Abstract
Background
Short QT syndrome (SQT) 1 is an inherited sudden death syndrome often associated with atrial fibrillation (AF). We examined the cellular basis for AF in a newly developed experimental atrial model of SQT1.
Methods
Action potentials (AP) were recorded from pectinate muscle (PM) and crista terminalis (CT) regions of coronary-perfused canine right atrial preparations, together with a pseudo-ECG. The IKr agonist PD-118057 (20 μM) was used to mimic the gain of function in IKr known to underlie SQT1.
Results
The IKr agonist significantly abbreviated AP duration (APD) of CT and PM and effective refractory period (ERP) measured in PM (n = 28). Spatial dispersion of repolarization (SDR), defined as inter-regional differences of APD, increased from 27±17 to 51±32 (p=0.002; n=28). AF could be induced by a single premature stimulus after, but not before exposure to PD-118057, in 26/28 (93%) preparations. Quinidine (10 μM), which prolonged APD and ERP, but not lidocaine (20 μM) or E-4031 (5 μM), which prolonged only ERP or APD, respectively, was effective in preventing the PD-118057-mediated AF. In the presence of PD-118057, isoproterenol (100 nM) further abbreviated both APD and ERP and facilitated induction of sustained AF in 5/6 preparations.
Conclusions
The IKr agonist recapitulates the electrophysiologic and arrhythmic manifestations of SQT1. Abbreviation of APD, ERP and amplification of SDR predispose to the development of AF by creating the substrate for reentry. Quinidine, but not E-4031 or lidocaine, was effective in preventing AF in this setting.
Keywords: Arrhythmia, Electrophysiology, Antiarrhythmic drugs, Quinidine, Isoproterenol
Introduction
The short QT syndrome (SQTS) is an inherited sudden cardiac death syndrome characterized by abnormally short QTc intervals (<360 ms) 1 and an increased propensity for development of atrial and ventricular tachyarrhythmias.2–6 Although a short QT interval is the hallmark of SQTS, these patients tend to have other distinguishing electrocardiogram (ECG) characteristics, including tall peaked, symmetrical or asymmetrical T waves and occasional depression of the PR interval.6 In the largest series available (n= 29),7 31% of patients presented with atrial fibrillation (AF) or flutter.
Thus far, mutations in 5 different genes encoding cardiac ion channels have been identified in familial or sporadic AF cases. Gain-of-function mutations in KCNH27, KCNQ18 and KCNJ29 genes encoding for the rapidly and slowly activating potassium channel currents (IKr and IKs) and the inward rectifier potassium channel current (IK1), give rise to the SQT1, SQT2 and SQT3 forms of SQTS. Our group was the first to identify a missense mutation (N588K) in KCNH2 in 2 unrelated families.7 This mutation has proved to be a hotspot for SQTS. Analysis of the current generated following transfection of the mutated channel into human embryonic kidney (HEK) cells showed that the mutation shifted inactivation of the KCNH2 channels out of the range of the action potential, thus producing a large gain of function in IKr. The gain of function in IKr is responsible for abbreviation of the action potential duration (APD).
A heterogeneous abbreviation of APD and refractoriness in different regions of ventricular myocardium is believed to create the substrate for reentrant arrhythmias within the ventricle. Evidence in support of this hypothesis in SQTS derives from two models involving the left ventricular coronary-perfused wedge preparations exposed to the K channel opener pinacidil10 or to the IKr agonist, PD-118057.11 It is noteworthy that the same point mutation in KCNH2 shown to be associated with sudden cardiac death (SCD), was reported by Hong and co-workers 12 to be associated exclusively with AF.
Pharmacotherapy of SQTS would appear to be fairly straightforward since many of the traditional antiarrhythmics can act to prolong APD via inhibition of IKr and thus antagonize the increase in net depolarizing current produced by the genetic defects. However, this logical approach proved to be less than straightforward when first implemented in the clinic. Attempts to prolong the QT interval using the selective IKr blocker, d-sotalol, in SQT1 patients met with failure.13 Subsequent studies identified quinidine as an effective agent for both ventricular fibrillation (VF) and AF associated with SQT1,14,13 suggesting a common mechanism. To our knowledge there are no experimental models of SQT syndrome in the atria and little in the way of experimental data that are helpful in advancing our understanding of the mechanism(s) underlying arrhythmogenesis in this syndrome or the approach to therapy of AF in these patients.
