Summary
Background
Large animal models that accurately mimic human hemophilia A (HA) are in great demand for developing and testing novel therapies to treat HA.
Objectives
To re-establish a line of sheep exhibiting a spontaneous bleeding disorder closely mimicking severe human HA, fully characterize their clinical presentation, and define the molecular basis for disease.
Patients/methods
Sequential reproductive manipulations were performed with cryopreserved semen from a deceased affected ram. The resultant animals were examined for hematologic parameters, clinical symptoms, and responsiveness to human FVIII (hFVIII). The full coding region of sheep FVIII mRNA was sequenced to identify the genetic lesion.
Results and conclusions
The combined reproductive technologies yielded 36 carriers and 8 affected animals. The latter had almost non-existent levels of FVIII:C and extremely prolonged aPTT, with otherwise normal hematologic parameters. These animals exhibited bleeding from the umbilical cord, prolonged tail and nail cuticle bleeding time, and multiple episodes of severe spontaneous bleeding, including hemarthroses, muscle hematomas and hematuria, all of which responded to hFVIII. Inhibitors of hFVIII were detected in four treated animals, further establishing the preclinical value of this model. Sequencing identified a premature stop codon and frame-shift in exon 14, providing a molecular explanation for HA. Given the decades of experience using sheep to study both normal physiology and a wide array of diseases and the high homology between human and sheep FVIII, this new model will enable a better understanding of HA and facilitate the development and testing of novel treatments that can directly translate to HA patients.
Keywords: hemophilia A, inhibitors, large animal model, molecular characterization, sheep
Introduction
Hemophilia A (HA) is an X-linked bleeding disorder caused by a deficiency/abnormality of coagulation factor VIII (FVIII). HA represents the most common inheritable disorder of coagulation, with an incidence of 1 in 5000–10000 male births [1]. The hemorrhagic phenotype of HA depends upon the genetic defect and functional level of circulating FVIII, with < 1 unit per dL of FVIII considered severe hemophilia [2]. These individuals experience recurrent hemarthrosis, causing chronic debilitating arthropathy, hematomas of subcutaneous connective tissue and muscle, and hematuria. Intracranial bleeding accounts for approximately 1/3 of the hemorrhagic deaths in these patients [3]. Current HA treatment consists of intravenous FVIII protein infusions to maintain hemostasis. These infusions must be given frequently throughout life due to the short half-life of FVIII. While this therapy allows many hemophiliacs to live relatively normal lives, it is far from ideal, due to the need for lifelong infusions, the high cost, and the formation of FVIII inhibitors in many patients, reducing the efficacy of subsequent factor infusions and complicating treatment [4]. These shortcomings have generated tremendous interest in developing novel HA therapies, such as factor concentrates with prolonged half-life, stem cell transplantation and gene therapy, which could offer longer-lasting benefit or permanent cure [5–11].
To evaluate the efficacy and safety of new treatments, a number of HA animal models have been developed, the most widely used of which are the murine models, created by gene targeting/knockout technology [12]. These models offer the convenience and cost benefits of a small animal model, but do not accurately recapitulate the human disease, since they often possess a relatively mild phenotype, frequently only exhibiting bleeding following trauma [12–15]. As a result, the spontaneous hematomas and hemarthroses seen in patients with severe HA are not observed in these models. While this facilitates maintenance of these mice, it also limits their use to studies on the efficacy of treatments for trauma-induced hemorrhage. Transient hemophilic rabbit models created by infusing plasma containing FVIII inhibitors have also been of great value for testing the ability of various bypass products to FVIII [16] to mediate hemostatic correction. In contrast to the murine models, several dog breeds with naturally occurring congenital coagulopathies exhibit symptoms closely mimicking those of humans with severe HA. These canine lines have been selectively bred, the molecular nature of the lesions defined, and their resultant coagulation defects characterized, providing a much needed, valuable large animal HA model [17–19]. Despite these colonies having been extremely helpful in evaluating the efficacy and safety of different therapy protocols, several limitations to this model still exist. As with other large animal models, the costs of production and maintenance are high, and it has proven difficult to produce adequate numbers of these animals to meet current experimental demand. In addition, several other characteristics of the dog make it complicated to directly translate findings in this model to the clinical setting. These include the need for scale-up to move from dogs to humans, differences in the physical location/sequestration of von Willebrand factor (VWF) in dogs and humans, and the robust immune response to even a single injection of human FVIII (hFVIII), which precludes the testing of protein or gene-based hFVIII therapeutics in this model [19–22].
In sheep, HA was first reported between 1979 and 1982, in male offspring of a single alpine white ewe at the Swiss Federal Institute of Technology [23–25]. These animals all died several hours postpartum due to severe bleeding from the umbilical cord [23–25]. Daughters and granddaughters of this ewe also gave birth to lambs exhibiting the same pathology. The affected animals exhibited extensive subcutaneous and intramuscular hematomas and spontaneous hemarthroses leading to reduced locomotion and symptoms of pain in standing up, restricting nursing activity. Laboratory tests showed an increase in the activated partial thromboplastin time (aPTT) and a decrease of FVIII levels to less than 1% of normal (using a modified one-stage aPTT-based assay). Unfortunately, due to the expense and effort of maintenance, only a small number of straws of semen were saved prior to this valuable resource passing into extinction.
Here we report the successful re-establishment of this unique line of HA sheep and characterization of their coagulation profile. We also describe for the first time the sequence of normal sheep FVIII mRNA, the HA-inducing molecular lesion, and a PCR-based method to screen for this mutation.
Because sheep have been successfully used for decades to study both normal physiology and a wide array of diseases, including those of hematopoiesis [26–29], we anticipate that this large animal model will not only contribute to a better understanding of HA's physiopathology, but will also become a useful preclinical resource for developing and testing novel HA treatments such as stem cell transplantation and gene therapy.
Materials and methods
These studies were approved by the University of Nevada, Reno, IACUC.
Re-establishment of HA sheep
The line of HA sheep was re-established from six straws of cryopreserved semen from a single male from the original Swiss colony using a variety of reproductive technologies [30–32], including multiple ovulation embryo transfer (MOET), in vitro fertilization (IVF), and intracytoplasmic sperm injection (ICSI). Details of these procedures appear in the Supporting Information.
