been proposed for proximodistal outgrowth of chick limb buds (Mercader et al., 2000) as well as anteroposterior extension of the posterior neural tube and somitogenesis (Del Corral et al., 2003; Moreno and Kintner, 2004). In each case the RA signal was hypothesized to be generated by Raldh2 expressed in mesoderm and the FGF signal was from Fgf8 expressed in tailbud ectoderm. Genetic studies in the mouse revealed that the RA signal needed for limb buds is indeed from Raldh2 and that RA travels from the lateral plate mesoderm to the adjacent limb bud ectoderm where it is needed for correct expression of Fgf8 in the apical ectodermal ridge (Mic et al., 2004b). As we have now shown that RA normally travels from the somitic mesoderm to the tailbud neuroectoderm, and that its presence there results in a smaller Fgf8 expression domain compared to Raldh2–/– embryos, it is now possible to propose that endogenous RA signaling controlled by Raldh2 performs a direct role in modulating the action of FGF signaling in the tailbud. In particular, our findings suggest that a balance between RA and FGF signaling in the tailbud stem cell zone may be needed to achieve the appropriate amount of cell proliferation in the developing tailbud neuroectoderm. In particular, we found that endogenous RA generated by Raldh2 in the somites is released and enters the tailbud neuroectoderm where it is needed to achieve a high level of cell proliferation in this tissue. In contrast, our findings indicate that endogenous RA is not required to regulate neuroectodermal cell proliferation further anterior in the neural tube. Studies on vitamin A deficient quail embryos lacking endogenous RA have also shown that RA is not required to maintain neuroectodermal cell proliferation in the neural tube at the comparable stage examined here (Wilson et al., 2003); the tailbud was not examined in those studies. We speculate that neuroectodermal cells of the neural tube may no longer require RA for cell proliferation as they have moved out of the range of FGF8 signaling which is highest in the tailbud.
We propose that nascent somitic mesoderm that has exited the tailbud sends an RA signal back to the tailbud neuroectoderm that limits Fgf8 expression and establishes the optimal rate of tailbud neuroectodermal cell proliferation needed to coordinate neural plate and somite formation. This model of mouse embryo posterior development involving opposing RA and FGF signals is consistent with a model proposed for avian posterior neural development (Del Corral et al., 2003), and extends the mechanism by demonstrating that there is a direct effect of somitic RA on tailbud neuroectoderm as well as trunk neuroectoderm. We further suggest that neuroectodermal cells in the tailbud exist in a low RA zone, but upon exiting anteriorly these cells enter a zone of high RA emanating from the directly adjacent somitic mesoderm which stimulates neuronal differentiation (i.e. Pax6 and Olig2 expression). Our results provide physiological insight into how RA normally functions in mammalian neural stem cell differentiation which may be useful to develop strategies for cell-based therapies through manipulation of stem cell lines.
4. Experimental procedures
4.1. Mice
Raldh2–/– embryos were generated from matings of heterozygous adult Raldh2–/+ mice carrying a targeted deletion of Raldh2 exons 3 and 4 that we have previously described (Mic et al., 2002). In those studies we demonstrated that Raldh2–/– embryos develop until E8.5 then undergo developmental arrest and die by E10.5. Mice carrying a RAREhsplacZ (RARE-lacZ) RA-reporter transgene in which lacZ (encoding β-galactosidase) is controlled by a retinoic acid response element (RARE) have been previously described (Rossant et al., 1991). Adult Raldh2–/+ mice carrying the RARE-lacZ transgene were generated by matings between the two lines, and these mice were used to generate Raldh2–/– embryos carrying one copy of RARE-lacZ.
4.2. Rescue of Raldh2–/– embryos by maternal dietary RA treatment
Maternal dietary RA rescue of Raldh2–/– embryos was performed by a slight modification of a previously described method (Mic et al., 2003). Briefly, all-trans-RA (Sigma) was dissolved in corn oil and mixed with powdered food at the following concentrations: 0.1 mg/g for treatment from E6.75–E7.75; 0.1 mg/g for treatment from E6.75–E8.25; for treatment from E6.75–E9.5 a low dose of 0.1 mg/g was used from E6.75–E8.25 and then the dose was raised to 0.25 mg/g for E8.25–E9.5. Such food was prepared fresh twice each day.