Methods
Arterially-perfused whole canine right atrium (RA)
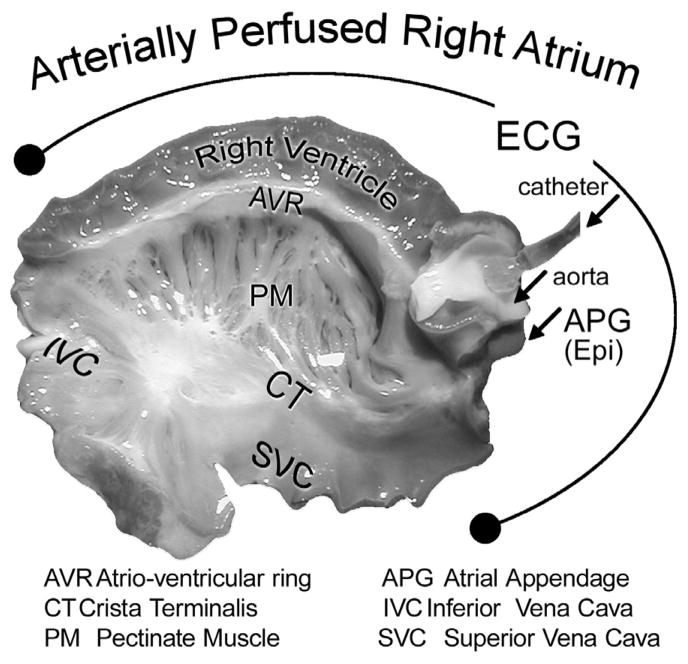
Mongrel dogs (≥1 year old) weighing 20–25 kg were anticoagulated with heparin and anesthetized with pentobarbital (30–35 mg/kg, i.v.). The chest was opened via a left thoracotomy, the heart excised, placed in a cardioplegic solution consisting of cold (4°C) Tyrode’s solution containing 8.5 mM [K+]o and transported to a dissection tray. The ostium of the right coronary artery was cannulated and the preparation was perfused with cold Tyrode’s solution (12–15°C) containing 8.5 [K+]0. The RA preparation (Fig. 1) was placed in a temperature-controlled bath, maintained at 37°C throughout all experiments and perfused and superfused at a rate of 5–10 and 8–10 ml/min, respectively, with Tyrode’s solution. The composition of the Tyrode’s solution was as follows (in mmol/L): NaCl 129, KCl 4, NaH2PO4 0.9, NaHCO3 20, CaCl2 1.8, MgSO4 0.5, D-glucose 5.5 (pH=7.4). Transmembrane action potential (AP) recordings were obtained either differentially or referenced to ground using standard and floating glass microelectrodes (2.7 M KCl, 10–25 MΩ DC resistance) connected to a high input impedance amplification system (WPI, Sarasota, FL). The signals were displayed on Tektronix oscilloscopes, amplified (model 1903–4 programmable amplifiers, Cambridge Electronic Design (CED, Philadelphia, PA), digitized (model 1401 AD/DA system, CED), analyzed (Spike 2 for Window) and stored on computer hard drive. A pseudo-ECG was recorded with 2 electrodes consisting of Ag/AgCl half cells were placed in the Tyrode’s solution bathing the preparation, 1.0 to 1.2 cm from opposite sides of the atrial or to the ventricular coronary-perfused preparation. The basic stimulus (S1) was delivered at progressively endocardium at a BCL of 500 ms. A premature stimulus (S2) was delivered briefer S1–S2 intervals (with 10 ms resolution; stimulation with a diastolic threshold of excitation X 2 amplitude) until the refractory period was reached in an attempt to induce AF. The effective refractory period (ERP) was defined as the longest S1-S2 interval at which S2 failed to capture. AF inducibility was examined under each condition separately (control, PD-118057 + Quinidine, + Lidocaine, + E-4301 or + Isoproterenol). APD dispersion was determined as the inter-regional atrial differences between the longest and shortest APD measured at 70% and 90% repolarization (APD70 and APD90, respectively), recording from the crista terminalis (CT) and the pectinate muscle (PM).
Figure 1.
Unfolded isolated canine coronary-perfused right atrial preparation.
The dominant frequency (DF) of activation during AF was determined using Fast Fourier transform (FFT) (3.93 sec duration, 8192 bins, providing 0.254-Hz spectral resolution, Spike 2 for Windows, Cambridge, England).
Drugs
PD-118057, a kind gift of Pfizer Pharmaceuticals, was prepared as a 10 mM stock in 100% Dimethyl Sulfoxide (DMSO) and added to Tyrode’s solution at final concentration of 20 μM. Quinidine, Lidocaine and E-4301 were prepared as a 20 mM stock in Millipore waterand added to Tyrode’s solution to achieve final concentrations of 10 μM, 20 uM and 5 μM, respectively. Isoproterenol was prepared as a stock solution of 1 mM in Millipore water and added to Tyrode’s solution to achieve final concentrations of 100 nM.
Statistical Analysis
Statistical analysis was performed through the use of a paired or unpaired Student’s t test and one way repeated-measures or multiple comparisons ANOVA followed by Bonferroni’s test, as appropriate. All data are expressed as mean ± standard deviation (SD). Statistical significance was assumed at P<0.05.
The authors had full access to and take responsibility for the integrity of the data. All authors have read and agree to the manuscript as written. All experiments were performed in conformance with the guidelines of the Institutional Animal Care Committee.