Clinical parameters of carriers and affected sheep
Shortly after birth and prior to administering hFVIII, blood was collected from all animals into citrated tubes, spun to obtain platelet-deficient plasma, and sent to the Animal Health Diagnostic Center (AHDC) at Cornell University, where standard clinical aPTT and prothrombin time (PT, Quick-test) tests were run. All clotting time tests were performed using a photo-optical clot detection instrument (Coag-A-Mate XM; bioMerieux, Inc, Durham, NC, USA) according to the manufacturer's recommendations for use with human plasma. The aPTT assay utilized Actin-FS reagent (Dade Behring, Marburg, Germany), while the one-stage PT was performed with rabbit brain thromboplastin reagent containing CaCl2 (Thromboplastin-LI; Helena Laboratories, Beaumont, TX, USA) [33]. Pooled normal sheep plasma was obtained from a flock of Suffolk sheep at Cornell University (n = 8) and stored frozen at −50 °C until use. These controls were then run in parallel with control (n = 4) and experimental sheep samples from the University of Nevada, Reno (UNR). Plasma samples were also obtained from these animals at intervals throughout their lives, prior to and following administration of hFVIII for each bleeding episode, and levels of FVIII (FVIII:C) coagulant activity were determined using a modified one-stage aPTT [34] and human FVIII-deficient plasma (George King Bio-Medical, Inc, Overland Park, KS, USA). Results for FVIII activity were reported as the percentage of the Cornell pooled sheep plasma as standard (set as 100% FVIII:C activity). To quantitate the levels of VWF present in the healthy control and affected animals, we used a double sandwich ELISA to detect sheep VWF antigen, employing methods that have previously been described in detail [35]. In this assay, both the capture and the sandwich antibodies were anti-human vWF polyclonal antibodies that cross-react with sheep VWF.
Inhibitor formation in hFVIII-treated animals
To assess formation of inhibitory antibodies to hFVIII, platelet-deficient plasma was collected, and the levels of inhibitory antibodies were quantified using a standard Bethesda Assay, measuring the ability of any antibodies present within the plasma of these animals to neutralize a defined quantity of hFVIII, using platelet-deficient normal human plasma as a source of a defined quantity of hFVIII. For quantification, we used the definition of one Bethesda unit (BU) = amount of antibody that neutralizes 50% of the FVIII present in a 1:1 mixture of the `patient's' plasma and normal human plasma after a 2 h incubation at 37 °C.
Sequencing normal sheep FVIII mRNA
RNA isolation, RT-PCR and cloning of RT-PCR products
Four grams of normal control sheep spleen were lysed in 40 mL Trizol reagent (Invitrogen, Carlsbad, CA, USA), minced and homogenized. Following overnight incubation, lysates were centrifuged, and the manufacturer's protocol (Invitrogen) was followed for the remainder of the RNA extraction: 10 μg of RNA were treated with DNAse (Turbo DNA-free™, Ambion®) according to the manufacturer's directions; 2 μg of DNA-free RNA were then used for reverse transcription using the Qiagen LongRange 2Step RT-PCR kit according to the manufacturer's protocol; and 3–5 μL of each resultant product were analyzed on a 1% agarose gel. The TOPO™ XL PCR Cloning Kit (Invitrogen) was then used to gel purify, clone and amplify these RT-PCR products. Briefly, following TOPO™ XL gel purification (Invitrogen), isolated DNA was immediately used in a TOPO™ XL cloning reaction and transformed into One Shot® Top 10 chemically competent E. coli according to the manufacturer's instructions (Invitrogen). Following overnight growth on FastMedia™ LB Agar-Kan (Fermentas)plates, five to ten colonies were picked for each RT-PCR product and used for plasmid purification and sequencing.
Plasmid isolation and sequencing
Plasmids isolated using a Qiagen Miniprep kit were sequenced by the Nevada Genomics Center (University of Nevada). Initially M13F, M13R and T7 primers supplied in the XL-TOPO Kit were used to sequence the plasmid inserts. After initial sequence data were obtained, new primers were designed in order to walk along the remaining RT-PCR inserts. Only trimmed sequence data were used for subsequent sequence analysis. Sequences for each region were aligned using the Geneious Basic 3.5.6 program to determine consensus sequences. The NCBI/BLAST website (http://blast.ncbi.nlm.nih.gov/Blast.cgi) was used to compile successive sequences into an overall consensus for FVIII mRNA.
Sequencing hemophilic sheep FVIII mRNA
For RNA isolation, RT-PCR and cloning, RNA was isolated from the spleen of a deceased hemophilic animal as described above. RT-PCR was performed essentially as for control sheep with the modification of using 8 μg RNA in the Turbo DNA-free treatment. In addition, because the reduced RNA quality of the deceased animal precluded amplification of fragments over 2 kb, primers were employed that amplified smaller fragments than those used for the wild-type FVIII sequence. Given the smaller size of the target amplicons, Platinum® High Fidelity Taq and reagents were used (Invitrogen); 3–5 μL of each product were analyzed on an agarose gel, and subsequently cloned with the Invitrogen TOPO 2.1 cloning kit.
Results
Re-establishment of HA sheep
To restore the line of HA sheep, six straws of frozen semen obtained from an affected Swiss Alpine White male were subjected to a panel of reproductive manipulations [30–32]. The fact that this semen had been cryopreserved for 10 years caused concerns as to whether it could in fact be used to re-establish this line of sheep. The first thawed semen straw was of poor quality, and the two ewes that were synchronized for use as MOET donors produced only unfertilized ova. As a result of the poor MOET outcome, we attempted ICSI with oocytes collected from superstimulated ewes. From 236 oocytes, ICSI produced 189 embryos, which were transferred surgically to the oviducts of synchronized recipients and produced 17 lambs. The straw of semen utilized for the second set of experiments was of higher quality. Three ewes were superstimulated for use as MOET donors, yielding 12 embryos, which, when transferred to four synchronized recipients, produced nine lambs. Semen was also used to perform IVF with oocytes from superstimulated ewes, producing 91 embryos that were transferred to 20 recipients. Seven recipients maintained pregnancy, producing 10 lambs. Of the 36 lambs produced from these two sets of experiments, 14 were unaffected males and 22 were females, all of which were obligate HA carriers.
To produce hemophiliacs, 20 female carriers were backcrossed using three straws of cryopreserved semen from the affected male employing either IVF or MOET. Eight MOET donors yielded 38 embryos, which were then transferred into 21 synchronized recipients, and produced 16 lambs. IVF was performed with 140 ova, yielding 54 embryos, which, when transferred into 15 recipients, produced three lambs. Co-transfer of one IVF and one MOET embryo into a single recipient produced one offspring. Overall, these two reproductive technologies produced eight hemophiliacs (seven females and one male), six carrier females, and six unaffected males.