4.3. In situ hybridization and retinoic acid detection
Whole-mount in situ hybridization was performed as described (Wilkinson, 1992). Detection of RA activity was performed in embryos carrying the RARE-lacZ RA-reporter gene by staining for β-galactosidase activity in situ with X-gal as substrate as described (Rossant et al., 1991). Stained embryos were embedded in 3% agarose and sectioned at 50 μm with a vibratome.
4.4. Cell proliferation assay
Wild-type and Raldh2–/– embryos embryos at the 10-somite stage (approximately E8.5) were treated with Dent's fixative (Methanol:dimethylsulfoxide 4:1), endogenous peroxidase activity was quenched by incubation with 5% hydrogen peroxide, and paraffin embedding was performed. Tissue sections (7 μm) were incubated with anti-phosphohistone 3 antibodies as a marker for mitosis (1:200 dilution of 1 μg/μl stock antibody giving a final concentration of 0.005 μg/μl; Upstate Cell Signaling Solutions, Lake Placid, NY). Sections were then incubated with peroxidase-linked anti-rabbit secondary antibodies, and finally with diaminobenzidine (DAB) substrate. Total cell numbers were determined by counter-staining with hematoxylin. To obtain mitotic indices (percentage cells undergoing mitosis) for the tailbud and neural tube, the numbers of hematoxylin-stained nuclei and phosphohistone 3-positive nuclei were counted in four sections from two wild-type and Raldh2–/– embryos. Mitotic indices were determined from the mean cell counts and are presented as mean±SEM; statistical significance was determined for raw data using the unpaired Student's t test.
Acknowledgements
We thank the following for mouse cDNAs used to prepare in situ hybridization probes: R. Lovell-Badge (Sox1 and Sox2), P. Gruss (Pax6), C. Stiles and D. Rowitch (Olig2), J. Rubenstein (Nkx6.1), G. Martin (Fgf8), M. Petkovich and Cytochroma, Inc. (Cyp26a1), and A. Mansouri (Uncx4.1). We also thank J. Rossant for providing RARE-lacZ mice. This work was funded by National Institutes of Health grant GM62848.
References
- Abu-Abed S, Dollé P, Metzger D, Beckett B, Chambon P, Petkovich M. The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev. 2001;15:226–240. doi: 10.1101/gad.855001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand N, Médevielle F, Pituello F. FGF signalling controls the timing of Pax6 activation in the neural tube. Development. 2000;127:4837–4843. doi: 10.1242/dev.127.22.4837. [DOI] [PubMed] [Google Scholar]
- Del Corral RD, Breitkreuz DN, Storey KG. Onset of neuronal differentiation is regulated by paraxial mesoderm and requires attenuation of FGF signaling. Development. 2002;129:1681–1691. doi: 10.1242/dev.129.7.1681. [DOI] [PubMed] [Google Scholar]
- Del Corral RD, Olivera-Martinez I, Goriely A, Gale E, Maden M, Storey K. Opposing FGF and retinoid pathways control ventral neural pattern, neuronal differentiation, and segmentation during body axis extension. Neuron. 2003;40:65–79. doi: 10.1016/s0896-6273(03)00565-8. [DOI] [PubMed] [Google Scholar]
- Dobbs-McAuliffe B, Zhao QS, Linney E. Feedback mechanisms regulate retinoic acid production and degradation in the zebrafish embryo. Mech. Dev. 2004;121:339–350. doi: 10.1016/j.mod.2004.02.008. [DOI] [PubMed] [Google Scholar]
- Durston AJ, Timmermans JPM, Hage WJ, Hendriks HFJ, de Vries NJ, Heideveld M, Nieuwkoop PD. Retinoic acid causes an anteroposterior transformation in the developing central nervous system. Nature. 1989;340:140–144. doi: 10.1038/340140a0. [DOI] [PubMed] [Google Scholar]