Results
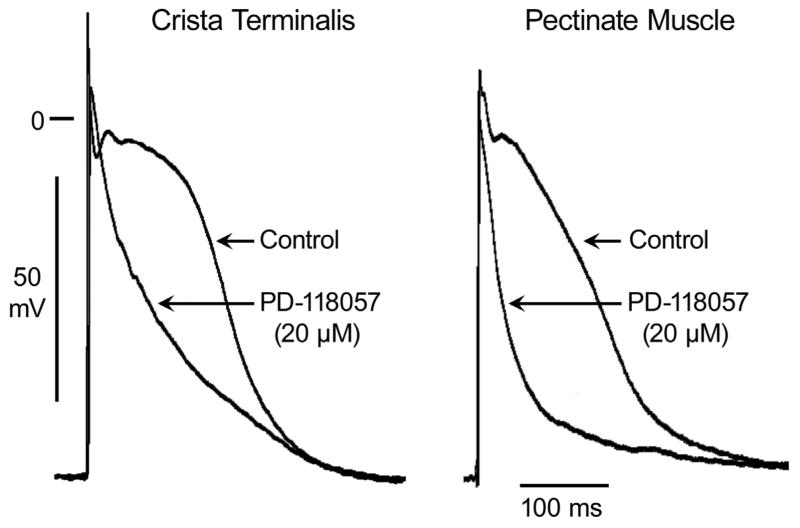
The IKr agonist PD-118057 abbreviated APD90 from 236± 23 to 195± 30 ms and APD70 from 164± 21 to 116± 30 ms in CT (p< 0.001; n= 28) and APD90 from 212± 20 to 139± 3 and APD70 from 142± 19 to 67± 25 in PM (p< 0.001; n= 28) (Fig 2). Abbreviation of APD was accompanied by abbreviation of ERP in PM from 149±12 to 100±10 (p< 0.001; n= 28) (Figs. 2 and 3). However, PD-118057 produced a greater abbreviation of APD70 in PM than in CT (Fig. 2). This differential abbreviation of APD in PM and CT, significantly increased the spatial dispersion of repolarization (SDR) from 27±17 to 51±32 (p= 0.002; n= 28) (Fig. 3).
Figure 2.
Heterogeneous abbreviation of action potential duration (APD) by IKr agonist PD-118057 (20 μM) in coronary-perfused canine right atrium. PD-118057 produced a greater abbreviation of APD in pectinate muscle than in crista terminalis.
Figure 3.
Composite data of the effect of PD-118057 (20 μM) to abbreviate effective refractory period (ERP) and differentially abbreviate action potential duration (APD) measured at 70 and 90% repolarization (APD70 and APD90) of pectinate muscle (PM) and crista terminalis (CT), thus increasing spatial dispersion of repolarization (SDR) in all 28 preparations. Reported ERP was recorded from PM region. * PD-118057 vs. respective control, p< 0.05
† vs. respective CT APD value, p< 0.05
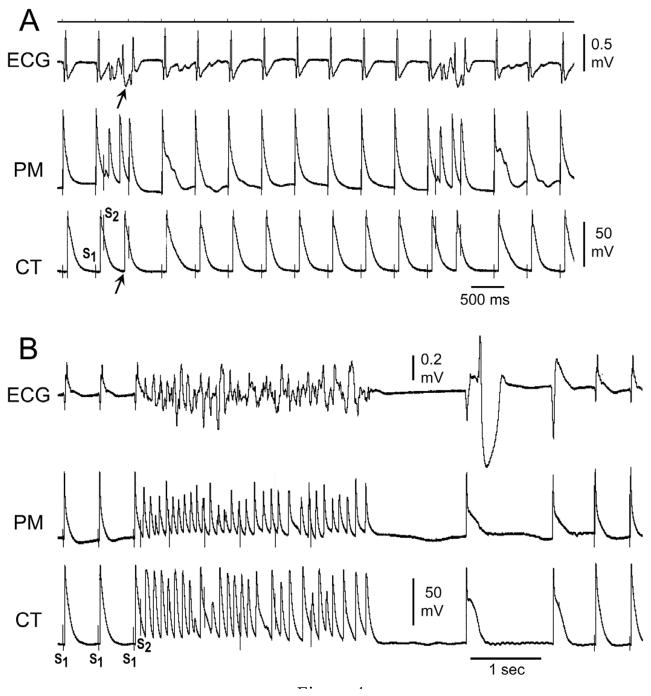
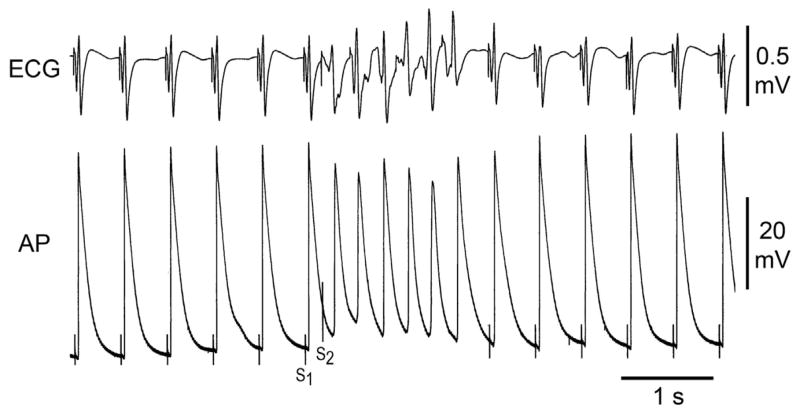
Under these conditions, with basic stimulation (S1) at a CL of 500 ms, we applied single premature stimuli (S2) to PM endocardium at progressively shorter S1–S2 intervals in an attempt to induce AF. A single extrastimulus applied to the PM region gave rise to non-sustained (< 30 s) AF in 26/28 (93%) preparations exposed to PD-118057 (Fig. 4). In only one of these, did non-sustained AF degenerate into sustained AF. In one preparation, permanent AF was induced. AF (>4 beats) could not be induced in any preparation under control conditions. Thus, PD-118057 via its actions to increase IKr, markedly abbreviated APD and increased SDR, leading to the development of AF.
Figure 4.