Clinical parameters and symptoms of carriers and affected sheep
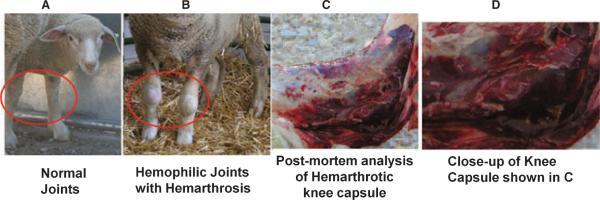
Carriers generated by our first round of reproductive manipulations did not exhibit any symptoms indicative of HA. In contrast, clinical symptoms consistent with HA were evident from the moment of birth in eight of the offspring of the carriers backcrossed with hemophilic sperm. All of these animals exhibited prolonged bleeding from the umbilical cord. Unfortunately, based on other animal models of HA, we were not anticipating such a severe phenotype, and attempted to tie off the cord in the first two lambs that were born. These animals died shortly after birth, and postmortem analysis revealed extensive internal bleeding as a result of birth trauma and continued hemorrhaging from the tied cord. All subsequent animals that exhibited prolonged umbilical cord bleeding were immediately administered human FVIII concentrate (hFVIII) (Koāte®-DVI, Talecris Biotherapeutics, Research Triangle Park, NC), after first drawing blood for the coagulation tests detailed below. Bleeding promptly ceased upon hFVIII administration in all animals. Due to the ineffectiveness of tying or clamping the cord, FVIII therapy was continued every 12 h until the umbilical cord dropped. All animals surviving birth exhibited extremely prolonged bleeding following routine tail clipping and hoof trimming. Despite the fragile nature of the hemophilic lambs, these procedures are required by the IACUC for all research sheep to prevent injury and infection. While the bleeding caused by these procedures is self-limiting in normal sheep, it proved life-threatening in the hemophiliacs. In addition to exhibiting severe bleeding following these routine procedures, all surviving sheep also developed clinical symptoms closely mimicking those of human patients with severe HA. Each animal had between two and six episodes of severe spontaneous bleeding, including: hemarthroses of the elbow, shoulder, hip and knee; multiple muscle hematomas, including one hematoma of the tongue (after attempting oral administration of iron); and two episodes of hematuria. Figure 1 shows an image of the knees of a normal healthy sheep (A), the hemarthrosis of the knee that spontaneously occurred in one of these sheep (B) and a postmortem analysis of the knee capsule of another animal that displayed hemarthrosis and internal bleeding (low magnification in C, high magnification in D). Most of the bleeding episodes improved/resolved upon administration of hFVIII. FVIII treatment was also necessary to stop bleeding resulting from routine ear tagging and tail clipping. Despite administration of hFVIII, five of these affected animals ultimately perished as a direct result of internal bleeding, two died from respiratory distress secondary to internal bleeding, and one of a post-transfusion reaction performed to alleviate severe anemia. Figure 2 depicts the lifespan of each of the hemophiliacs and the cause of death. A summary of the clinical symptoms during the hemophiliacs' lives appears in Table 1. Despite the varied lifespan of these animals, all exhibited prolonged umbilical cord bleeding, bleeding after ear tagging, bleeding after tail clipping, and hematomas, with the number of hematomas increasing as a function of lifespan. Because animal 703 exhibited pronounced bleeding following hoof trimming, subsequent animals were subjected to hoof trimming only after prophylactic treatment and/or by specialized personnel. All but one animal (aside from the two that died at birth) developed three to four hemarthroses during their lives, and two of the six animals that lived beyond birth exhibited hematuria.
Fig. 1.
Hemarthrosis in HA sheep. Spontaneous hemarthrosis occurred frequently in these animals. (A) Normal knees; (B) hemarthrosis of the knees; (C) postmortem analysis of the knee capsule of one animal that displayed hemarthrosis and internal bleeding; (D) close-up of the postmortem of the knee capsule shown in panel C.
Fig. 2.
Lifespan and causes of death in hemophilic sheep colony.
Table 1.
Clinical symptoms of hemophilic sheep
| Sheep number | 708 | 705 | 711 | 720 | 704 | 709 | 703 | 710 |
|---|---|---|---|---|---|---|---|---|
| Umbilical cord bleeding | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Bleeding after tail clipping | N/A | N/A | Yes | Yes | Yes | Yes | Yes | Yes |
| Bleeding after ear tagging | N/A | N/A | Yes | Yes | Yes | Yes | Yes | Yes |
| Hematomas | N/A | N/A | 1 | 1 | 3 | 2 | 4 | 5 |
| Hemarthroses | N/A | N/A | 4 | 4 | 3 | No | 4 | 4 |
| Bleeding after trimming hooves | N/A | N/A | N/A | N/A | N/A | N/A | Yes | N/A |
| Internal bleeding | 1 | 1 | No | No | 1 | 1 | No | 1 |
| Hematuria | N/A | N/A | No | No | No | 1 | No | 1 |
| Total hFVIII administered (U) | 75 | 77 | 2888 | 3207 | 9693 | 1597 | 15 697 | 13 501 |
| FVIII inhibitors (Bethesda units) | N/A | N/A | No | No | 3 | 1.3 | 0.5 | 13.2 |
| Months alive | 0 | 0 | 2 | 2 | 7 | 8 | 14 | 17 |
N/A, not applicable.
Laboratory parameters of carriers and affected sheep
Blood was drawn at intervals from the obligate carriers and sent for a panel of laboratory coagulation tests. When compared with pooled control sheep plasma (n = 4), carriers exhibited slightly increased aPTT (36.8 ± 4.3; normal = 31 ± 1.1), and normal PT and platelet number. Levels of fibrinogen, FIX, VWF activity and VWF:ag were also normal. However, all of these animals exhibited slightly decreased levels of FVIII:C (83.6 ± 24%; normal = 119 ± 13.3%). Immediately following birth of the animals from the second round of reproductive manipulations, all animals that exhibited excessive/prolonged umbilical cord bleeding had blood drawn prior to the administration of hFVIII. Platelet-deficient plasma was prepared and shipped to AHDC, where coagulation tests were performed, including aPTT and PT, as well as tests for levels of platelets, FVIII:C, FIX, fibrinogen and VWF. These tests revealed that these animals had extremely prolonged aPTT (92 ± 1.1, normal = 30.3) and almost non-existent levels of FVIII:C, with normal levels of platelets and VWF. Although some residual FVIII:C activity appeared to be present in these animals, this is most likely due to the exclusive use of aPTT-based assays for quantitating FVIII:C. In our hands, this assay had a sensitivity of roughly 2.3%. While FIX and fibrinogen levels were lower than expected for normal adults, the levels observed in these newborn hemophiliacs were consistent with the documented reduction in the plasma levels of these factors that occurs during the neonatal period in humans and in sheep [36–38]. In agreement with this conclusion, the levels of these two critical plasma factors normalized in all lambs by the end of the neonatal period. Table 2 summarizes the coagulation parameters of the carriers (Phase 1 animals) and affected newborn hemophiliacs (Phase 2 animals).
Table 2.
