Abstract
Recently we showed a critical role for Vascular Adhesion Protein-1 (VAP-1) in rodents during acute ocular inflammation, angiogenesis, and diabetic retinal leukostasis. However, the expression of VAP-1 in the human eye is unknown. VAP-1 localization was investigated by immunohistochemistry. Five μm thick sections were generated from human ocular tissues embedded in paraffin. Sections were incubated overnight with primary mAbs against VAP-1 (5μg/ml), smooth muscle actin (1μg/ml), CD31 or isotype-matched IgG at 4°C. Subsequently, a secondary mAb was used for 30min at room temperature, followed by Dako Envision + HRP (AEC) System for signal detection. The stained sections were examined using light microscopy and the signal intensity was quantified by two masked evaluators and graded into 4 discrete categories. In all examined ocular tissues, VAP-1 staining was confined to the vasculature. VAP-1 labeling showed the highest intensity in both arteries and veins of neuronal tissues; retina, and optic nerve, and the lowest intensity in the iris vasculature (p<0.05). Scleral and choroidal vessels showed moderate staining for VAP-1. VAP-1 intensity was significantly higher in the arteries compared to veins (p<0.05). Furthermore, VAP-1 staining in arteries co-localized with both CD31 and smooth muscle actin (sm-actin) staining, suggesting expression of VAP-1 in endothelial cells, smooth muscle cells or potentially pericytes. In conclusion, Immunohistochemistry reveals constitutive expression of VAP-1 in human ocular tissues. VAP-1 expression is exclusive to the vasculature with arteries showing significantly higher expression than veins. Furthermore, VAP-1 expression in the ocular vasculature is heterogeneous, with the vessels of the optic nerve and the retina showing highest expressions. These results characterize VAP-1 expression in human ocular tissues.
Keywords: Inflammation, adhesion molecules, morphology, vasculature
Introduction
Vascular adhesion protein-1 (VAP-1), a 170kDa homodimeric sialylated glycoprotein, is a unique adhesion molecule that plays an important role in leukocyte trafficking to lymphoid organs, specifically during the transmigration step (Bono, Salmi et al. 1998; Smith, Salmi et al. 1998; Salmi, Yegutkin et al. 2001). In addition to being an adhesion molecule, VAP-1 belongs to a group of enzymes, known as semicarbazide sensitive amine oxidases (SSAO), which catalyze the formation of reactive oxygen species (Yu, Wright et al. 2003). VAP-1 was originally discovered in inflamed synovial vessels, and it appears to be induced at sites of inflammation (Salmi and Jalkanen 1992; Jalkanen and Salmi 1993; Jalkanen and Salmi 1993; Salmi, Kalimo et al. 1993). Altered levels of SSAO activity in humans have been reported in a number of diseases, including rheumatoid arthritis, diabetes, and atherosclerosis (O’Sullivan, Unzeta et al. 2004).
Under physiological conditions, VAP-1 is predominantly expressed in high endothelial venules (HEV) of peripheral lymph nodes, however, capillaries of nonlymphatic human tissues, such as kidney and heart, also express VAP-1 (Salmi, Kalimo et al. 1993; Salmi and Jalkanen 2006). In addition to endothelial cells, VAP-1 is expressed on adipocytes, dendritic cells and smooth muscle cells (Salmi, Kalimo et al. 1993; Jaakkola, Kaunismaki et al. 1999). However, the expression and localization of VAP-1 in the human eye remains to be investigated.
Devastating eye diseases, such as uveitis (Rosenbaum, McDevitt et al. 1980; Smith, Hart et al. 1998), age-related macular degeneration (AMD) (Shen, Yu et al. 1998; Sakurai, Taguchi et al. 2003), and diabetic retinopathy (DR), are of vascular and inflammatory nature. Leukocyte endothelial interaction is a key component in the pathogenesis of these diseases (Hafezi-Moghadam, Noda et al. 2007). Recently, we showed that VAP-1 is expressed on the endothelium of retinal vessels in rodents and that it plays a critical role in the recruitment of leukocytes to the eye during acute inflammatory conditions, such as the endotoxin-induced uveitis (EIU) (Noda, Miyahara et al. 2008). In a laser-induced model of choroidal neovascularization (CNV), we found that inhibition of VAP-1 effectively reduces cytokine expression, macrophage recruitment, and the development of CNV (Noda, She et al. 2008). Furthermore, in experimentally-induced DR, VAP-1 inhibition significantly reduced leukocyte transmigration rate in the retina (Noda, Nakao et al. 2009).