A: PD-118057 induces a greater abbreviation of action potential duration (APD) in pectinate muscle (PM) than in crista terminalis (CT), facilitating the induction of atrial fibrillation (AF). A single extrastimulus applied to the pectinate muscle region gives rise to a response in PM but not CT, resulting in the generation of a single reentrant extrasystole (arrow). B: With further exposure to PD-118057, a single premature stimulus to the pectinate muscle region of same arterially-perfused canine atrial preparation leads to induction of atrial fibrillation (AF). Once again, the IKr agonist is observed to abbreviate APD of PM more than that of CT.
We next examined the effectiveness of various antiarrhythmic drugs to suppress AF in the model. We selected drugs representing Vaughan Williams Class IA (quinidine), Class IB (lidocaine) and Class III (the IKr blocker E-4301). The effects of these agents were evaluated after induction of AF using PD-118057. Each agent was added to the coronary perfusate in the continued presence of PD-118057. Table 1 summarizes the effect of these drugs on APD70 in PM and CT, ERP and AF inducibility.
Table 1.
Effect of lidocaine, E-4031, quinidine and isoproterenol on atrial electrophysiological parameters and atrial fibrillation (AF) inducibility in an SQT1 model of AF.
| Lidocaine (20 μM) (n=5) |
E-4031 (5 μM) (n=5) |
Quinidine (10 μM) (n=5) |
Isoproterenol (100 nM) (n=6) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | PD- 118057 (20 μM) |
+Lidocaine | Control | PD- 118057 (20 μM) |
+E-4031 | Control | PD- 118057 (20 μM) |
+Quinidine | Control | PD- 118057 (20 μM) |
+Isoproterenol | |
| APD70CT (ms) | 187±13 | 126±17* | 115±42 | 170±16 | 116±25* | 146±26† | 171±24 | 96±42* | 134±25† | 160±36 | 110±51* | 53±22† |
| APD70PM (ms) | 145±12 | 100±15* | 83±12† | 154±24 | 56±27* | 96±28† | 124±20 | 45±10* | 76±10† | 137±30 | 62±27* | 22±13† |
| ERP PM (ms) | 152±16 | 101±7* | 158±22† | 158±16 | 105±8* | 113±10† | 148±13 | 100±17* | 174±24† | 141±17 | 100±12* | 73±8† |
| AF/AT | 0/5 | 5/5 (AF) | 1/5 (AF) 4/5 (AT) | 0/5 | 5/5 (AF) | 4/5 (AF) | 0/5 | 5/5 (AF) | 0/5 | 0/6 | 6/6 (AF) | 6/6 (AF) |
APD70: Action potential duration measured at 70% repolarization, AF: atrial fibrillation, AT: atrial tachycardia; CT: crista terminalis, ERP: effective refractory period, PM: pectinate muscle
PD-118057 vs. control; p< 0.05
Lidocaine/E-4031/Quinidine/Isoproterenol vs. PD-118057; p< 0.05
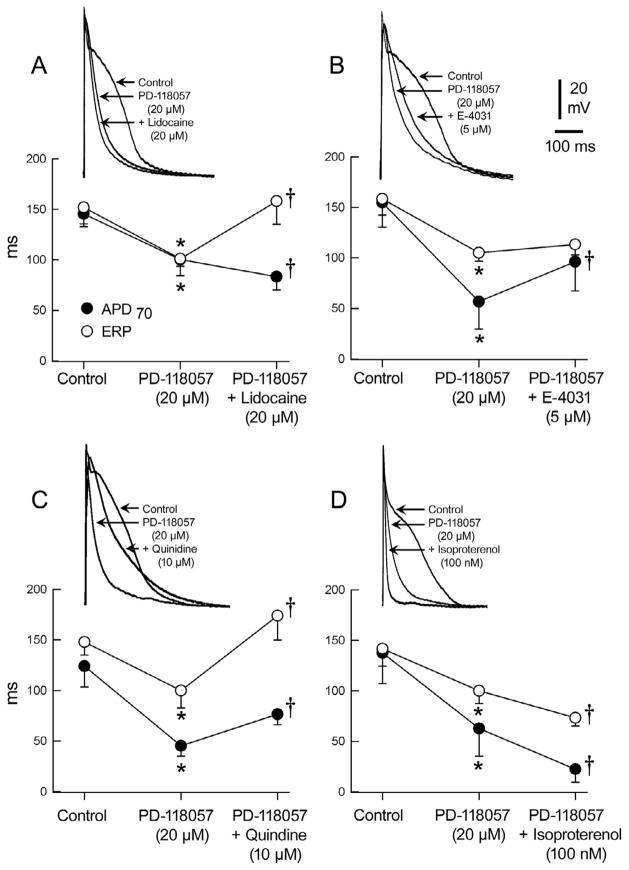
Lidocaine, a selective sodium channel current (INa) blocker, was effective in reversing the IKr agonist-induced abbreviation of ERP, but not APD, whereas E-4031, a selective IKr blocker, reversed both the PD-118057-induced abbreviation of APD and ERP, but not to control levels. Quinidine, possessing both IKr and INa inhibitory effects, similarly reversed both the APD and ERP abbreviation, and was the only agent effective in preventing the induction of AF in all cases (Fig. 5). Lidocaine, despite restoring ERP to control values, permitted induction of brief episodes of atrial tachycardia in 4/5 preparations (Fig. 6) and AF in 1/5.
Figure 5.