Xxxxxxxxxxxxxxxxx
| Phase 1 | Evaluation of carriers | |||||
|---|---|---|---|---|---|---|
| aPTT (s) | PT (s) | Fibrinogen (mg dL −1) | FVIII:C (%)* | F IX(%) | VWF:Ag(%) | |
| Normal ewes (n = 4) | 31 ± 1.1 | 26.2 ± 1.3 | 155 ± 17.3 | 119 ± 13.3 | 160 ± 33 | 106 ± 12 |
| Carrier ewes (n = 22) | 36.8 ± 4.3 | 24.6 ± 1.3 | 172 ± 25 | 83.6 ± 24 | 140 ± 33 | 121 ± 8.7 |
| Phase 2 | Evaluation of newborn hemophiliacs | |||||
|---|---|---|---|---|---|---|
| aPTT (s) | PT (s) | Fibrinogen (mg dL−1) | FVIII:C (%)* | F IX(%) | VWF:Ag(%) | |
| Normal ewes (n = 4) | 30.3 | 36.4 | 237 | 120 | 100 | 100 |
| Hemophiliacs (n = 8) (7 female, 1 male) | 92 ± 1.1 | 52 ± 5.2 | 103 ± 5 | 2.3 ± 0.8 | 57 ± 10 | 110 ± 20 |
| Other offspring (n = 6) (all male) | 36.5 ± 2.1 | 34.2 ± 2 | 341 ± 51 | 83.1 ± 11 | 81.4 ± 8 | 100.5 ± 14 |
In our hands, the sensitivity of the aPTT test used to quantitate the levels of FVIII:C was 2.3%.
Administration of human factor VIII (hFVIII) to hemophilic sheep
Treatment with hFVIII (Koāte® -DVI, Talecris Biotherapeutics) commenced immediately following birth of the hemophiliacs to prevent death due to unabated bleeding from the umbilical cord. This treatment consisted of between two and seven infusions of a total dose of 181–448 units of hFVIII, depending upon the length of time it took for the umbilical cord to drop off and the animal's birth weight, respectively. This dose was calculated based on the manufacturer's product literature, aiming to increase FVIII levels to between 25 and 50% of normal. In all cases, administration of hFVIII resulted in cessation of bleeding (if present) and prevented further bleeding from the umbilical cord. After recovering from umbilical cord bleeding, the hemophilic lambs suffered throughout the remainder of their lifetime from a variety of bleeding symptoms that are characteristic of HA, as discussed in detail above. Each of these events required treatment with hFVIII, with the dose being adjusted depending upon the observed symptom. Specifically, prior to and following tail clipping, hFVIII was given to increase FVIII levels to 25–50% of normal, while for ear tagging, hFVIII was dosed to achieve levels of 50% of normal. Each time blood was drawn from these animals, to provide protection, hFVIII was dosed to increase FVIII levels to 10% of normal. Each observed hemarthrosis was treated with a dose of hFVIII that would produce FVIII levels of 50% of normal. Hematomas were treated identically to hemarthroses, with the exception that the hFVIII dosage was increased to produce 100% correction in cases where multiple consecutive hematomas were observed (animal # 704). In the two observed cases of hematuria, hFVIII was dosed to increase FVIII levels to 100% of normal. Table 1 provides the total cumulative dose of hFVIII administered to each animal throughout its lifetime. As can be seen, the number of events experienced and, hence, the amount of hFVIII given was a direct function of the length of the animal's life, because the symptoms appeared at a similar frequency in each of the animals. As a result, animals that lived longer were likely to exhibit more events requiring treatment with hFVIII.
Inhibitor formation in hFVIII-treated animals
We next examined whether repeated administration of hFVIII resulted in the formation of inhibitory antibodies in the hemophilic sheep. Platelet-deficient plasma was collected from all hemophilic animals at intervals throughout their respective lifetimes and Bethesda assays were performed at AHDC to identify and quantitate inhibitory antibodies. As shown in Table 1, only the four animals that lived for longer than 2 months developed inhibitors to hFVIII, and the levels of these inhibitors varied markedly among these animals. Animal #710 exhibited the highest levels of inhibitors: 13.2 Bethesda units. This animal was the longest survivor and received the second highest cumulative dose of hFVIII (13 501 units); animal #703, which received the highest dose of hFVIII (15 697 U) and survived for 14 months, generated inhibitory antibodies at a titer of only 0.5 Bethesda units, suggesting that factors others than the time and/or total hFVIII dose may be responsible for the resultant inhibitor titer. Indeed, at the age of 4 months, animal #703 had received a total of 2001 hFVIII units in 10 treatments, and had no detectable inhibitory antibodies, whereas at the same age #710 had a titer of 2.6 Bethesda units upon receiving a cumulative dose of 1544 hFVIII units during 12 treatments. In two of the remaining animals, the levels of inhibitors were 1.3 Bethesda units after a cumulative dose during 8 months of 1597 units of hFVIII (#709) and 3 Bethesda units as a result of receiving a total of 9693 units of hFVIII during 7 months (#704).
Sequencing normal sheep FVIII mRNA
While these prior analyses demonstrated that the hematologic parameters and clinical presentation of these sheep closely mimic those seen in human severe HA patients, the normal sheep FVIII mRNA had to be sequenced to characterize the hemophilia-inducing mutation. We performed reverse transcription (RT) on normal sheep spleen mRNA using oligo-dT to prime cDNA synthesis from the 3′-polyA tail, and another RT using a primer designed from the limited public sequence data from part of sheep exon 14. The resultant cDNA products were cloned and subjected to overlapping sequencing with primers designed from a bovine exon map we created by aligning the bovine genomic sequence with human exon sequence data. Multiple rounds of PCR and sequencing were performed, designing primers (Supplementary Table 2) based on these presumed bovine exon sequences and sequence data we were obtaining from the sheep mRNA. This approach allowed us to walk along the mRNA and obtain the complete coding sequence for sheep FVIII (sFVIII).
The sFVIII coding sequence is 6765 nucleotides, which, based on predictions from ORF Finder (NCBI) and comparison with published human sequence data, is translated into a 2235 amino acid (a.a.) protein. The nucleotide sequence has been submitted to GenBank (accession #GQ200609). BLAST alignment to hFVIII at the a.a. level revealed a high degree of identity in all regions except the B domain, which in humans is dispensable for clotting activity. Specifically, the A1 domain (sheep a.a. 1–332) showed 81% identity; A2 (a.a. 371–732) 88% identity; A3 (a.a. 1593–1922) 87% identity; C1 domain (a.a. 1923–2075) 90% identity; and C2 (a.a. 2075–2235) 86% identity. In contrast, the B domain (a.a. 733–1551) exhibited only 47% identity. A summary of the degree of identity at the a.a. level of each region in the human and sheep proteins appears in Table 3, as well as comparisons with published dog, mouse and pig FVIII sequences.
Table 3.