Here, we study the expression of VAP-1 in the human eye and further assess its distribution in vessels from different ocular tissues.
Materials & Methods
Tissue Samples
Paraffin embedded blocks of normal human ocular tissues were obtained from the MEEI stored archive of samples. All materials were used under the protocol approved by the Institutional Review Board (IRB) of Massachusetts Eye and Ear Infirmary (MEEI) and in accordance to the Declaration of Helsinki.
Immunohistochemistry
VAP-1 tissue localization was examined in 5μm sections generated from the above described tissue samples. The slides were deparaffinized and hydrated through exposure with graded alcohols (100% then 95%) followed by water. Endogenous peroxidase activity was then blocked by placing the sections in 0.3% hydrogen peroxide (Sigma Aldrich, ST Louis, MO, US) for 15min and subsequently with 10% normal goat serum (Invitrogen, Carlsbad, CA) for 1 hour to block nonspecific binding. Subsequently, sections were incubated with primary monoclonal antibodies (mAb) against either VAP-1 raised in mouse (5μg/ml; BD biosciences, Franklin Lakes, NJ), endothelial CD31 (1/50, Abcam, Cambridge, MA) or smooth muscle actin (1/100, Abcam, Cambridge, MA) raised in rabbit at 4C° overnight. For CD31 staining, antigen retrieval was obtained through heated water bath at 97°C for 10min. As a negative control, the primary antibodies were replaced with non-immune mouse IgG (Dako, Carpinteria, CA).
Thereafter, the sections were incubated for 30min at room temperature with HRP-labeled polymer conjugated secondary antibody against mouse IgG (Dako). For signal detection, the sections were incubated with the ready-to-use AEC substrate-chormogen solution for 5min according to the manufacturer’s protocol (Dako Envision + System − HRP (AEC) then washed with distilled water. Finally, sections were counterstained with hematoxylin (Sigma) for 3min, followed by washing with distilled water and mounting with the hard-set media (Vectra, Burlingame, CA). Photomicrographs were taken with a 5 MP color digital camera (Q-color; Olympus, Lake Success, NY) mounted on a microscope (Leica, Deerfield, IL).
Alternatively for immunofluorescence, the sections were secondarily stained with Alexa Fluor 488 goat anti-mouse IgG and Alexa Fluor 546 goat anti-rabbit IgG (1/200, Invitrogen) and the photomicrographs were taken using a digital high sensitivity camera (Hamamatsu, ORCA-ER C4742-95, Japan).
Data Reduction and Statistical Analysis
Histological sections were examined under light microscopy at a magnification of 320× and graded by two independent experimenters. VAP-1 signal intensity was divided into 3 different groups: −, no evidence for VAP-1 staining; +, moderate; and ++, strong staining. To compare the results from different groups, grading was performed by two independent evaluators and averaged for each eye and plotted as 0, 1 and 2, respectively.
For statistical analysis, the results were divided into two groups (0, no staining or higher, positive staining). A Chi-square test was used to calculate the degree of confidence with which the data supports the null hypothesis. Probability values (p) less than 0.05 were considered statistically significant.