Effects of PD-118057 on action potential morphology and effective refractory period (ERP) recorded from the pectinate muscle region of the arterially-perfused canine atrial preparation and the effect of various antiarrhythmic drugs. Upper traces show representative traces and graphs below present the composite data with each drug. A: Lidocaine (20 μM) did not prolong APD but significantly prolonged ERP. B: Although E- 4031(5 μM) prolonged APD and ERP, it lacked the ability to reverse APD and ERP back to control conditions. C: Quinidine (10 μM) prolonged both APD and ERP, but only ERP reversed back to control conditions. D: Isoproterenol (100 nM) accentuated the effect of PD-118057 (20 μM) further abbreviating APD70 and reducing the effective refractory period (ERP). * PD-118057 vs. control; p< 0.05
†Lidocaine/E-4031/Quinidine/Isoproterenol vs. PD-118057; p< 0.05
Figure 6.
Lidocaine (20 μM) added to PD-118057 containing coronary perfusate prevented AF, but permitted induction of short episodes of atrial tachycardia with a single premature stimulus to the pectinate muscle region.
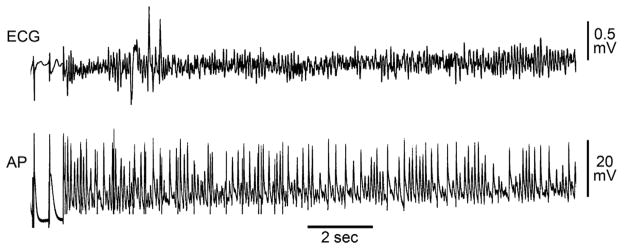
Addition of isoproterenol (100 nM) to the coronary-perfusate containing PD-118057 further abbreviated ERP and APD70 in both CT and PM (Table 1 and Fig. 5D). With PD-118057 alone, a premature stimulus was effective in inducing non-sustained AF in 6/6 preparations. After adding isoproterenol, a premature stimulus induced sustained AF in 5/6 preparations (Fig. 7). The dominant frequency of atrial activation was much faster after isoproterenol (12± 1 vs. 8± 0.8 Hz; p= 0.002). In the absence of PD-118057, isoproterenol alone did not induce sustained AF in 8/8 preparations, although non-sustained AF (<30 sec) could be induced in 2/8 preparations.
Figure 7.
Isoproterenol (100 nM) in the presence of PD-118057 (20 μM) permits induction of very rapid sustained (>30sec) atrial fibrillation (AF).
Discussion
To our knowledge, this is the first experimental model of SQT syndrome involving atria. Using PD-118057, an IKr agonist, we were able to recapitulate the clinical atrial arrhythmic manifestations of SQT1 and examine the cellular mechanisms underlying the development of AF as well as the mechanism of action of antiarrhythmic drug therapy for AF under SQT1 conditions. The model also enabled us to test the hypothesis that sympathetic stimulation may contribute to or aggravate the atrial arrhythmic burden in patients with shorter than normal QT intervals.
Although AF is the most common arrhythmia in humans, our knowledge about the ionic mechanisms underlying this arrhythmia is far from complete. Genetic syndromes and ion channel mutations can provide insight into our understanding of the basic mechanisms contributing to the development of AF. In our SQT1 model, abbreviation of APD facilitated the development of the substrate for reentry by abbreviating ERP and reducing wavelength as well as by amplifying SDR, secondary to heterogeneous abbreviation of APD. Interestingly, patients with lone AF were found in one study 15 to have shorter QT intervals compared to control. These findings demonstrated that even in the absence of genetically identifiable channelopathies, patients with AF and no structural heart disease have significantly shorter QT intervals than their age- and gender-matched healthy counterparts.15
Human atrial cells isolated from patients with AF have been found to have reduced levels of L-type calcium current (ICa,L) and transient outward current (Ito) and increased levels of inward rectifier potassium current (IK1) and acetylcholine-activated potassium current (IK-ACh).16 Activation of IK-ACh was found to accelerate AF in humans.17 Gain of function in IKs due to mutations in KCNQ118 and KCNE219,20 genes were found to be associated with familial AF. The common pathway in these studies as well as in SQT patients is the increase in K+ conductance in the atrium abbreviating APD and ERP. Our study provides evidence in support of the hypothesis that amplified atrial heterogeneity leading to SDR contributes to the development of AF in SQTS.
Transmural dispersion of repolarization (TDR) has been shown to exist in the canine right atrium and to play a role in arrhythmogenesis.21, 22 It is possible that accentuation of atrial TDR could have contributed to the development of atrial arrhythmias in our investigation. We did not record transmural dispersion of repolarization in the current study because of technical difficulties (one has to flip the preparations over several times during the course of an experiment in order to accomplish this).
It is not clear why PD-118057 caused a greater abbreviation of repolarization in PM compared to CT. The densities of IKr and IKs are similar in canine PM and CT23 and the extent of APD prolongation induced by IKr inhibition in both PM and CT is similar as well.22
It is noteworthy that whereas AF is often initiated by rapid firing foci arising from the pulmonary veins taking advantage of a substrate in the left atrium, in other cases it has been shown that the trigger and substrate for AF may arise from different regions of either atria.24–26 The present study provides evidence in support of the thesis that AF can arise from a substrate and trigger located exclusively in the right atrium.
Isoproterenol alone did not induce sustained AF in any of our preparations. However, when added in the presence of the IKr agonist PD-118057, we were able to induce sustained AF. Our results are in accord with previous studies demonstrating that sympathetic stimulation by itself is not very effective in inducing AF,27,28 but that it plays a role in the maintenance of AF 28. Our study suggests that adrenergic interventions or hyper-adrenergic states may place SQTS patients at higher risk for life-threatening arrhythmic events.