- Fujii H, Sato T, Kaneko S, Gotoh O, Fujii-Kuriyama Y, Osawa K, Kato S, Hamada H. Metabolic inactivation of retinoic acid by a novel P450 differentially expressed in developing mouse embryos. Eur. Mol. Biol. Org. J. 1997;16:4163–4173. doi: 10.1093/emboj/16.14.4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb DI. Signposts on the neural path. Nat. Biotechnol. 2002;20:1208–1210. doi: 10.1038/nbt1202-1208. [DOI] [PubMed] [Google Scholar]
- Guan KM, Chang H, Rolletschek A, Wobus AM. Embryonic stem cell-derived neurogenesis-Retinoic acid induction and lineage selection of neuronal cells. Cell Tissue Res. 2001;305:171–176. doi: 10.1007/s004410100416. [DOI] [PubMed] [Google Scholar]
- Lewandoski M, Sun X, Martin GR. Fgf8 signalling from the AER is essential for normal limb development. Nat. Genet. 2000;26:460–463. doi: 10.1038/82609. [DOI] [PubMed] [Google Scholar]
- Lu QR, Sun T, Zhu Z, Ma N, Garcia M, Stiles CD, Rowitch DH. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- Maden M. Retinoid signalling in the development of the central nervous system. Nat. Rev. Neurosci. 2002;3:843–853. doi: 10.1038/nrn963. [DOI] [PubMed] [Google Scholar]
- Mansouri A, Yokota Y, Wehr R, Copeland NG, Jenkins NA, Gruss P. Paired-related murine homeobox gene expressed in the developing sclerotome, kidney, and nervous system. Dev. Dyn. 1997;210:53–65. doi: 10.1002/(SICI)1097-0177(199709)210:1<53::AID-AJA6>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Marquardt T, Pfaff SL. Cracking the transcriptional code for cell specification in the neural tube. Cell. 2001;106:651–654. doi: 10.1016/s0092-8674(01)00499-8. [DOI] [PubMed] [Google Scholar]
- Mercader N, Leonardo E, Piedra ME, Martínez-A C, Ros MA, Torres M. Opposing RA and FGF signals control proximodistal vertebrate limb development through regulation of Meis genes. Development. 2000;127:3961–3970. doi: 10.1242/dev.127.18.3961. [DOI] [PubMed] [Google Scholar]
- Mic FA, Haselbeck RJ, Cuenca AE, Duester G. Novel retinoic acid generating activities in the neural tube and heart identified by conditional rescue of Raldh2 null mutant mice. Development. 2002;129:2271–2282. doi: 10.1242/dev.129.9.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Benbrook DM, Duester G. Retinoid activation of retinoic acid receptor but not retinoid X receptor is sufficient to rescue lethal defect in retinoic acid synthesis. Proc. Natl. Acad. Sci. USA. 2003;100:7135–7140. doi: 10.1073/pnas.1231422100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mic FA, Molotkov A, Molotkova N, Duester G. Raldh2 expression in optic vesicle generates a retinoic acid signal needed for invagination of retina during optic cup formation. Dev. Dyn. 2004a;231:270–277. doi: 10.1002/dvdy.20128. [DOI] [PubMed] [Google Scholar]
- Mic FA, Sirbu IO, Duester G. Retinoic acid synthesis controlled by Raldh2 is required early for limb bud initiation and then later as a proximodistal signal during apical ectodermal ridge formation. J. Biol. Chem. 2004b;279:26698–26706. doi: 10.1074/jbc.M401920200. [DOI] [PubMed] [Google Scholar]
- Molotkov A, Fan X, Deltour L, Foglio MH, Martras S, Farrés J, Parés X, Duester G. Stimulation of retinoic acid production and growth by ubiquitously-expressed alcohol dehydrogenase Adh3. Proc. Natl. Acad. Sci. USA. 2002;99:5337–5342. doi: 10.1073/pnas.082093299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon AM, Capecchi MR. Fgf8 is required for outgrowth and patterning of the limbs. Nat. Genet. 2000;26:455–459. doi: 10.1038/82601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno TA, Kintner C. Regulation of segmental patterning by retinoic acid signaling during Xenopus somitogenesis. Dev. Cell. 2004;6:205–218. doi: 10.1016/s1534-5807(04)00026-7. [DOI] [PubMed] [Google Scholar]
- Muhr J, Graziano E, Wilson S, Jessell TM, Edlund T. Convergent inductive signals specify midbrain, hindbrain, and spinal cord identity in gastrula stage chick embryos. Neuron. 1999;23:689–702. doi: 10.1016/s0896-6273(01)80028-3. [DOI] [PubMed] [Google Scholar]