Results
Exclusive Expression of VAP-1 in the Vasculature of the Human Eye
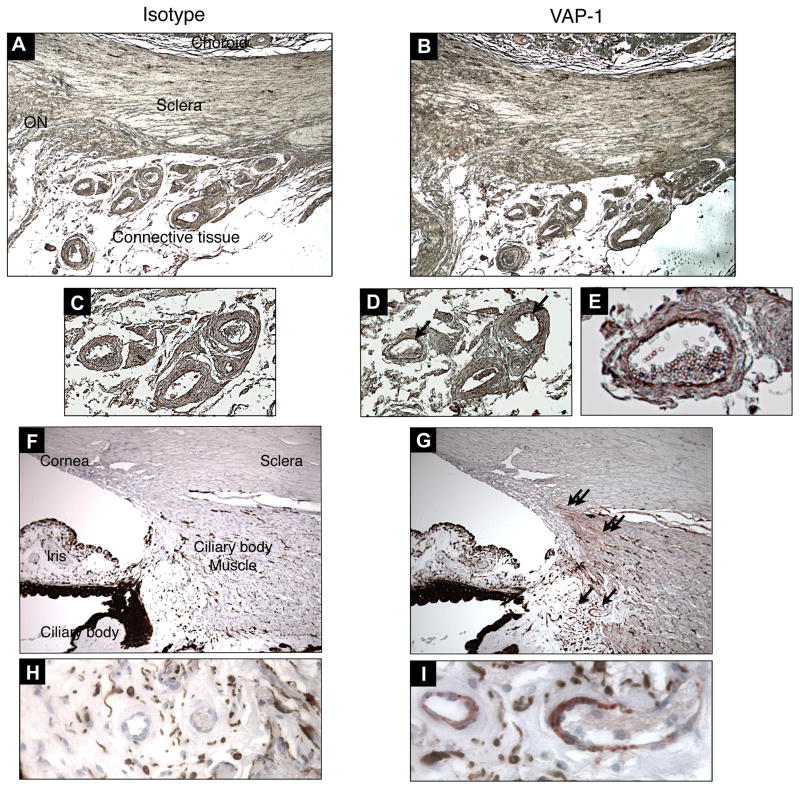
To determine VAP-1 expression in the human eye, immunohistochemistry was performed on normal human ocular tissues. The ocular tissues were obtained post-mortally from donors with known systemic conditions (n=7 eyes, 6 individuals; Table 1). In various ocular tissues, such as sclera and choroid, VAP-1 specific signal was almost exclusively confined to the vasculature, whereas non-immune isotype control did not show any staining. Particularly, VAP-1 was observed in the inner and medial layers, but not the outer adventitial layer of the main branches of the ophthalmic artery. In contrast, VAP-1 expression wasn’t detectable in small capillaries (Fig. 1A–E).
Table 1.
Demographic data and case information (4A and 4B are eyes related to the same case). N/A, not available; CVD, cardiovascular disease; ICH, intracerebral hemorrhage; SLE, systemic lupus erythematosus; HTN, hypertension.
| Case# | Gender | Age | Primary Cause of Death | Medical History | Ocular Diagnosis |
|---|---|---|---|---|---|
| 1 | M | 26 | N/A | Hodgkins Lymphoma | Normal |
| 2 | F | 42 | N/A | Metastasized breast cancer | Normal |
| 3 | F | 49 | N/A | Septicemia, SLE | Normal |
| 4A | M | 51 | CVD | N/A | Normal/donor |
| 4B | M | 51 | CVD | N/A | Normal/donor |
| 5 | F | 65 | ICH | HTN | Normal with few retinal hemorrhages |
| 6 | F | 76 | N/A | HTN | Normal |
Figure 1. VAP-1 Immunostaining in the Eye.
Paraffin sections obtained from normal human eyes were stained with non-immune isotype-matched control mAb (A,C,F&H) or anti VAP-1 mAb (B,D,E,G&I). Single arrows depict VAP-1 expression (red) on the vessels of the connective tissue outside the eye (B,D&E) and the ciliary body (G&I), while double arrows depict VAP-1 expression on the smooth muscle cells of the ciliary body (G). Magnification of A and B, ×50; C, D, F and G, ×100; E, ×320; H and I, ×640. ON, optic nerve head.