Summary of the degree of identity of sheep FVIII at the amino acid level of each region in human, dog, mouse and pig proteins
| Region | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | A2 | B | A3 | C1 | C2 | |||||||
| Species | Identity | Positivity | Identity | Positivity | Identity | Positivity | Identity | Positivity | Identity | Positivity | Identity | Positivity |
| Human | 81 | 87 | 88 | 94 | 47 | 59 | 87 | 93 | 90 | 94 | 86 | 93 |
| Dog | 76 | 86 | 88 | 94 | 67 | 77 | 85 | 92 | 90 | 94 | 82 | 91 |
| Mouse | 77 | 86 | 80 | 89 | 45 | 60 | 82 | 89 | 88 | 93 | 82 | 91 |
| Pig | 79 | 88 | 87 | 92 | 48 | 58 | 88 | 93 | 92 | 95 | 82 | 91 |
A subsequent comparison, considering conservative a.a. changes, indicated even higher homology: A1, 87% positivity; A2, 94%; A3, 93%; C1, 94%; and C2, 93%.Even with this approach, the B domain still exhibited only 59% conservation between the two species (Table 3). These analyses establish that a high degree of homology exists between hFVIII and sFVIII, and explain the ability of hFVIII to correct the phenotype of these sheep. Further analysis of the sheep sequence focusing on regions of the hFVIII protein that are known to serve as antigens for triggering inhibitory anti-FVIII antibodies [39] revealed a higher degree of identity between sFVIII and hFVIII than between the canine and hFVIII proteins at three of these six sites. Specifically, sFVIII showed 91% identity at the A2 inhibitor site (human a.a. 454–508) vs. 81% for the dog; 93% identity at the A3 inhibitor site (human a.a. 1778–1823) vs. 86% for the dog; and 90% identity at the 2nd C2 inhibitor site (human a.a. 2248–2312) vs. 87% for the dog. It thus seems reasonable to speculate that this higher degree of identity at these highly immunogenic sites within the FVIII protein may explain, at least in part, the development of inhibitors in only some of the sheep receiving hFVIII, and the relatively low titer of these inhibitors. This is in marked contrast to HA dogs, in which even a single infusion of hFVIII reliably produces a robust anti-hFVIII response with inhibitors in the range of hundreds of Bethesda units [40]. Two things, however, suggest caution be used when interpreting the present studies on inhibitor formation to the xenogeneic hFVIII in sheep. The first of these comes from studies examining the administration of human FIX (hFIX) to non-hemophilia B (HB) monkeys, in which the difference of only 11 a.a. between the native monkey FIX and the exogenously supplied hFIX is sufficient to trigger antibody formation. However, these studies were further complicated by the fact that these anti-hFIX antibodies then cross-reacted with the endogenous monkey FIX, thus creating a non-human primate model for acquired HB [41,42]. The second point comes from recent studies in HB dogs, showing that it is possible to induce tolerance to hFIX following either protein administration [43] or gene therapy [44] when treatment commences in the neonatal period. Thus,it is likely that the neonatal approach could also be used to make HA dogs tolerant of hFVIII and could potentially apply to the HA sheep.
Sequencing hemophilic sheep FVIII mRNA, characterization of mutation, and development of a diagnostic PCR-based RFLP for HA in sheep
Having established the sequence of wild-type sFVIII, we next determined the molecular lesion present in the hemophiliacs. mRNA isolated from the spleen of a deceased hemophiliac was RT'ed using a panel of primers (Supplementary Table 1) and subjected to multiple rounds of PCR with primers designed from the wild-type sFVIII sequence (Supplementary Table 2). This analysis identified an 11 bp region in exon 14 that differed between the wild-type and the hemophiliac. Importantly, this difference introduced a premature stop codon at base position 3112–4 in exon 14, as is seen in some human HA patients. This mutation also included a single nucleotide insertion-induced frame shift, creating five additional stop codons within the next 183 bp, precluding protein translation past this point, and providing a molecular explanation for the severe phenotype. Figure 3 shows the sequence of the region of the sFVIII gene harboring the mutation responsible for HA in these sheep.
Fig. 3.
Sequence of the region of the sheep FVIII gene harboring the mutation responsible for hemophilia A in these sheep. The boxed region indicates the frame shift-inducing mutation, and bold italicized triplets indicate the stop codons introduced by the mutation/frameshift.
Once we established the nature of the mutation, we commenced developing a method to identify both homozygous affected animals and heterozygous carriers. While wild-type animals do not possess a PacI restriction site in this genomic region, the hemophilia-inducing mutation creates a single PacI site within this region. Therefore, we designed PCR primers to amplify the 240 bp spanning the mutation. The PCR products were digested with PacI and analyzed by gel electrophoresis. Figure 4 shows results obtained with a representative normal (control) sheep, a carrier female and a hemophiliac, and demonstrates this method allows us to unambiguously establish which sheep are wild-type, heterozygous or homozygous for the HA mutation. This PCR-based assay will greatly facilitate studies using these sheep to explore gene- and stem cell-based HA therapies, because it is possible to screen not only newborns, but also fetuses in utero using a small volume of amniotic fluid, just as is done for prenatal diagnosis of genetic diseases in humans.
Fig. 4.
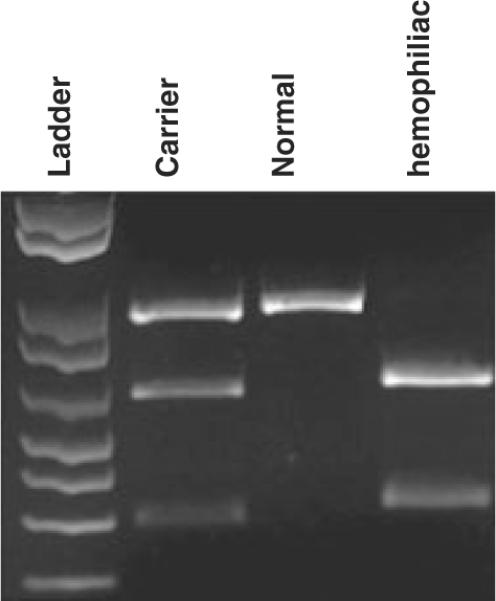
Diagnostic PCR RFLP. PCR primers were designed to amplify a 240 bp region of the genome spanning the area harboring the mutation. Since wild-type animals do not possess a PacI site in this region of their genome, the mutation present in the hemophiliacs creates a single site for this restriction enzyme within this region. Therefore, following amplification, PCR products were subjected to digestion with PacI and analyzed by agarose gel electrophoresis. The results obtained with a representative normal (control) sheep, a carrier female generated from our first round of reproductive manipulations, and a hemophiliac generated from the second round of reproduction, are shown, demonstrating that this method allows us to rapidly and unequivocally establish whether the sheep are wild-type, heterozygous, or homozygous for the HA mutation.
Discussion
Currently, HA treatment consists of administering frequent intravenous infusions of the short-lived FVIII protein to maintain hemostasis. While current therapeutic products for HA offer reliable prophylactic and therapeutic efficacy, they do not cure the underlying disease, and their high cost precludes many hemophiliacs worldwide from receiving regular prophylactic treatment. This unfortunate reality coupled with the high risk of treatment failure due to inhibitor formation over time results in a worldwide mortality rate for individuals with severe HA that is still four to six times that of the healthy male population [45,46], highlighting the urgent need for novel therapies that allow long-term or even permanent cure of HA.