- Niederreither K, McCaffery P, Dräger UC, Chambon P, Dollé P. Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (RALDH-2) gene during mouse development. Mech. Dev. 1997;62:67–78. doi: 10.1016/s0925-4773(96)00653-3. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Subbarayan V, Dollé P, Chambon P. Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat. Genet. 1999;21:444–448. doi: 10.1038/7788. [DOI] [PubMed] [Google Scholar]
- Niederreither K, Vermot J, Schuhbaur B, Chambon P, Dollé P. Retinoic acid synthesis and hindbrain patterning in the mouse embryo. Development. 2000;127:75–85. doi: 10.1242/dev.127.1.75. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop PD. Activation and organization of the central nervous system in amphibians. Part III. synthesis of a new working hypothesis. J. Exp. Zool. 1952;120:83–108. [Google Scholar]
- Novitch BG, Wichterle H, Jessell TM, Sockanathan S. A requirement for retinoic acid-mediated transcriptional activation in ventral neural patterning and motor neuron specification. Neuron. 2003;40:81–95. doi: 10.1016/j.neuron.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Pevny LH, Sockanathan S, Placzek M, Lovell-Badge R. A role for SOX1 in neural determination. Development. 1998;125:1967–1978. doi: 10.1242/dev.125.10.1967. [DOI] [PubMed] [Google Scholar]
- Rossant J, Zirngibl R, Cado D, Shago M, Giguère V. Expression of a retinoic acid response element-hsplacZ transgene defines specific domains of transcriptional activity during mouse embryogenesis. Genes Dev. 1991;5:1333–1344. doi: 10.1101/gad.5.8.1333. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Meno C, Fujii H, Nishino J, Shiratori H, Saijoh Y, Rossant J, Hamada H. The retinoic acid-inactivating enzyme CYP26 is essential for establishing an uneven distribution of retinoic acid along the anterio-posterior axis within the mouse embryo. Genes Dev. 2001;15:213–225. doi: 10.1101/gad.851501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasai Y, De Robertis EM. Ectodermal patterning in vertebrate embryos. Dev. Biol. 1997;182:5–20. doi: 10.1006/dbio.1996.8445. [DOI] [PubMed] [Google Scholar]
- Sive HL, Draper BW, Harland RM, Weintraub H. Identification of a retinoic acid-sensitive period during primary axis formation in Xenopus laevis. Genes Dev. 1990;4:932–942. doi: 10.1101/gad.4.6.932. [DOI] [PubMed] [Google Scholar]
- Stern CD. Induction and initial patterning of the nervous system-the chick embryo enters the scene. Curr. Opin. Genet. Dev. 2002;12:447–451. doi: 10.1016/s0959-437x(02)00324-6. [DOI] [PubMed] [Google Scholar]
- Sun X, Meyers EN, Lewandoski M, Martin GR. Targeted disruption of Fgf8 causes failure of cell migration in the gastrulating mouse embryo. Genes Dev. 1999;13:1834–1846. doi: 10.1101/gad.13.14.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takebayashi H, Nabeshima Y, Yoshida S, Chisaka O, Ikenaka K. The basic helix-loop-helix factor Olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr. Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Lieberam I, Porter JA, Jessell TM. Directed differentiation of embryonic stem cells into motor neurons. Cell. 2002;110:385–397. doi: 10.1016/s0092-8674(02)00835-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG. Whole mount in situ hybridization of vertebrate embryos. In: Wilkinson DG, editor. In Situ Hybridization: A Practical Approach. IRL Press; Oxford: 1992. pp. 75–83. [Google Scholar]
- Wilson L, Gale E, Maden M. The role of retinoic acid in the morphogenesis of the neural tube. J. Anat. 2003;203:357–368. doi: 10.1046/j.1469-7580.2003.00230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]