Our experimental model also provides insight into the optimal approach to therapy in the management of AF in SQTS and the mechanistic foundation for this approach. Quinidine was found to be most effective in prolonging ERP and in preventing the induction of AF, due to its combined actions to inhibit IKr and INa. This combination of ion channel effects has recently been highlighted as an effective atrial-selective approach in the management of AF.29–31 Gaita et al.13 demonstrated the efficacy of quinidine in patients with SQTS. Hydroquinidine caused QT prolongation, increased ventricular ERP and rendered VF non inducible, whereas the Class IC and III antiarrhythmic drugs failed to do so. Moreover, quinidine also restored QT-RR relationship towards the normal range.32 In a one year follow up, patients treated with hydroquinidine remained asymptomatic and no further episodes of ventricular arrhythmia were detected. Although the effectiveness quinidine has only been demonstrated in SQT1, there is reason to believe that it may be effective in the other forms of SQTS. Prolongation of QT interval by quinidine has been reported in one patient with SQT4.33 In contrast, IKr blockers such as sotalol were not effective.13 IKr antagonists were also not effective in preventing and terminating vagally-mediated AF in dog 34 or in a goat AF model produced by prolonged atrial tachypacing.35, 36 During a short action potential, fewer channels are activated and therefore IKr blockers may be less effective.35 This may in part explain why E-4031 in our model as well as sotalol in SQT patients does not prevent induction of arrhythmias. Lidocaine, an INa blocker was also not effective in preventing induction of atrial arrhythmias in our model as in clinical AF.37 Despite restoring ERP to normal, atrial arrhythmias were still inducible after lidocaine (although not as easily as before), suggesting that dispersion of repolarization may be more important than APD in the generation of AF. Only blockade of both potassium and sodium currents prevented the induction of AF. Thus, the wedge model not only mimics the clinical syndrome in its ability to produce atrial arrhythmias, but is also consistent with the efficacy of anti arrhythmic therapy in the clinic.
It is noteworthy that the lack of an effect of sotalol in SQT1 cases associated with an N588K mutation in KCNH2 is due to the fact that the gain of function in IKr current caused by the missense mutation also desensitizes the channel to traditional IKr blockers such as sotalol. Heterologous expression studies have shown that the N588K mutation in KCNH2 dramatically increases IKr density and reduces the affinity of the channel to d-sotalol by 20-fold.7 Similarly, the affinity of E-4031, was shown to be reduced.38 The reduced sensitivity to the IKr blockers is attributed to the +90 mV shift in the voltage-dependence of inactivation of human ether-a-go-go-related gene (HERG) channels.39 The inactivated state of the channel normally stabilizes the interaction of the channel with most IKr blockers. Thus, failure of rectification of current due to loss of inactivation of the channel renders the IKr blockers in N588K KCNH2 channels largely ineffective.7, 38, 39 Consistent with this observation, heterologous expression showed that affinity of mutant channel for an open-state channel blocker like quinidine is relatively high.32,38 Indeed, the N588K mutation reduces the affinity of quinidine for the IKr channel by only 5.8-fold.
Limitations of the Study
Drug-mediated accentuation of IKr, although capable of recapitulating the electrophysiologic and arrhythmic manifestations of the SQT1, may not produce a change in IKr qualitatively equivalent to that encountered in all forms of congenital SQT1. Of note, a specific genetic model of the disease would have the same limitation in that it would not be representative of other genetic forms of the syndrome.
Extrapolation of our results obtained from in vitro animal models to the clinic or to the understanding of in vivo phenomenon must be done with great caution. The absence of exogenous autonomic modulators and other hormones absent in isolated Tyrode’s perfused preparations may influence the results.
Acknowledgments
Financial Support: Supported by NIH grant HL47678 from NHLBI (CA) and by New York State and Florida Grand Lodges Free and Accepted Masons. Dr. Eyal Nof was a recipient of fellowship grants from BIOTRONIK® and the “American Physicians Fellowship for Medicine” in Israel.
We gratefully acknowledge the expert technical assistance of Judy Hefferon and Robert Goodrow, Jr.