Outside of the vessels, VAP-1 expression was also observed in the smooth muscle cells of the ciliary body (Fig. 1G). No VAP-1 staining was observed in the RPE layer of any of the eyes.
VAP-1 Distribution in Normal Human Ocular Tissues
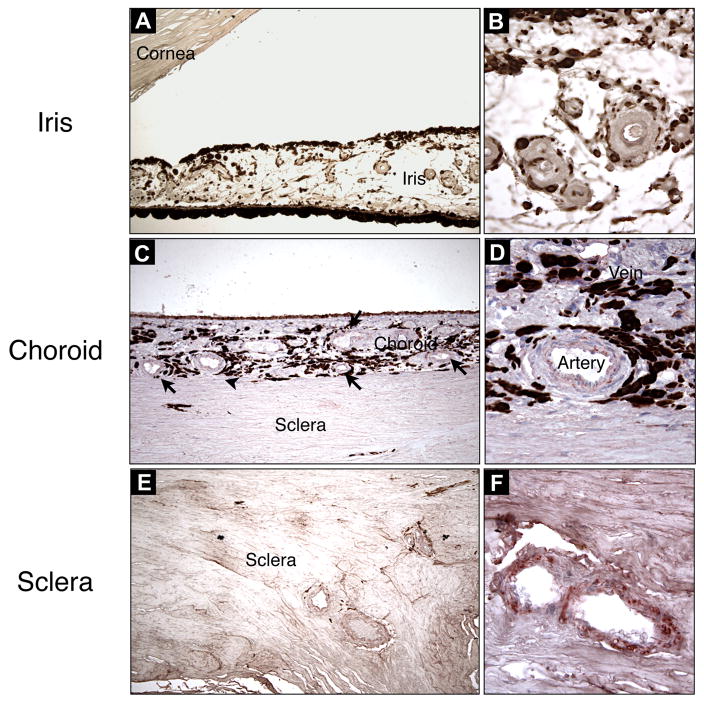
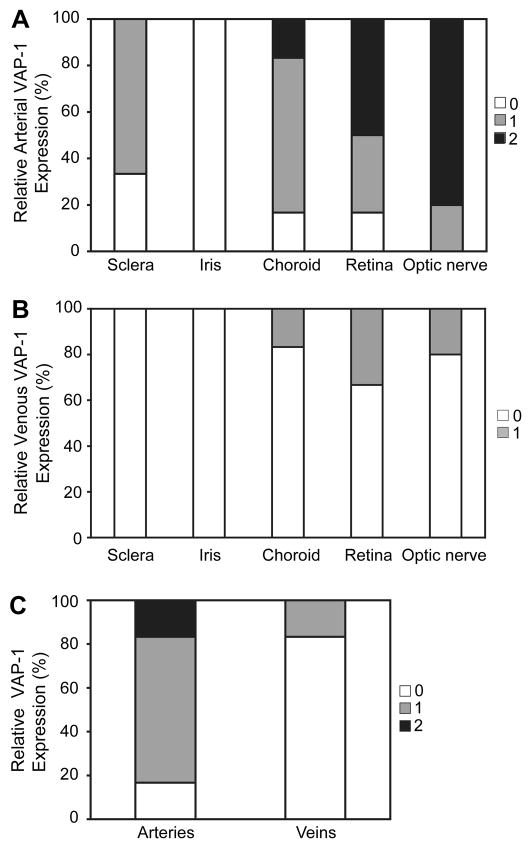
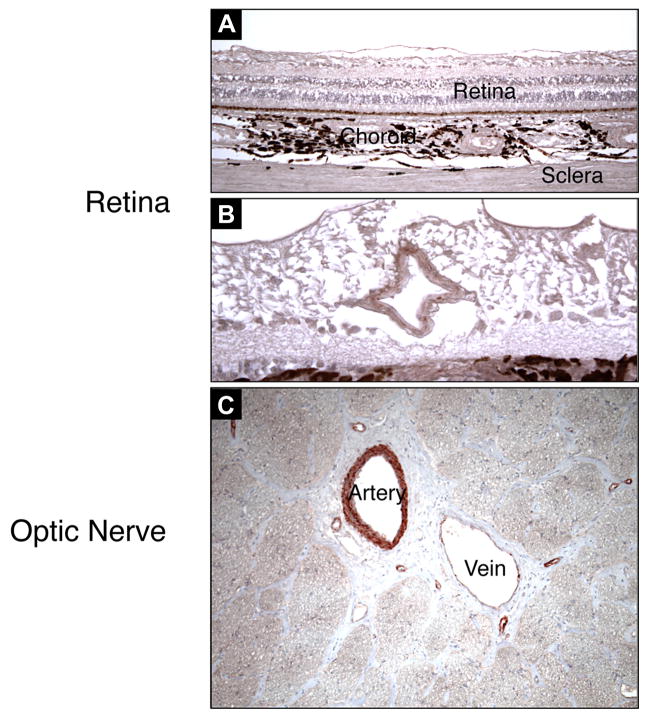
To compare the vascular VAP-1 expression in different ocular tissues, VAP-1 signal intensity was quantified by grading (Table 2). No appreciable staining for VAP-1 was observed in the iris vessels; in either arteries or veins (n=4) (Fig. 2A&B, 4A&B). Compared to the iris arteries, arteries of the scleral (n=7) and choroidal tissues (n=6) showed significantly higher VAP-1 staining (p<0.05) (Fig. 2C–F, 4A), and arteries of neuronal tissues -the retina (n=6) and optic nerve (n=7)- showed the most prominent staining (p<0.05, p<0.01 respectively) (Fig. 3A–C, 4A). In contrast, no significant difference was observed in VAP-1 expression between veins of all the tissue groups (Fig. 4B, p>0.1).
Table 2.
Summary of VAP-1 expression in different ocular tissues divided into arteries and veins. Ø, tissue not available; −, +, and ++, intensity of VAP-1 staining.
| Case # | Arteries |
Veins |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sclera | Iris | Choroid | Retina | Optic N. | Sclera | Iris | Choroid | Retina | Optic N. | |
| 1 | + | − | − | − | + | − | − | − | − | − |