Here we describe the re-establishment of a line of sheep with a spontaneous bleeding disorder that accurately recapitulates both the genetics and the clinical symptoms of the severe form of human HA. Sheep share many important physiological and developmental characteristics with humans, making them a particularly relevant model in which to study therapies for HA [28,29]. Sheep are fairly close in size to humans, weighing roughly 8l bs at birth and 150–170 lbs as adults, which is likely to obviate the need for scale-up to move from experiments in sheep to human trials. Furthermore, the development of the sheep immune system closely parallels that of humans [47], making sheep well suited for studying the immunological aspects of HA therapy and the mechanisms of FVIII inhibitor formation.
Similarly to human patients, this line of sheep exhibited a severe bleeding phenotype with spontaneous hemarthrosis leading to reduced locomotion, muscular hematomas, and episodes of hematuria and internal bleeding leading to death. The severity and frequency of the bleeding episodes were fairly constant between animals. For instance, umbilical cord bleeding at birth or during the first 12 h of life was a common trait amongst all HA sheep. Although in humans this occurrence is rare, as no significant trauma is inflicted to the umbilical cord during birth, in sheep the normal delivery process entails the tearing of the umbilical cord, potentially contributing to more significant bleeding. Furthermore, the increase in abdominal pressure caused by the effort of the lamb standing up soon after birth may also play a role in the bleeding through the umbilical cord in the absence of efficient hemostasis. Tying the umbilical cord resulted in internal bleeding and death of the two animals in which this procedure was attempted. The internal bleeding was due possibly to the extensive manipulation of the animals during the procedure. All of the HA animals had normal levels of platelets and VWF. Levels of fibrinogen and FIX, while lower than would be expected for adults, were consistent with the reduced levels of these factors that are routinely observed in humans and sheep during the neonatal period [36–38]. The PT was slightly elongated, which is normal for this newborn period, while the aPTT was extremely prolonged at birth. Despite the severe symptoms displayed by these animals, the levels of FVIII were 2.3 ± 0.8%. This is likely to be due to the exclusive use of one-stage aPTT-based assays for quantitating FVIII:C. This assay is challenging to perform and is often inaccurate at very low levels of FVIII:C [48]. Coupling this with the lack of experience with the model and the absence of appropriate reagents (such as hemophilic sheep plasma to serve as diluent) is likely to have led to an artificial elevation of the measured levels and accounts for the assay, in our hands, only havinga sensitivity of roughly 2.3%. Replacement therapy with hFVIII resulted in rapid clinical improvement, demonstrating that there was no association with other inherited deficiencies of plasma proteins in these animals.
While these animals harbored the same mutation, displayed the same severe symptoms and were exposed to identical treatments during the first hFVIII exposures, different levels of inhibitors were found following hFVIII replacement therapy. Also, we were unable to find a direct correlation between duration of therapy, total hFVIII dose, and inhibitor titer. Of note is that, because sFVIII protein is not yet available, hFVIII was given to these animals. Thus, these studies only demonstrate the ability of these animals to form antibodies against hFVIII. Studies administrating sFVIII will be necessary to prove that the hemophilic animals have the ability to form antibodies to FVIII of the same species. Once sFVIII protein becomes available, DNA microsatellite analysis of the animals forming inhibitors may yield clues regarding which genotypes are more prone to develop inhibitors.
The uniqueness of this model is also derived from the nature of the mutation found in these animals. Murine models have been generated through knockout/deletion technology, and the naturally occurring dog colonies exhibit gene inversions [49]. Similarly to mutations seen in human patients [50], we identified, in this line of animals, a premature stop codon with a frame shift mutation, making this sheep colony the first large animal model to possess a mutation of this kind. As such, a hypothetical advantage of this new model is that these animals could be used to test novel therapies based on read-through compounds, which is not feasible in the dog or murine models. Because large animals such as sheep allow the performance of long-term studies, questions such as long-term efficacy and safety of novel treatments can be properly addressed.
We anticipate that the availability of this animal model that closely parallels normal human weight and physiology, and in which the severity of the disease clearly resembles that of humans, will provide researchers in the field with an ideal and invaluable preclinical resource.
Supplementary Material
Acknowledgements
This work was supported by National Institutes of Health Grant R01HL073737-01. The Nevada Genomics Center is supported by NIH Grant P20 RR-016464 from the INBRE Program of the National Center for Research Resources.
We would like to thank J. Catalfamo, Director of Comparative Coagulation at the Animal Health Diagnostic Center at Cornell University, for his insightful help with all the coagulation tests performed in these animals.
Footnotes
Addendum C.D. Porada* and G. Almeida-Porada*: design and supervision of all studies, characterization of hemophilic phenotype, cloning and sequencing of normal and hemophilic sheep FVIII, data analysis and interpretation, and writing of the manuscript. C. Sanada*, J.A. Wood*, W. Liu§, J.Desai* and J.N. Lozier¶: cloning and sequencing of normal and hemophilic sheep FVIII to characterize disease-causing mutation. H. Glimp*, N.Frederick* and L. Millsap*: responsible for care of hemophilic lambs, administering hFVIII treatment, and synchronizing ewes for egg donation and for MOET. D.C. Kraemer†, C.R. Long†, C. Bormann†, S.L. Menges†, C. Hanna†, G. Flores-Foxworth†, T. Shin† and M.E. Westhusin†: performed all reproductive manipulations (IVF, artificial insemination, ICSI, and MOET) to re-establish line of hemophilia A sheep. E.D. Zanjani*: contributed to data analysis and interpretation. V. Pliska‡, G. Stranzinger‡ and H. Joerg‡: identification and characterization of original colony of hemophilia A sheep in Switzerland; and provided frozen semen to allow re-establishment of line.
Disclosure of Conflict of Interests The authors state that they have no conflict of interests.
Supporting information Additional Supporting Informationmay be found in the online version of this article:
Data S1. Reproductive manipulations to re-establish HA sheep.
Table S1. Primers used to reverse transcribe sheep FVIII mRNA.