Abbreviations
- AF
atrial fibrillation
- APG
Atrial Appendage
- APD
action potential duration
- APD70
Action potential duration measured at 70% repolarization
- APD90
Action potential duration measured at 90% repolarization
- AT
atrial tachycardia
- AVR
atrio-ventricular ring
- CT
crista terminalis
- DF
dominant frequency
- DMSO
Dimethyl Sulfoxide
- ECG
electrocardiogram
- ERP
effective refractory period
- FFT
Fast Fourier transform
- HERG
human ether-a-go-go-related gene
- ICa,L
L- type calcium current
- IK-Ach
acetylcholine-activated potassium current
- IK1
inward rectifier potassium channel current
- INa
sodium channel current
- Ito
transient outward current
- IVC
Inferior Vena Cava
- PM
pectinate muscle
- RA
right atrium
- SCD
sudden cardiac death
- SD
standard deviation
- SDR
spatial dispersion of repolarization
- SQTS
short QT syndrome
- SVC
Superior Vena Cave
- TDR
Transmural dispersion of repolarization
- VF
ventricular fibrillation
Footnotes
Conflict of Interest: None
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Viskin S, Zeltsner D, Ish-Shalom M, et al. Is idiopathic ventricular fibrillation a short QT syndrome? Comparison of QT intervals of patients with idiopathic ventricular fibrillation and healthy controls. Heart Rhythm. 2004;1:587–91. doi: 10.1016/j.hrthm.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 2.Gussak I, Brugada P, Brugada J, et al. Idiopathic short QT interval: a new clinical syndrome? Cardiology. 2000;94:99–102. doi: 10.1159/000047299. [DOI] [PubMed] [Google Scholar]
- 3.Gussak I, Brugada P, Brugada J, et al. ECG phenomenon of idiopathic and paradoxical short QT intervals. Cardiac Electrophysiol Rev. 2002;6:49–53. doi: 10.1023/a:1017931020747. [DOI] [PubMed] [Google Scholar]
- 4.Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: a familial cause of sudden death. Circulation. 2003;108:965–70. doi: 10.1161/01.CIR.0000085071.28695.C4. [DOI] [PubMed] [Google Scholar]
- 5.Bjerregaard P, Gussak I. Short QT syndrome: mechanisms, diagnosis and treatment. Nat Clin Pract Cardiovasc Med. 2005;2:84–7. doi: 10.1038/ncpcardio0097. [DOI] [PubMed] [Google Scholar]
- 6.Gussak I, Bjerregaard P. Short QT syndrome-5 years of progress. J Electrocardiol. 2005;38:375–7. doi: 10.1016/j.jelectrocard.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 7.Brugada R, Hong K, Dumaine R, et al. Sudden death associated with short-QT syndrome linked to mutations in HERG. Circulation. 2004;109:30–5. doi: 10.1161/01.CIR.0000109482.92774.3A. [DOI] [PubMed] [Google Scholar]
- 8.Bellocq C, Van Ginneken AC, Bezzina CR, et al. Mutation in the KCNQ1 gene leading to the short QT-interval syndrome. Circulation. 2004;109:2394–7. doi: 10.1161/01.CIR.0000130409.72142.FE. [DOI] [PubMed] [Google Scholar]
- 9.Priori SG, Pandit SV, Rivolta I, et al. A novel form of short QT syndrome (SQT3) Is caused by a mutation in the KCNJ2 gene. Circ Res. 2005;96:800–7. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- 10.Extramiana F, Antzelevitch C. Amplified transmural dispersion of repolarization as the basis for arrhythmogenesis in a canine ventricular-wedge model of short QT syndrome. Circulation. 2004;110:3661–6. doi: 10.1161/01.CIR.0000143078.48699.0C. [DOI] [PubMed] [Google Scholar]
- 11.Patel C, Antzelevitch C. Cellular basis for arrhythmogenesis in an experimental model of the SQT1 form of the short QT syndrome. Heart Rhythm. 2008;5:585–90. doi: 10.1016/j.hrthm.2008.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hong K, Bjerregaard P, Gussak I, et al. Short QT syndrome and atrial fibrillation caused by mutation in KCNH2. J Cardiovasc Electrophysiol. 2005;16:394–6. doi: 10.1046/j.1540-8167.2005.40621.x. [DOI] [PubMed] [Google Scholar]
- 13.Gaita F, Giustetto C, Bianchi F, et al. Short QT syndrome: pharmacological treatment. J Am Coll Cardiol. 2004;43:1494–9. doi: 10.1016/j.jacc.2004.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Giustetto C, Di Monte F, Wolpert C, et al. Short QT syndrome: clinical findings and diagnostic-therapeutic implications. Eur Heart J. 2006;27:2440–7. doi: 10.1093/eurheartj/ehl185. [DOI] [PubMed] [Google Scholar]
- 15.Poglajen G, Fister M, Radovancevic B, et al. Short QT Interval and Atrial Fibrillation in Patients Without Structural Heart Disease. J Am Coll Cardiol. 2006;47:1905–7. doi: 10.1016/j.jacc.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 16.Bosch RF, Zeng X, Grammer JB, et al. Ionic mechanisms of electrical remodeling in human atrial fibrillation. Cardiovasc Res. 1999;44:121–31. doi: 10.1016/s0008-6363(99)00178-9. [DOI] [PubMed] [Google Scholar]
- 17.Atienza F, Almendral J, Moreno J, et al. Activation of inward rectifier potassium channels accelerates atrial fibrillation in humans: evidence for a reentrant mechanism. Circulation. 2006;114:2434–42. doi: 10.1161/CIRCULATIONAHA.106.633735. [DOI] [PubMed] [Google Scholar]
- 18.Chen YH, Xu SJ, Bendahhou S, et al. KCNQ1 gain-of-function mutation in familial atrial fibrillation. Science. 2003;299:251–4. doi: 10.1126/science.1077771. [DOI] [PubMed] [Google Scholar]