| 2 | + | Ø | Ø | Ø | Ø | − | Ø | Ø | Ø | Ø |
| 3 | + | − | ++ | ++ | ++ | − | − | + | + | + |
| 4A | − | − | + | + | ++ | − | − | − | − | − |
| 4B | − | Ø | + | + | ++ | − | Ø | − | − | − |
| 5 | + | − | + | ++ | ++ | − | − | − | − | − |
| 6 | + | Ø | + | ++ | Ø | − | Ø | − | + | Ø |
Figure 2. AEC (3-Amino-9-ethylcarbazole) Staining of VAP-1 in Various Ocular Tissues.
VAP-1 staining was evaluated in different tissues of the eye and selectively found in choroidal (C&D) and scleral vessels (E&F), but not in iris vessels (A&B). Magnification of A, C and E, ×100; B, D, and F, ×320.
Figure 4. Quantification of VAP-1 Expression in Arteries and Veins in Various Ocular Tissues.
Highest levels of VAP-1 expression were found in the arteries of the retina and optic nerve (A). However, VAP-1 was not detectable in arteries (A) and veins (B) of the iris.
(C) Quantification of VAP-1 expression in the choroidal vessels. VAP-1 expression was significantly higher in arteries than veins.
Figure 3. AEC Staining of VAP-1 in Ocular Neuronal Tissues.
VAP-1 is strongly expressed in vessels of neuronal tissues, the retina (A&B), and the optic nerve (C). Magnification of A and C, ×100; B, ×200.
Furthermore, VAP-1 expression was compared in arteries and veins. Interestingly, VAP-1 expression is significantly higher in arteries than veins in all examined tissues (p<0.05), except for the iris vessels (Fig. 4C).