Table S2. Primers used to clone sheep wild-type (WT) and hemophilic (H) FVIII fragments for sequencing.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- 1.Mannucci PM, Tuddenham EG. The hemophilias-from royal genes to gene therapy. N Engl J Med. 2001;344:1773–9. doi: 10.1056/NEJM200106073442307. [DOI] [PubMed] [Google Scholar]
- 2.White GC, II, Rosendaal F, Aledort LM, Lusher JM, Rothschild C, Ingerslev J. Definitions in hemophilia. Recommendation of the scientific subcommittee on factor VIII and factor IX of the scientific and standardization committee of the International Society on Thrombosis and Haemostasis. Thromb Haemost. 2001;85:560. [PubMed] [Google Scholar]
- 3.Agaliotis D, Zaiden RA, Ozturk S. Hemophilia, Overview. In: Besa EC, editor. eMedicine from WebMD, Medscape's continually updated clinical reference. 2006. [Google Scholar]
- 4.Kaveri SV, Dasgupta S, Andre S, Navarrete AM, Repesse Y, Wootla B, Lacroix-Desmazes S. Factor VIII inhibitors: role of von Willebrand factor on the uptake of factor VIII by dendritic cells. Haemophilia. 2007;13(Suppl 5):61–4. doi: 10.1111/j.1365-2516.2007.01575.x. [DOI] [PubMed] [Google Scholar]
- 5.Nichols TC, Dillow AM, Franck HW, Merricks EP, Raymer RA, Bellinger DA, Arruda VR, High KA. Protein replacement therapy and gene transfer in canine models of hemophilia A, hemophilia B, von willebrand disease, and factor VII deficiency. ILAR J. 2009;50:144–67. doi: 10.1093/ilar.50.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arruda VR. Toward gene therapy for hemophilia A with novel adenoviral vectors: successes and limitations in canine models. J Thromb Haemost. 2006;4:1215–7. doi: 10.1111/j.1538-7836.2006.01964.x. [DOI] [PubMed] [Google Scholar]
- 7.Doering CB, Denning G, Dooriss K, Gangadharan B, Johnston JM, Kerstann KW, McCarty DA, Spencer HT. Directed engineering of a high-expression chimeric transgene as a strategy for gene therapy of hemophilia A. Mol Ther. 2009 doi: 10.1038/mt.2009.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi Q, Fahs SA, Wilcox DA, Kuether EL, Morateck PA, Mareno N, Weiler H, Montgomery RR. Syngeneic transplantation of hematopoietic stem cells that are genetically modified to express factor VIII in platelets restores hemostasis to hemophilia A mice with preexisting FVIII immunity. Blood. 2008;112:2713–21. doi: 10.1182/blood-2008-02-138214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ide LM, Gangadharan B, Chiang KY, Doering CB, Spencer HT. Hematopoietic stem-cell gene therapy of hemophilia A incorporating a porcine factor VIII transgene and nonmyeloablative conditioning regimens. Blood. 2007;110:2855–63. doi: 10.1182/blood-2007-04-082602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lipshutz GS, Sarkar R, Flebbe-Rehwaldt L, Kazazian H, Gaensler KM. Short-term correction of factor VIII deficiency in a murine model of hemophilia A after delivery of adenovirus murine factor VIII in utero. Proc Natl Acad Sci USA. 1999;96:13324–9. doi: 10.1073/pnas.96.23.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ponder KP. Gene therapy for hemophilia. Curr Opin Hematol. 2006;13:301–7. doi: 10.1097/01.moh.0000239700.94555.b1. [DOI] [PubMed] [Google Scholar]
- 12.Bi L, Lawler AM, Antonarakis SE, High KA, Gearhart JD, Kazazian HH., Jr. Targeted disruption of the mouse factor VIII gene produces a model of haemophilia A. Nat Genet. 1995;10:119–21. doi: 10.1038/ng0595-119. [DOI] [PubMed] [Google Scholar]
- 13.Bi L, Sarkar R, Naas T, Lawler AM, Pain J, Shumaker SL, Bedian V, Kazazian HH., Jr. Further characterization of factor VIII-deficient mice created by gene targeting: RNA and protein studies. Blood. 1996;88:3446–50. [PubMed] [Google Scholar]
- 14.Hakobyan N, Kazarian T, Valentino LA. Synovitis in a murine model of human factor VIII deficiency. Haemophilia. 2005;11:227–32. doi: 10.1111/j.1365-2516.2005.01080.x. [DOI] [PubMed] [Google Scholar]
- 15.Valentino LA, Hakobyan N, Kazarian T, Jabbar KJ, Jabbar AA. Experimental haemophilic synovitis: rationale and development of a murine model of human factor VIII deficiency. Haemophilia. 2004;10:280–7. doi: 10.1111/j.1365-2516.2004.00899.x. [DOI] [PubMed] [Google Scholar]
- 16.Turecek PL, Gritsch H, Richter G, Auer W, Pichler L, Schwarz HP. Assessment of bleeding for the evaluation of therapeutic preparations in small animal models of antibody-induced hemophilia and von Willebrand disease. Thromb Haemost. 1997;77:591–9. [PubMed] [Google Scholar]
- 17.Hough C, Kamisue S, Cameron C, Notley C, Tinlin S, Giles A, Lillicrap D. Aberrant splicing and premature termination of transcription of the FVIII gene as a cause of severe canine hemophilia A: similarities with the intron 22 inversion mutation in human hemophilia. Thromb Haemost. 2002;87:659–65. [PubMed] [Google Scholar]
- 18.Lozier JN, Dutra A, Pak E, Zhou N, Zheng Z, Nichols TC, Bellinger DA, Read M, Morgan RA. The Chapel Hill hemophilia A dog colony exhibits a factor VIII gene inversion. Proc Natl Acad Sci USA. 2002;99:12991–6. doi: 10.1073/pnas.192219599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brooks MB, Barnas JL, Fremont J, Ray J. Cosegregation of a factor VIII microsatellite marker with mild hemophilia A in Golden Retriever dogs. J Vet Intern Med. 2005;19:205–10. doi: 10.1892/0891-6640(2005)19<205:coafvm>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 20.Rawle FE, Lillicrap D. Preclinical animal models for hemophilia gene therapy: predictive value and limitations. Semin Thromb Hemost. 2004;30:205–13. doi: 10.1055/s-2004-825634. [DOI] [PubMed] [Google Scholar]
- 21.High K. Gene transfer for hemophilia: can therapeutic efficacy in large animals be safely translated to patients? J Thromb Haemost. 2005;3:1682–91. doi: 10.1111/j.1538-7836.2005.01460.x. [DOI] [PubMed] [Google Scholar]
- 22.Pijnappels MI, Briet E, van der Zwet GT, Huisden R, van Tilburg NH, Eulderink F. Evaluation of the cuticle bleeding time in canine haemophilia A. Thromb Haemost. 1986;55:70–3. [PubMed] [Google Scholar]
- 23.Neuenschwander S, Kissling-Albrecht L, Heiniger J, Backfisch W, Stranzinger G, Pliska V. Inherited defect of blood clotting factor VIII (haemophilia A) in sheep. Thromb Haemost. 1992;68:618–20. [PubMed] [Google Scholar]