- 19.Yang Y, Xia M, Jin Q, et al. Identification of a KCNE2 gain-of-function mutation in patients with familial atrial fibrillation. Am J Hum Genet. 2004;75:899–905. doi: 10.1086/425342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lundby A, Ravn LS, Svendsen JH, et al. KCNQ1 mutation Q147R is associated with atrial fibrillation and prolonged QT interval. Heart Rhythm. 2007;4:1532–41. doi: 10.1016/j.hrthm.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 21.Schuessler RB, Kawamoto T, Hand DE, et al. Simultaneous epicardial and endocardial activation sequence mapping in the isolated canine right atrium. Circulation. 1993;88:250–63. doi: 10.1161/01.cir.88.1.250. [DOI] [PubMed] [Google Scholar]
- 22.Burashnikov A, Mannava S, Antzelevitch C. Transmembrane action potential heterogeneity in the canine isolated arterially-perfused atrium: effect of IKr and Ito/IKur block. Am J Physiol. 2004;286:H2393–H2400. doi: 10.1152/ajpheart.01242.2003. [DOI] [PubMed] [Google Scholar]
- 23.Feng J, Yue L, Wang Z, et al. Ionic mechanisms of regional action potential heterogeneity in the canine right atrium. Circ Res. 1998;83:541–51. doi: 10.1161/01.res.83.5.541. [DOI] [PubMed] [Google Scholar]
- 24.Haissaguerre M, Sanders P, Hocini M, et al. Catheter ablation of long-lasting persistent atrial fibrillation: critical structures for termination. J Cardiovasc Electrophysiol. 2005;16:1125–37. doi: 10.1111/j.1540-8167.2005.00307.x. [DOI] [PubMed] [Google Scholar]
- 25.Tsai CF, Tai CT, Hsieh MH, et al. Initiation of atrial fibrillation by ectopic beats originating from the superior vena cava: electrophysiological characteristics and results of radiofrequency ablation. Circulation. 2000;102:67–74. doi: 10.1161/01.cir.102.1.67. [DOI] [PubMed] [Google Scholar]
- 26.Sanders P, Berenfeld O, Hocini M, et al. Spectral analysis identifies sites of high-frequency activity maintaining atrial fibrillation in humans. Circulation. 2005;112:789–97. doi: 10.1161/CIRCULATIONAHA.104.517011. [DOI] [PubMed] [Google Scholar]
- 27.Liu L, Nattel S. Differing sympathetic and vagal effects on atrial fibrillation in dogs: role of refractoriness heterogeneity. Am J Physiol. 1997;273:H805–H816. doi: 10.1152/ajpheart.1997.273.2.H805. [DOI] [PubMed] [Google Scholar]
- 28.Sharifov OF, Fedorov VV, Beloshapko GG, et al. Roles of adrenergic and cholinergic stimulation in spontaneous atrial fibrillation in dogs. J Am Coll Cardiol. 2004;43:483–90. doi: 10.1016/j.jacc.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 29.Burashnikov A, Antzelevitch C. How do atrial-selective drugs differ from antiarrhythmic drugs currently used in the treatment of atrial fibrillation? J Atrial Fibrillation. 2008;1:98–107. doi: 10.4022/jafib.v1i1.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Burashnikov A, Antzelevitch C. New pharmacological strategies for the treatment of atrial fibrillation. Ann Noninvasive Electrocardiol. 2009;14:290–300. doi: 10.1111/j.1542-474X.2009.00305.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burashnikov A, Di Diego JM, Sicouri S, et al. Atrial-selective effects of chronic amiodarone in the management of atrial fibrillation. Heart Rhythm. 2008;5:1735–42. doi: 10.1016/j.hrthm.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wolpert C, Schimpf R, Giustetto C, et al. Further insights into the effect of quinidine in short QT syndrome caused by a mutation in HERG. J Cardiovasc Electrophysiol. 2005;16:54–8. doi: 10.1046/j.1540-8167.2005.04470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Antzelevitch C, Pollevick GD, Cordeiro JM, et al. Loss-of-function mutations in the cardiac calcium channel underlie a new clinical entity characterized by ST-segment elevation, short QT intervals, and sudden cardiac death. Circulation. 2007;115:442–9. doi: 10.1161/CIRCULATIONAHA.106.668392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rivard L, Sinno H, Shiroshita-Takeshita A, et al. The pharmacological response of ischemia-related atrial fibrillation in dogs: evidence for substrate-specific efficacy. Cardiovasc Res. 2007;74:104–13. doi: 10.1016/j.cardiores.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 35.Blaauw Y, Gogelein H, Tieleman RG, et al. “Early” class III drugs for the treatment of atrial fibrillation: efficacy and atrial selectivity of AVE0118 in remodeled atria of the goat. Circulation. 2004;110:1717–24. doi: 10.1161/01.CIR.0000143050.22291.2E. [DOI] [PubMed] [Google Scholar]
- 36.Duytschaever M, Blaauw Y, Allessie M. Consequences of atrial electrical remodeling for the anti-arrhythmic action of class IC and class III drugs. Cardiovasc Res. 2005;67:69–76. doi: 10.1016/j.cardiores.2005.02.019. [DOI] [PubMed] [Google Scholar]
- 37.Marrouche NF, Reddy RK, Wittkowsky AK, et al. High-dose bolus lidocaine for chemical cardioversion of atrial fibrillation: a prospective, randomized, double-blind crossover trial. Am Heart J. 2000;139:E8–11. doi: 10.1067/mhj.2000.106169. [DOI] [PubMed] [Google Scholar]
- 38.McPate MJ, Duncan RS, Witchel HJ, et al. Disopyramide is an effective inhibitor of mutant HERG K+ channels involved in variant 1 short QT syndrome. J Mol Cell Cardiol. 2006;41:563–6. doi: 10.1016/j.yjmcc.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 39.Cordeiro JM, Brugada R, Wu YS, et al. Modulation of IKr inactivation by mutation N588K in KCNH2: A link to arrhythmogenesis in short QT syndrome. Cardiovasc Res. 2005;67:498–509. doi: 10.1016/j.cardiores.2005.02.018. [DOI] [PubMed] [Google Scholar]