Localization of VAP-1 in Vascular Endothelium, Smooth Muscle Cells, and Pericytes
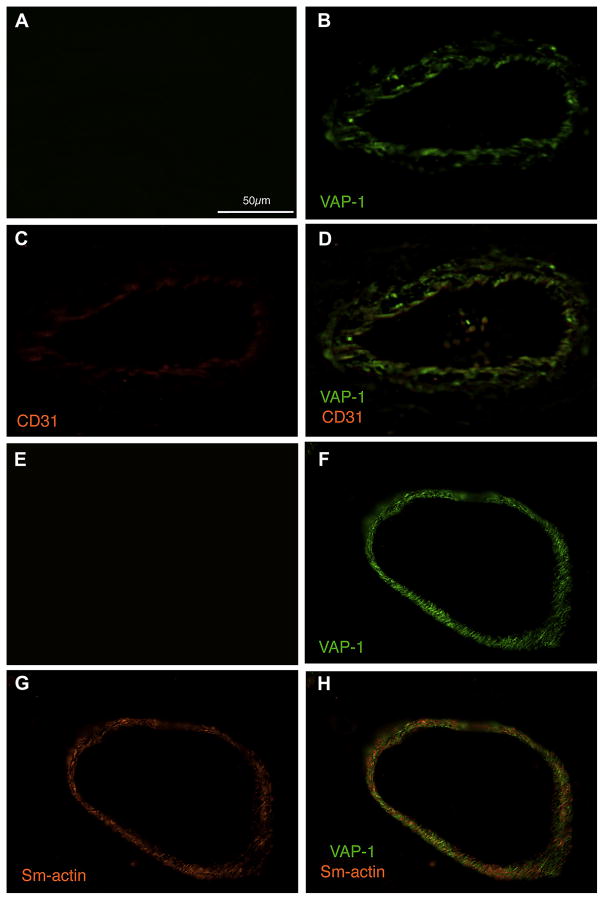
To further investigate the cellular distribution of VAP-1, we performed co-immuno staining of VAP-1 with either CD31, a marker for endothelial cells, or smooth muscle actin, a marker expressed in smooth muscle cells and pericytes (Mancuso, Davis et al. 2006). In line with previous studies in various other human tissues (Jaakkola, Kaunismaki et al. 1999), in the eye, VAP-1 expression was found in vascular endothelium, smooth muscle cells, and pericytes (Fig. 5).
Figure 5. Cellular Localization of VAP-1 in Ocular Vessels.
Paraffin sections were co-immunostained with antibodies against endothelial CD31 (C&D), smooth muscle actin (G&H), and VAP-1 (B,D,F&H). VAP-1 colocalized in endothelial cells (D), smooth muscle cells and pericytes (H) compared to the negative isotype control (A&E). Scale bar, 50μm.
Discussion
Important eye diseases, such as AMD, DR, and uveitis, have a vascular and inflammatory component (Donoso, Kim et al. 2006). Adhesion molecules are key mediators of inflammatory leukocyte-endothelial interaction in these diseases (Sakurai, Taguchi et al. 2003). Recently, we showed a critical role for VAP-1 in acute ocular inflammation (Noda, Miyahara et al. 2008), angiogenesis (Noda, She et al. 2008), and diabetic retinal leukostasis (Noda, Nakao et al. 2009). However, the expression and function of VAP-1 in the human eye was unknown. In this work we investigated the distribution of VAP-1 in normal human ocular tissues.
In the eye, we found VAP-1 is predominantly expressed in the vasculature. Interestingly, arteries show significantly higher levels of VAP-1 staining than veins, suggesting a specialized role for this molecule in diseases with primary arterial involvement. Furthermore, VAP-1 appears not to be expressed under constitutive conditions in capillaries and smaller venules, or its expression is much lower than in arteries. Next to the vascular endothelium, VAP-1 is also expressed in smooth muscle cells within the vascular wall or in the ciliary body and also in pericytes (Jaakkola, Kaunismaki et al. 1999).