- 24.Backfisch W, Neuenschwander S, Giger U, Stranzinger G, Pliska V. Carrier detection of ovine hemophilia A using an RFLP marker, and mapping of the factor VIII gene on the ovine X-chromosome. J Hered. 1994;85:474–8. doi: 10.1093/oxfordjournals.jhered.a111503. [DOI] [PubMed] [Google Scholar]
- 25.Neuenschwander S, Pliska V. Factor VIII in blood plasma of haemophilic sheep: analysis of clotting time-plasma dilution curves. Haemostasis. 1994;24:27–35. doi: 10.1159/000217077. [DOI] [PubMed] [Google Scholar]
- 26.Zanjani ED, Pallavicini MG, Ascensao JL, Flake AW, Langlois RG, Reitsma M, MacKintosh FR, Stutes D, Harrison MR, Tavassoli M. Engraftment and long-term expression of human fetal hematopoietic stem cells in sheep following transplantation in utero. J Clin Invest. 1992;89:1178–88. doi: 10.1172/JCI115701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perkett EA, Lyons RM, Moses HL, Brigham KL, Meyrick B. Transforming growth factor-beta activity in sheep lung lymph during the development of pulmonary hypertension. J Clin Invest. 1990;86:1459–64. doi: 10.1172/JCI114862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abraham WM. Modeling of asthma, COPD and cystic fibrosis in sheep. Pulm Pharmacol Ther. 2008;21:743–54. doi: 10.1016/j.pupt.2008.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Morrison JL. Sheep models of intrauterine growth restriction: fetal adaptations and consequences. Clin Exp Pharmacol Physiol. 2008;35:730–43. doi: 10.1111/j.1440-1681.2008.04975.x. [DOI] [PubMed] [Google Scholar]
- 30.Bormann C, Long C, Menges S, Hanna C, Foxworth G, Westhusin M, Pliska V, Stranzinger G, Glimp H, Millsap L, Porada C, Almeida-Porada G, Kraemer D. Reestablishment of an extinct strain of sheep utilizing assisted reproductive technologies. Reprod Fertil Dev. 2007;21:153. [Google Scholar]
- 31.Bormann C, Long C, Menges S, Hanna C, Foxworth G, Shin T, Westhusin M, Pliska V, Stranzinger G, Joerg H, Glimp H, Millsap L, Porada C, Almeida-Porada G, Kraemer D. Reestablishment of an extinct strain of sheep from a limited supply of frozen semen. Reprod Fertil Dev. 2006;18:201. [Google Scholar]
- 32.Shin T, Long C, Foxworth G, Hanna C, Menges S, Bormann C, Almeida-Porada G, Porada C, Glimp H, Millsap L, Westhusin M, Kraemer D. A simple modified ICSI technique resulting in high pregnancy rates in merino sheep (Ovis Aries). Society for the Study of Reproduction; 38th Annual Meeting.2005. [Google Scholar]
- 33.Triplett DA, Harms CS. Coagulation screening assays. In: Triplett DA, editor. Procedures for the coagulation laboratory. American Society of Clinical Pathologists Press; Chicago: 1981. pp. 1–21. [Google Scholar]
- 34.Arkin CF, Bovil EG, Brandt JT, Rock WA, Triplett DA. Factors affecting the performance of Factor VIII coagulant activity assays. Arch Pathol Lab Med. 1992;116:908–15. [PubMed] [Google Scholar]
- 35.Benson R, Catalfamo JL, Dodds WJ. A multispecies enzyme-linked immunosorbent assay for von Willebrand's factor. J Lab Clin Med. 1992;119:420–7. [PubMed] [Google Scholar]
- 36.Jain N. Essentials of Veterinary Hematology. Williams & Wilkins; Media: 1993. [Google Scholar]
- 37.Williams W. Williams Hematology. McGraw-Hill Medical; 2006. [Google Scholar]
- 38.Journeycake JM, Buchanan GR. Coagulation disorders. Pediatr Rev/Am Acad Pediatr. 2003;24:83–91. doi: 10.1542/pir.24-3-83. [DOI] [PubMed] [Google Scholar]
- 39.Ananyeva NM, Lacroix-Desmazes S, Hauser CA, Shima M, Ovanesov MV, Khrenov AV, Saenko EL. Inhibitors in hemophilia A: mechanisms of inhibition, management and perspectives. Blood Coagul Fibrinolysis. 2004;15:109–24. doi: 10.1097/00001721-200403000-00001. [DOI] [PubMed] [Google Scholar]
- 40.Littlewood JD, Barrowcliffe TW. The development and characterisation of antibodies to human factor VIII in haemophilic dogs. Thromb Haemost. 1987;57:314–21. [PubMed] [Google Scholar]
- 41.Benincosa LJ, Chow FS, Tobia LP, Kwok DC, Davis CB, Jusko WJ. Pharmacokinetics and pharmacodynamics of a humanized monoclonal antibody to factor IX in cynomolgus monkeys. J Pharmacol Exp Ther. 2000;292:810–6. [PubMed] [Google Scholar]
- 42.Tomokiyo K, Teshima K, Nakatomi Y, Watanabe T, Mizuguchi J, Nozaki C, Nakagaki T, Miyamoto S, Funatsu A, Iwanaga S. Induction of acquired factor IX inhibitors in cynomolgus monkey (Macaca fascicularis): a new primate model of hemophilia B. Thromb Res. 2001;102:363–74. doi: 10.1016/s0049-3848(01)00253-5. [DOI] [PubMed] [Google Scholar]
- 43.Russell KE, Olsen EH, Raymer RA, Merricks EP, Bellinger DA, Read MS, Rup BJ, Keith JC, Jr, McCarthy KP, Schaub RG, Nichols TC. Reduced bleeding events with subcutaneous administration of recombinant human factor IX in immune-tolerant hemophilia B dogs. Blood. 2003;102:4393–8. doi: 10.1182/blood-2003-05-1498. [DOI] [PubMed] [Google Scholar]
- 44.Xu L, Mei M, Haskins ME, Nichols TC, O'Donnell P, Cullen K, Dillow A, Bellinger D, Ponder KP. Immune response after neonatal transfer of a human factor IX-expressing retroviral vector in dogs, cats, and mice. Thromb Res. 2007;120:269–80. doi: 10.1016/j.thromres.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 45.Aronson DL. Cause of death in hemophilia A patients in the United States from 1968 to 1979. Am J Hematol. 1988;27:7–12. doi: 10.1002/ajh.2830270103. [DOI] [PubMed] [Google Scholar]
- 46.Chorba TL, Holman RC, Strine TW, Clarke MJ, Evatt BL. Changes in longevity and causes of death among persons with hemophilia A. Am J Hematol. 1994;45:112–21. doi: 10.1002/ajh.2830450204. [DOI] [PubMed] [Google Scholar]
- 47.Miyasaka M, Trnka Z. Lymphocyte migration and differentiation in a large-animal model: the sheep. Immunol Rev. 1986;91:87–114. doi: 10.1111/j.1600-065x.1986.tb01485.x. [DOI] [PubMed] [Google Scholar]
- 48.Cinotti S, Paladino E, Morfini M. Accuracy of FVIII: C assay by one-stage method can be improved using hemophilic plasma as diluent. J Thromb Haemost. 2006;4:828–33. doi: 10.1111/j.1538-7836.2006.01880.x. [DOI] [PubMed] [Google Scholar]
- 49.Ovlisen K, Kristensen AT, Tranholm M. In vivo models of haemophilia - status on current knowledge of clinical phenotypes and therapeutic interventions. Haemophilia. 2008;14:248–59. doi: 10.1111/j.1365-2516.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- 50.Vidal F, Farssac E, Altisent C, Puig L, Gallardo D. A novel mutation (2409delT) in exon 14 of the factor VIII gene causes severe haemophilia A. Hum Hered. 2000;50:266–7. doi: 10.1159/000022928. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.