The difference between arterial and venous expression might for instance be relevant in the pathogenesis of DR, where capillary non-perfusion, due to leukocyte plugging at the capillary entrance has been postulated as an important component (Schroder, Palinski et al. 1991; Miyamoto, Khosrof et al. 1999). Adhesion molecules, such as Intracellular Adhesion Molecule-1 (ICAM-1) or P-selectin, which cause leukocyte adhesion in postcapillary venules may not sufficiently explain ischemia due to capillary non-perfusion (Miyamoto, Khosrof et al. 1999). The higher expression of VAP-1 in arteries together with the specialized role of this molecule for leukocyte transmigration may open new possibilities for targeting this molecule in ocular diseases. Unaddressed in this study however is VAP-1’s enzymatic activity, which might also differ from the expression of the molecule in the various vessels.
Our studies also indicate that in addition to the endothelium, smooth muscle cells and pericytes express VAP-1. Since arteries have both endothelial and smooth muscle cells, while veins have mainly endothelial cells, this might in part explain the higher level of VAP-1 expression in arteries compared to veins.
Interestingly we also found considerable heterogeneity in the vascular expression of VAP-1 within the various regions of the eye. While vessels of the optic nerve head expressed the highest amounts of the molecule, the iris vessels did not show detectable expression. The broad expression of VAP-1 in the posterior section of the human eye and the critical role of this molecule in CNV formation (Noda, She et al. 2008) and experimental DR (Noda, Nakao et al. 2009) suggests involvement of this molecule in human disease.
Our results show constitutive expression of VAP-1 in the human eye. This molecule is known to contribute to inflammatory leukocyte recruitment and angiogenesis in experimental models of ocular disease. Future studies will be needed to address the expression and function of VAP-1 in human diseases.
Acknowledgments
The authors would like to thank Ms. Marion Athanas and Dr. Pooja Bhat, members of the David D. Cogan Ophthalmic Pathology Laboratory at the Massachusetts Eye and Ear Infirmary for providing the human specimens, Mr. Norman Michaud and Ms. Sreedevi Mallemadugula for their expert technical assistance.
This work was supported by research funds from RTECH-UENO, Bausch & Lomb Vitreoretinal Fellowships to (K.N. and S.N.), NIH grants HL086933 (Alan Cross) and AI050775 (AH-M), and NEI core grant EY14104. We are indebted to the Massachusetts Lions Foundation for generous funds provided for laboratory equipment used in this project. We would like to thank Research to Prevent Blindness and American Health Assistance Foundation. We are grateful to the Marion W. and Edward F. Knight AMD Fund.
References
- Bono P, Salmi M, et al. Cloning and characterization of mouse vascular adhesion protein-1 reveals a novel molecule with enzymatic activity. J Immunol. 1998;160(11):5563–71. [PubMed] [Google Scholar]
- Donoso LA, Kim D, et al. The role of inflammation in the pathogenesis of age-related macular degeneration. Surv Ophthalmol. 2006;51(2):137–52. doi: 10.1016/j.survophthal.2005.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafezi-Moghadam A, Noda K, et al. VLA-4 blockade suppresses endotoxin-induced uveitis: in vivo evidence for functional integrin up-regulation. Faseb J. 2007;21(2):464–74. doi: 10.1096/fj.06-6390com. [DOI] [PubMed] [Google Scholar]
- Jaakkola K, Kaunismaki K, et al. Human vascular adhesion protein-1 in smooth muscle cells. Am J Pathol. 1999;155(6):1953–65. doi: 10.1016/S0002-9440(10)65514-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalkanen S, Salmi M. A novel endothelial cell molecule mediating lymphocyte binding in humans. Behring Inst Mitt. 1993;(92):36–43. [PubMed] [Google Scholar]
- Jalkanen S, Salmi M. Vascular adhesion protein-1 (VAP-1)--a new adhesion molecule recruiting lymphocytes to sites of inflammation. Res Immunol. 1993;144(9):746–9. doi: 10.1016/0923-2494(93)80060-c. discussion 754–62. [DOI] [PubMed] [Google Scholar]
- Mancuso MR, Davis R, et al. Rapid vascular regrowth in tumors after reversal of VEGF inhibition. J Clin Invest. 2006;116(10):2610–21. doi: 10.1172/JCI24612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto K, Khosrof S, et al. Prevention of leukostasis and vascular leakage in streptozotocin-induced diabetic retinopathy via intercellular adhesion molecule-1 inhibition. Proc Natl Acad Sci U S A. 1999;96(19):10836–41. doi: 10.1073/pnas.96.19.10836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, Miyahara S, et al. Inhibition of vascular adhesion protein-1 suppresses endotoxin-induced uveitis. Faseb J. 2008;22(4):1094–103. doi: 10.1096/fj.07-9377com. [DOI] [PubMed] [Google Scholar]
- Noda K, Nakao S, et al. Vascular adhesion protein-1 regulates leukocyte transmigration rate in the retina during diabetes. Exp Eye Res. 2009 doi: 10.1016/j.exer.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda K, She H, et al. Vascular adhesion protein-1 blockade suppresses choroidal neovascularization. Faseb J. 2008;22(8):2928–35. doi: 10.1096/fj.07-105346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Sullivan J, Unzeta M, et al. Semicarbazide-sensitive amine oxidases: enzymes with quite a lot to do. Neurotoxicology. 2004;25(1–2):303–15. doi: 10.1016/S0161-813X(03)00117-7. [DOI] [PubMed] [Google Scholar]
- Rosenbaum JT, McDevitt HO, et al. Endotoxin-induced uveitis in rats as a model for human disease. Nature. 1980;286(5773):611–3. doi: 10.1038/286611a0. [DOI] [PubMed] [Google Scholar]
- Sakurai E, Taguchi H, et al. Targeted disruption of the CD18 or ICAM-1 gene inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2003;44(6):2743–9. doi: 10.1167/iovs.02-1246. [DOI] [PubMed] [Google Scholar]
- Salmi M, Jalkanen S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science. 1992;257(5075):1407–9. doi: 10.1126/science.1529341. [DOI] [PubMed] [Google Scholar]
- Salmi M, Jalkanen S. Developmental regulation of the adhesive and enzymatic activity of vascular adhesion protein-1 (VAP-1) in humans. Blood. 2006;108(5):1555–61. doi: 10.1182/blood-2005-11-4599. [DOI] [PubMed] [Google Scholar]
- Salmi M, Kalimo K, et al. Induction and function of vascular adhesion protein-1 at sites of inflammation. J Exp Med. 1993;178(6):2255–60. doi: 10.1084/jem.178.6.2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmi M, Yegutkin GG, et al. A cell surface amine oxidase directly controls lymphocyte migration. Immunity. 2001;14(3):265–76. doi: 10.1016/s1074-7613(01)00108-x. [DOI] [PubMed] [Google Scholar]
- Schroder S, Palinski W, et al. Activated monocytes and granulocytes, capillary nonperfusion, and neovascularization in diabetic retinopathy. Am J Pathol. 1991;139(1):81–100. [PMC free article] [PubMed] [Google Scholar]
- Shen WY, Yu MJ, et al. Expression of cell adhesion molecules and vascular endothelial growth factor in experimental choroidal neovascularisation in the rat. Br J Ophthalmol. 1998;82(9):1063–71. doi: 10.1136/bjo.82.9.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DJ, Salmi M, et al. Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule. J Exp Med. 1998;188(1):17–27. doi: 10.1084/jem.188.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Hart PH, et al. Basic pathogenic mechanisms operating in experimental models of acute anterior uveitis. Immunol Cell Biol. 1998;76(6):497–512. doi: 10.1046/j.1440-1711.1998.00783.x. [DOI] [PubMed] [Google Scholar]
- Yu PH, Wright S, et al. Physiological and pathological implications of semicarbazide-sensitive amine oxidase. Biochim Biophys Acta. 2003;1647(1–2):193–9. doi: 10.1016/s1570-9639(03)00101-8. [DOI] [PubMed] [Google Scholar]